Whole Flour of Purple Maize as a Functional Ingredient of Gluten-Free Bread: Effect of In Vitro Digestion on Starch and Bioaccessibility of Bioactive Compounds
Abstract
:1. Introduction
2. Materials and Methods
2.1. Flour Samples
2.2. Chemical Composition
2.3. Viscosity and Flour Pasting Properties
2.4. Bread Making
2.5. Determination of Anthocyanins, Polyphenols, Ferulic Acid, and Antioxidant Capacity
2.6. In Vitro Static Digestion
2.6.1. Starch Digestion
2.6.2. Digestion of Antioxidant Compounds
2.7. Statistical Analysis
3. Results and Discussion
3.1. Flour Chemical Composition
3.2. Viscosity and Flour Pasting Properties
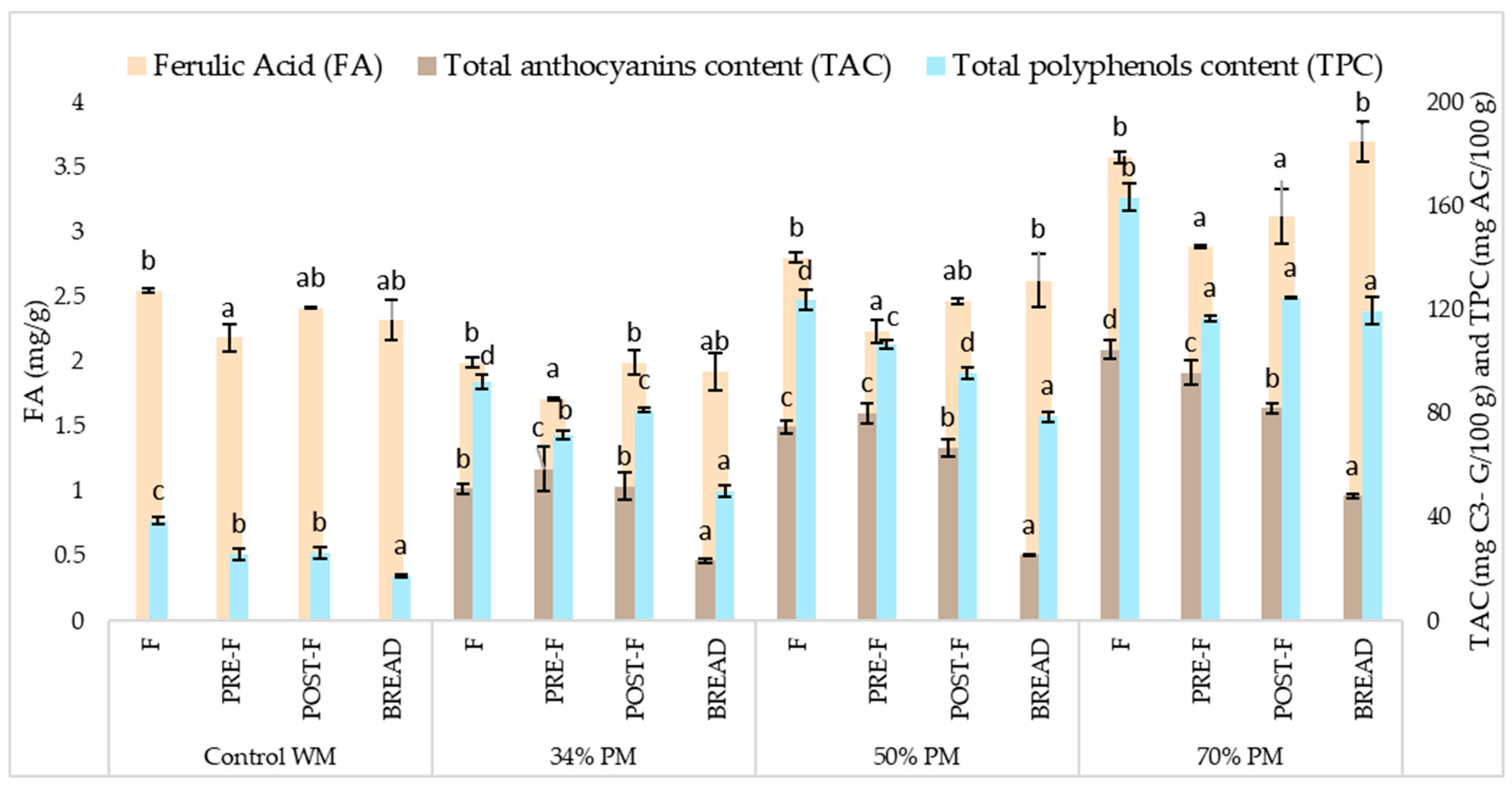
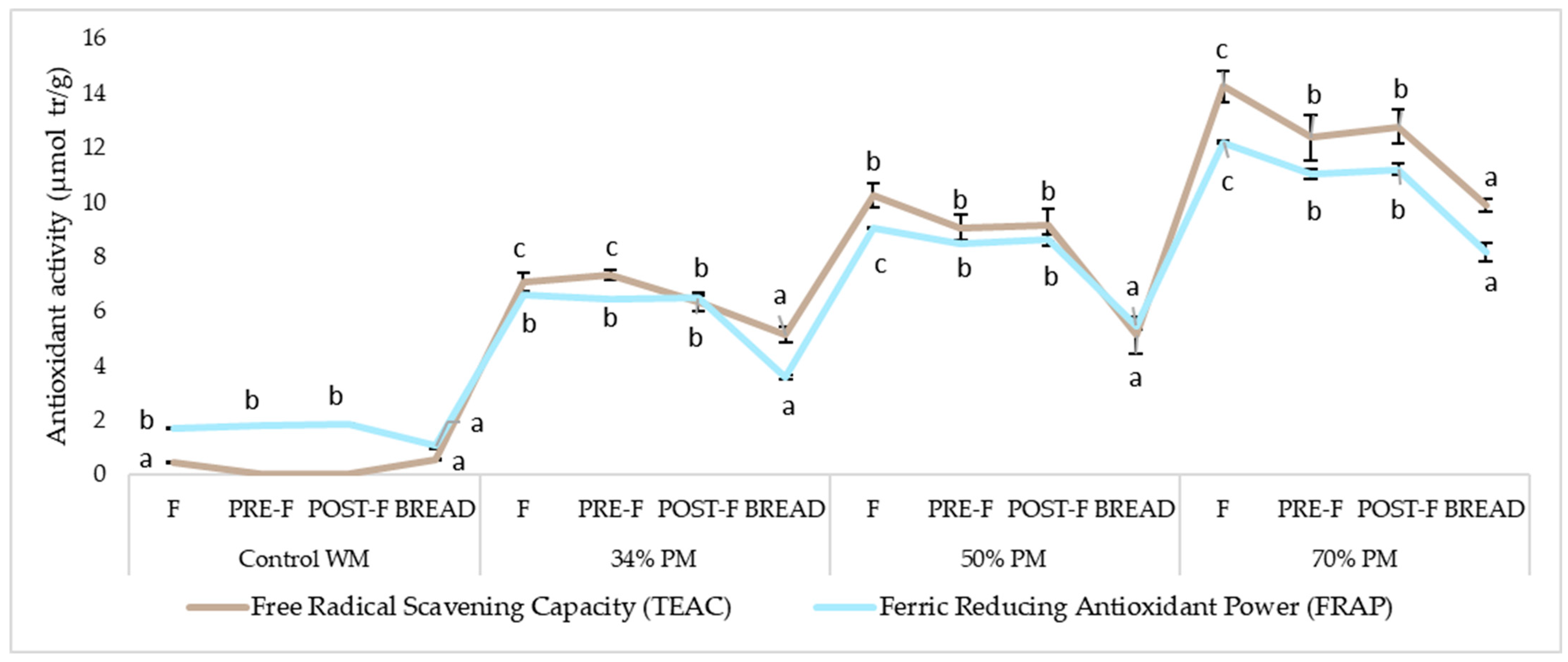
3.3. Effect of Bread Making on Anthocyanins, Polyphenols, Ferulic Acid and Antioxidant Capacity
3.3.1. Raw Flours
3.3.2. Effect of Kneading and Fermentation
3.3.3. Effect of Baking
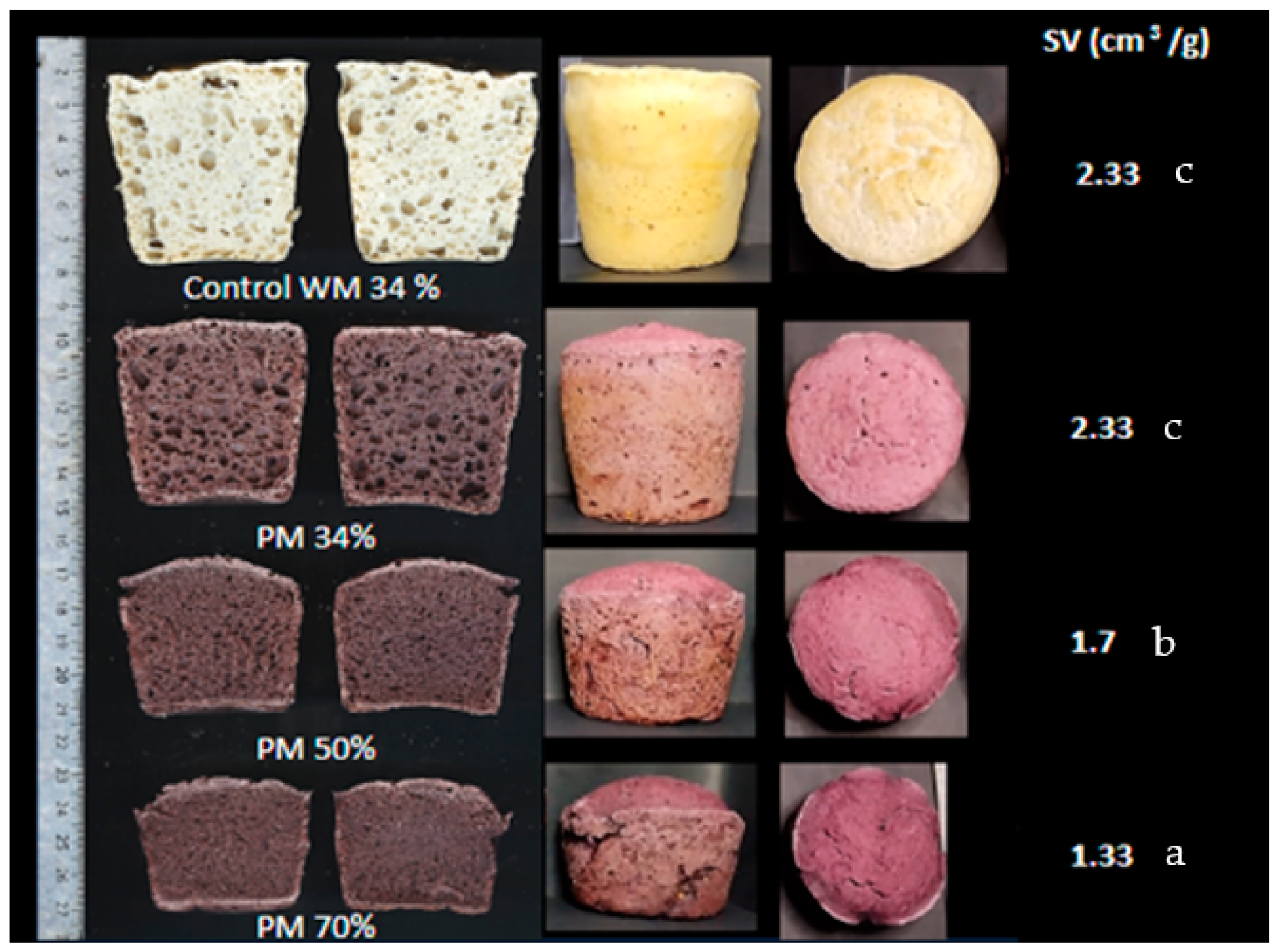
3.4. Bread Quality
3.4.1. Specific Volume and Crumb Texture
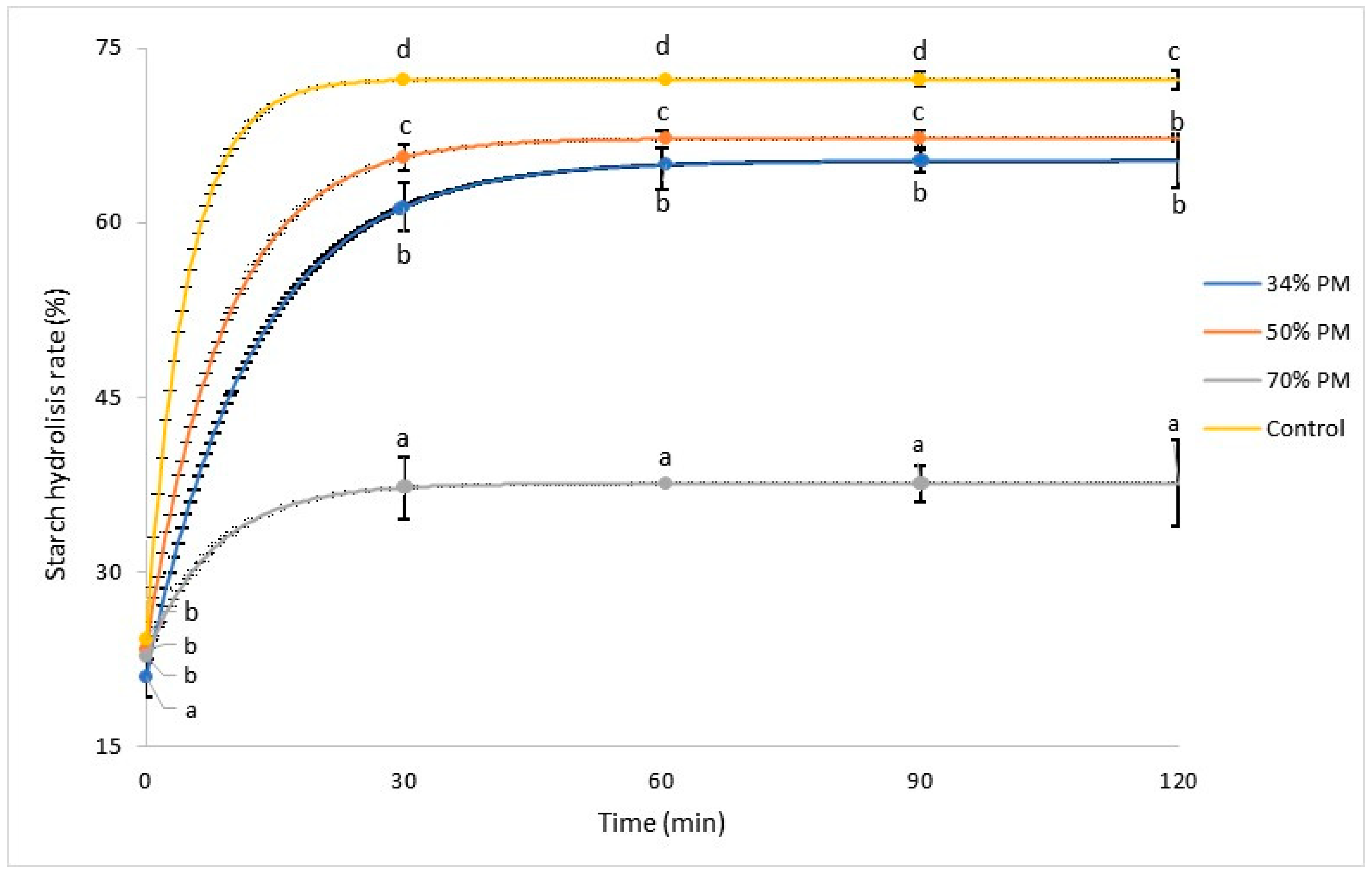
3.4.2. Starch Digestibility of Breads
3.4.3. In Vitro Digestibility of Antioxidant Compounds of Breads
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| PM | Purple maize |
| WM | White maize |
| RF | Rice flour |
| CF | Cassava flour |
| RDS | Rapidly digestible starch |
| SDS | Slowly digestible starch |
| RS | Resistant starch |
| PT | Pasting temperature |
| PV | Peak viscosity |
| FV | Final viscosity |
| TV | Trough viscosity |
| BD | Breakdown |
| SB | Setback |
| PRE-F | Pre fermented |
| POST-F | Post fermented |
| SV | Specific volume |
| CT | Crumb texture |
| N | Newton force |
| TAC | Total anthocyanin content |
| TPC | Total polyphenol content |
| FA | Ferulic acid |
| TEAC | Trolox Equivalent Antioxidant Capacity |
| FRAP | Ferric Reducing Antioxidant Power |
| DNS | 3,5-dinitrosalicylic acid |
| TH | Total hydrolysis |
| PB | Potentially bioavailable |
| B | Bioaccessible |
References
- Khemiri, S.; Khelifi, N.; Nunes, M.C.; Ferreira, A.; Gouveia, L.; Smaali, I.; Raymundo, A. Microalgae biomass as an additional ingredient of gluten-free bread: Dough rheology, texture quality and nutritional properties. Algal Res. 2020, 50, 101998. [Google Scholar] [CrossRef]
- da Costa Borges, V.; Fernandes, S.S.; da Rosa Zavareze, E.; Haros, C.M.; Hernandez, C.P.; Guerra Dias, A.R.; de las Mercedes Salas-Mellado, M. Production of gluten free bread with flour and chia seeds (Salvia hispânica L.). Food Biosci. 2021, 43, 101294. [Google Scholar] [CrossRef]
- Boateng, I.D.; Mustapha, A.; Kuehnel, L.; Daubert, C.R.; Kumar, R.; Agliata, J.; Flint-Garcia, S.; Wan, C.; Somavat, P. From purple corn waste (pericarp) to polyphenol-rich extract with higher bioactive contents and superior product qualities using two-step optimization techniques. Ind. Crop. Prod. 2023, 200, 116871. [Google Scholar] [CrossRef]
- Lao, F.; Sigurdson, G.T.; Giusti, M.M. Health Benefits of Purple Corn (Zea mays L.) Phenolic Compounds. Compr. Rev. Food Sci. Food Saf. 2017, 16, 234–246. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.H.; Lee, C.H.; Wong, S.; Ong, P.Y.; Hamdan, N.; Azmi, N.A. UPLC-orbitrap-MS/MS based characterization of phytochemical compounds from Malaysia purple corn (Zea mays). Biocatal. Agric. Biotechnol. 2021, 32, 101922. [Google Scholar] [CrossRef]
- Minekus, M.; Alminger, M.; Alvito, P.; Ballance, S.; Bohn, T.; Bourlieu, C.; Carrière, F.; Boutrou, R.; Corredig, M.; Dupont, D.; et al. A standardised static in vitro digestion method suitable for food—An international consensus. Food Funct. 2014, 5, 1113–1124. [Google Scholar] [CrossRef] [PubMed]
- Bustos, M.C.; Vignola, M.B.; Pérez, G.T.; León, A.E. In vitro digestion kinetics and bioaccessibility of starch in cereal food products. J. Cereal Sci. 2017, 77, 243–250. [Google Scholar] [CrossRef]
- Cosme, P.; Rodríguez, A.B.; Espino, J.; Garrido, M. Plant Phenolics: Bioavailability as a Key Determinant of Their Potential Health-Promoting Applications. Antioxidants 2020, 9, 1263. [Google Scholar] [CrossRef]
- Rocchetti, G.; Giuberti, G.; Gallo, A.; Bernardi, J.; Marocco, A.; Lucini, L. Effect of dietary polyphenols on the in vitro starch digestibility of pigmented maize varieties under cooking conditions. Food Res. Int. 2018, 108, 183–191. [Google Scholar] [CrossRef]
- Camelo-Méndez, G.A.; Agama-Acevedo, E.; Tovar, J.; Bello-Pérez, L.A. Functional study of raw and cooked blue maize flour: Starch digestibility, total phenolic content and antioxidant activity. J. Cereal Sci. 2017, 76, 179–185. [Google Scholar] [CrossRef]
- Bender, D.; Schönlechner, R. Innovative approaches towards improved gluten-free bread properties. J. Cereal Sci. 2020, 91, 102904. [Google Scholar] [CrossRef]
- Burbano, J.J.; Cabezas, D.M.; Correa, M.J. Effect of walnut flour addition on rheological, thermal and microstructural properties of a gluten free-batter. LWT 2022, 154, 112819. [Google Scholar] [CrossRef]
- Bolsa de Cereales de Córdoba. Anuario Meteorológico 2016. Red de Estaciones Meteorológicas del Departamento de Información Agroeconómica. Available online: https://es.scribd.com/document/336995943/Anuario-Meteorologico-2016 (accessed on 18 August 2023).
- American Association of Cereal Chemists International (AACC). Approved Methods of Analysis, 11th ed.; Methods 08–01.01, 44–19.01, 46–13.01, 30–25.01, 32–40.01 and 74-09; AACC International: St. Paul, MN, USA, 2010. [Google Scholar]
- Lee, J.; Durst, R.W.; Wrolstad, R.E.; Wrolstad, R.E. Determination of Total Monomeric Anthocyanin Pigment Content of Fruit Juices, Beverages, Natural Colorants, and Wines by the pH Differential Method: Collaborative Study. J. AOAC Int. 2005, 88, 1269–1278. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Martinez, L.X.; Oliart-Ros, R.M.; Valerio-Alfaro, G.; Lee, C.-H.; Parkin, K.L.; Garcia, H.S. Antioxidant activity, phenolic compounds and anthocyanins content of eighteen strains of Mexican maize. LWT-Food Sci. Technol. 2009, 42, 1187–1192. [Google Scholar] [CrossRef]
- Podio, N.S.; Baroni, M.V.; Wunderlin, D.A. Relation between polyphenol profile and antioxidant capacity of different Argentinean wheat varieties. A Boosted Regression Trees study. Food Chem. 2017, 232, 79–88. [Google Scholar] [CrossRef] [PubMed]
- Navarro, J.L.; Biglione, C.; Paesani, C.; Moiraghi, M.; León, A.E.; Steffolani, M.E. Effect of wheat pearling process on composition and nutritional profile of flour and its bread-making performance. Int. J. Food Sci. Technol. 2022, 57, 249–257. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Original Contribution antioxidant activity applying an improved abts radical cation decolorization assay. Free. Radic. Biol. Med. 1999, 26, 1231–1327. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef]
- Mansilla, P.S.; Nazar, M.C.; Pérez, G.T. Comparison of Flour Starch Properties in Half-Sib Families of Opaque-2 Maize (Zea mays L.) from Argentina. Cereal Chem. 2017, 94, 942–949. [Google Scholar] [CrossRef]
- Mansilla, P.S.; Bongianino, N.F.; Nazar, M.C.; Pérez, G.T. Agronomic and chemical description of open-pollinated varieties of opaque-2 and purple maize (Zea mays L.) adapted to semiarid region of Argentina. Genet. Resour. Crop. Evol. 2021, 68, 2351–2366. [Google Scholar] [CrossRef]
- Liu, C.Y.; Amani, R.; Sulaiman, S.; Mahmood, K.; Ariffin, F.; Nafchi, A.M. Formulation and characterization of physicochemical, functional, morphological, and antioxidant properties of cassava-based rice analogue. Food Sci. Nutr. 2022, 10, 1626–1637. [Google Scholar] [CrossRef] [PubMed]
- Monthe, O.C.; Grosmaire, L.; Nguimbou, R.M.; Dahdouh, L.; Ricci, J.; Tran, T.; Ndjouenkeu, R. Rheological and textural properties of gluten-free doughs and breads based on fermented cassava, sweet potato and sorghum mixed flours. LWT 2019, 101, 575–582. [Google Scholar] [CrossRef]
- Trehan, S.; Singh, N.; Kaur, A. Characteristics of white, yellow, purple corn accessions: Phenolic profile, textural, rheological properties and muffin making potential. J. Food Sci. Technol. 2018, 55, 2334–2343. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Tian, J.; Kong, X.; Wu, D.; Chen, S.; Liu, D.; Ye, X. Proanthocyanidins from Chinese berry leaves modified the physicochemical properties and digestive characteristic of rice starch. Food Chem. 2021, 335, 127666. [Google Scholar] [CrossRef] [PubMed]
- Sivam, A.S.; Sun-Waterhouse, D.; Waterhouse, G.I.; Quek, S.; Perera, C.O. Physicochemical Properties of Bread Dough and Finished Bread with Added Pectin Fiber and Phenolic Antioxidants. J. Food Sci. 2011, 76, 97–107. [Google Scholar] [CrossRef] [PubMed]
- Suriano, S.; Balconi, C.; Valoti, P.; Redaelli, R. Comparison of total polyphenols, profile anthocyanins, color analysis, carotenoids and tocols in pigmented maize. LWT 2021, 144, 111257. [Google Scholar] [CrossRef]
- Nankar, A.N.; Dungan, B.; Paz, N.; Sudasinghe, N.; Schaub, T.; Holguin, F.O.; Pratt, R.C. Quantitative and qualitative evaluation of kernel anthocyanins from southwestern United States blue corn. J. Sci. Food Agric. 2016, 96, 4542–4552. [Google Scholar] [CrossRef]
- Colombo, F.; Cappa, C.; Bani, C.; Magni, M.; Biella, S.; Restani, P.; Di Lorenzo, C. Characterization of color, phenolic profile, and antioxidant activity of Italian pigmented rice varieties after different technological treatments. Food Biosci. 2023, 53, 102674. [Google Scholar] [CrossRef]
- Reinaldo, J.S.; Milfont, C.H.; Gomes, F.P.; Mattos, A.L.; Medeiros, F.G.; Lopes, P.F.; Filho, M.d.S.M.S.; Matsui, K.N.; Ito, E.N. Influence of grape and acerola residues on the antioxidant, physicochemical and mechanical properties of cassava starch biocomposites. Polym. Test. 2021, 93, 107015. [Google Scholar] [CrossRef]
- Rodríguez, M.; Bustos, M.; Mansilla, P.; Monsierra, L.; de Pascual-Teresa, T. Study on bioaccesibility and bioactivity of health promoting compounds from purple corn of two varieties of Spanish and Argentinian origin after in vitro digestion. In Proceedings of the 7th International Conference on Food Digestion, Cork, Ireland, 3–5 May 2020. [Google Scholar]
- Salvador-Reyes, R.; Clerici, M.T.P.S. Peruvian Andean maize: General characteristics, nutritional properties, bioactive compounds, and culinary uses. Food Res. Int. 2020, 130, 108934. [Google Scholar] [CrossRef]
- Ktenioudaki, A.; Alvarez-Jubete, L.; Gallagher, E. A Review of the Process-Induced Changes in the Phytochemical Content of Cereal Grains: The Breadmaking Process. Crit. Rev. Food Sci. Nutr. 2015, 55, 611–619. [Google Scholar] [CrossRef] [PubMed]
- Girard, A.L.; Awika, J.M. Effects of edible plant polyphenols on gluten protein functionality and potential applications of polyphenol–gluten interactions. Compr. Rev. Food Sci. Food Saf. 2020, 19, 2164–2199. [Google Scholar] [CrossRef] [PubMed]
- Angelino, D.; Cossu, M.; Marti, A.; Zanoletti, M.; Chiavaroli, L.; Brighenti, F.; Del Rio, D.; Martini, D. Bioaccessibility and bioavailability of phenolic compounds in bread: A review. Food Funct. 2017, 8, 2368–2393. [Google Scholar] [CrossRef]
- Ou, J.; Wang, M.; Zheng, J.; Ou, S. Positive and negative effects of polyphenol incorporation in baked foods. Food Chem. 2019, 284, 90–99. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Sun, Y.; Zhang, H.; Mai, Q.; Zhang, B.; Li, H.; Deng, Z. The degradation rules of anthocyanins from eggplant peel and antioxidant capacity in fortified model food system during the thermal treatments. Food Biosci. 2020, 38, 100701. [Google Scholar] [CrossRef]
- Huang, D.; Ming, R.; Yao, S.; Li, L.; Huang, R.; Tan, Y. Identification of anthocyanins in the fruits of Kadsura coccinea using UPLC-MS/MS-based metabolomics. Biochem. Syst. Ecol. 2021, 98, 104324. [Google Scholar] [CrossRef]
- McDougall, G.J.; Dobson, P.; Jordan-Mahy, N. Effect of different cooking regimes on rhubarb polyphenols. Food Chem. 2010, 119, 758–764. [Google Scholar] [CrossRef]
- Hryhorenko, N.; Krupa-Kozak, U.; Bączek, N.; Rudnicka, B.; Wróblewska, B. Gluten-free bread enriched with whole-grain red sorghum flour gains favourable technological and functional properties and consumers acceptance. J. Cereal Sci. 2023, 110, 103646. [Google Scholar] [CrossRef]
- Gu, R.; Chang, X.; Bai, G.; Li, X.; Di, Y.; Liu, X.; Sun, L.; Wang, Y. Effects of household cooking methods on changes of tissue structure, phenolic antioxidant capacity and active component bioaccessibility of quinoa. Food Chem. 2021, 350, 129138. [Google Scholar] [CrossRef]
- Tian, W.; Chen, G.; Tilley, M.; Li, Y. Changes in phenolic profiles and antioxidant activities during the whole wheat bread-making process. Food Chem. 2021, 345, 128851. [Google Scholar] [CrossRef]
- Canelo-Álvarez, F.; Figueroa-Cárdenas, J.d.D.; Martínez-Cruz, E.; Pérez-Robles, J.F.; Villa, G.A.; Mariscal-Moreno, R.M.; Medina, J.J.V. Effect of nixtamalization on gluten-free whole corn, dough viscoelasticity, and the bread-making quality of leavened corn bread. Int. J. Gastron. Food Sci. 2023, 31, 100648. [Google Scholar] [CrossRef]
- Sciarini, L.S.; Steffolani, M.E.; León, A.E. El rol del gluten en la panificación y el desafío de prescindir de su aporte en la elaboración de pan. Agriscientia 2016, 33, 61–74. [Google Scholar] [CrossRef]
- Brites, C.; Trigo, M.J.; Santos, C.; Collar, C.; Rosell, C.M. Maize-Based Gluten-Free Bread: Influence of Processing Parameters on Sensory and Instrumental Quality. Food Bioprocess Technol. 2010, 3, 707–715. [Google Scholar] [CrossRef]
- Sigüenza-Andrés, T.; Gallego, C.; Gómez, M. Can cassava improve the quality of gluten free breads? LWT 2021, 149, 111923. [Google Scholar] [CrossRef]
- Zhu, F.; Cai, Y.Z.; Sun, M.; Corke, H. Effect of phytochemical extracts on the pasting, thermal, and gelling properties of wheat starch. Food Chem. 2009, 112, 919–923. [Google Scholar] [CrossRef]
- Macfarlane, G.T.; Macfarlane, S. Bacteria, Colonic Fermentation, and Gastrointestinal Health. J. AOAC Int. 2012, 95, 50–60. [Google Scholar] [CrossRef]
- Aalim, H.; Wang, D.; Luo, Z. Black rice (Oryza sativa L.) processing: Evaluation of physicochemical properties, in vitro starch digestibility, and phenolic functions linked to type 2 diabetes. Food Res. Int. 2021, 141, 109898. [Google Scholar] [CrossRef]
- Sun, L.; Miao, M. Dietary polyphenols modulate starch digestion and glycaemic level: A review. Crit. Rev. Food Sci. Nutr. 2019, 60, 541–555. [Google Scholar] [CrossRef]
- Alvarez-Poblano, L.; Roman-Guerrero, A.; Vernon-Carter, E.J.; Alvarez-Ramirez, J. Exogenous addition of muicle (Justicia spicigera Schechtendal) extract to white maize tortillas affects the antioxidant activity, texture, color, and in vitro starch digestibility. LWT 2020, 133, 110120. [Google Scholar] [CrossRef]
- Herrera-Balandrano, D.D.; Wang, J.; Chai, Z.; Zhang, X.; Wang, J.; Wang, N.; Huang, W. Impact of in vitro gastrointestinal digestion on rabbiteye blueberry anthocyanins and their absorption efficiency in Caco-2 cells. Food Biosci. 2023, 52, 102424. [Google Scholar] [CrossRef]
- Sęczyk, Ł.; Sugier, D.; Świeca, M.; Gawlik-Dziki, U. The effect of in vitro digestion, food matrix, and hydrothermal treatment on the potential bioaccessibility of selected phenolic compounds. Food Chem. 2021, 344, 128581. [Google Scholar] [CrossRef] [PubMed]
- Pinto, D.; Moreira, M.M.; Švarc-Gajić, J.; Vallverdú-Queralt, A.; Brezo-Borjan, T.; Delerue-Matos, C.; Rodrigues, F. In-vitro gastrointestinal digestion of functional cookies enriched with chestnut shells extract: Effects on phenolic composition, bioaccessibility, bioactivity, and α-amylase inhibition. Food Biosci. 2023, 53, 102766. [Google Scholar] [CrossRef]
- Karaś, M.; Jakubczyk, A.; Szymanowska, U.; Złotek, U.; Zielińska, E. Digestion and bioavailability of bioactive phytochemicals. Int. J. Food Sci. Technol. 2017, 52, 291–305. [Google Scholar] [CrossRef]
- Eran Nagar, E.; Okun, Z.; Shpigelman, A. In vitro bioaccessibility of polyphenolic compounds: The effect of dissolved oxygen and bile. Food Chem. 2023, 404, 134490. [Google Scholar] [CrossRef]
- Baroni, M.V.; Fabani, M.P.; Adan, F.; Podio, N.S.; Wunderlin, D.A. Effect of geographical location, processing and simulated digestion on antioxidant characteristics of quince (Cydonia oblonga). Heliyon 2022, 8, e11435. [Google Scholar] [CrossRef]
- Andrade, J.K.S.; Barros, R.G.C.; Gualberto, N.C.; de Oliveira, C.S.; Shanmugam, S.; Narain, N. Influence of in vitro gastrointestinal digestion and probiotic fermentation on the bioaccessibility of gallic acid and on the antioxidant potential of Brazilian fruit residues. LWT 2022, 153, 112436. [Google Scholar] [CrossRef]
- Pešić, M.B.; Milinčić, D.D.; Kostić, A.; Stanisavljević, N.S.; Vukotić, G.N.; Kojić, M.O.; Gašić, U.M.; Barać, M.B.; Stanojević, S.P.; Popović, D.A.; et al. In vitro digestion of meat- and cereal-based food matrix enriched with grape extracts: How are polyphenol composition, bioaccessibility and antioxidant activity affected? Food Chem. 2019, 284, 28–44. [Google Scholar] [CrossRef]
- Cheng, J.; Tang, D.; Yang, H.; Wang, X.; Lin, Y.; Liu, X. The effects of mulberry polyphenols on the digestibility and absorption properties of pork myofibrillar protein in vitro. Meat Sci. 2023, 202, 109205. [Google Scholar] [CrossRef]
- Ajayi, F.F.; Alnuaimi, A.; Hamdi, M.; Mostafa, H.; Wakayama, M.; Mudgil, P.; Maqsood, S. Metabolomics approach for the identification of bioactive compounds released from young and mature soybean upon in vitro gastrointestinal digestion and their effect on health-related bioactive properties. Food Chem. 2023, 420, 136050. [Google Scholar] [CrossRef]
- Palavecino, P.M.; Ribotta, P.D.; León, A.E.; Bustos, M.C. Gluten-free sorghum pasta: Starch digestibility and antioxidant capacity compared with commercial products. J. Sci. Food Agric. 2019, 99, 1351–1357. [Google Scholar] [CrossRef]
- Rumpf, J.; Burger, R.; Schulze, M. Statistical evaluation of DPPH, ABTS, FRAP, and Folin-Ciocalteu assays to assess the antioxidant capacity of lignins. Int. J. Biol. Macromol. 2023, 233, 123470. [Google Scholar] [CrossRef] [PubMed]
| Flour 1 | Protein (%) | Lipids (%) | Ash (%) | Carbohydrates (%) |
|---|---|---|---|---|
| PM | 10.08 ± 0.56 b | 3.78 ± 0.11 b | 1.78 ± 0.09 b | 84.45 ± 0.60 a |
| WM | 8.44 ± 0.12 a | 4.42 ± 0.29 b | 1.69 ± 0.02 b | 85.45 ± 0.17 a |
| CF | nd | nd | nd | 99.51 ± 0.18 c |
| RF | 9.24 ± 0.25 ab | 2.25 ± 0.17 a | 1.51 ± 0.04 a | 86.99 ± 0.26 b |
| Flour 2 | PV (cP) | TV (cP) | BD (cP) | FV (cP) | SB (cP) | PT (°C) |
|---|---|---|---|---|---|---|
| Rice | 3759.0 ± 44.3 f | 2471.3 ± 32.6 i | 1287.7 ± 36.7 e | 5193.7 ± 39.8 j | 2722.3 ± 54.1 g | 81.2 ± 0.4 g |
| Cassava | 6380.0 ± 88.6 h | 2101.0 ± 17.4 h | 4279.0 ± 83.5 g | 2970.2 ± 42.0 h | 869.2 ± 51.2 c | 71.2 ± 0.3 a |
| WM | 2353.0 ± 102.4 e | 1147.7 ± 22.7 g | 1205.3 ± 122.0 e | 2797.3 ± 57.0 g | 1649.7 ± 60.6 e | 77.8 ± 0.5 f |
| PM | 1049.0 ± 27.0 b | 536.3 ± 18.8 a | 512.6 ± 9.5 d | 1255.3 ± 44.8 a | 719.0 ± 26.4 a | 76.9 ± 0.4 e |
| 34% WM | 1306.3 ± 37.2 c | 889.0 ± 18.0 ef | 417.3 ± 22.8 c | 1769.0 ± 31.7 e | 880.0 ± 14.7 c | 74.3 ± 0.1 c |
| 34% PM | 1409.0 ± 18.0 d | 866.6 ± 7.3 de | 542.3 ± 17.3 d | 1754.7 ± 14.4 e | 888.0 ± 11.5 cd | 74.5 ± 0.1 c |
| 50% CF-50% RF | 4158.7 ± 26.4 g | 2626.7 ± 50.2 j | 1532.0 ± 27.7 f | 4455.3 ± 47.0 i | 1828.7 ± 63.5 f | 74.0 ± 0.4 bc |
| 50% WM | 1095.3 ± 9.8 b | 830.3 ± 2.3 d | 265.0 ± 8.8 a | 1654.3 ± 7.0 d | 824.0 ± 5.2 bc | 74.2 ± 0.1 c |
| 50% PM | 1021.3 ± 20.9 b | 709.3 ± 10.5 c | 312.0 ± 11.2 ab | 1489.0 ± 22.0 c | 779.6 ± 11.5 ab | 73.4 ± 0.1 b |
| 70% WM | 1318.0 ± 34.7 c | 923.6 ± 30.6 f | 394.3 ± 4.1 bc | 1876.0 ± 56.5 f | 952.3 ± 26.1 d | 75.4 ± 0.4 d |
| 70% PM | 876.0 ± 38.6 a | 603.3 ± 29.9 b | 272.6 ± 10.2 a | 1355.7 ± 69.3 b | 752.3 ± 39.5 a | 74.5 ± 0.4 c |
| Flour 2 | TAC (mg c3-GE/100 g) | TPC (mg AG/100 g) | FRAP (µmol tr/g) | TEAC (µmol tr/g) | FA (mg/g) |
|---|---|---|---|---|---|
| RF | nd | 49.24 ± 1.15 b | 2.39 ± 0.03 b | nd | 1.39 ± 0.05 a |
| CF | nd | nd | nd | nd | nd |
| WM | nd | 65.73 ± 5.34 c | 2.59 ± 0.04 c | nd | 5.80 ± 0.03 h |
| PM | 149.27 ± 5.12 d | 222.39 ± 7.39 g | 16.88 ± 0.07 g | 20.21 ± 0.76 d | 4.73 ± 0.04 g |
| CONTROL 3 | nd | 38.60 ± 1.58 a | 1.71 ± 0.01 a | nd | 2.54 ± 0.01 c |
| F34% PM | 50.87 ± 1.78 a | 91.86 ± 2.80 d | 6.57 ± 0.01 d | 7.06 ± 0.34 a | 1.99 ± 0.03 b |
| F50% PM | 74.72 ± 2.59 b | 123.50 ± 3.91 e | 9.07 ± 0.02 e | 10.25 ± 0.44 b | 2.80 ± 0.04 d |
| F70% PM | 104.54 ± 3.60 c | 163.00 ± 5.30 f | 12.20 ± 0.04 f | 14.24 ± 0.57 c | 3.57 ± 0.04 e |
| Formulation 2 | RDS (g/100 g) | SDS (g/100 g) | RS (g/100 g) | TH (%) |
|---|---|---|---|---|
| 34% PM | 56.39 ± 0.05 b | 8.90 ± 1.76 c | 34.70 ± 1.81 b | 65.30 ± 1.81 b |
| 50% PM | 62.33 ± 0.72 c | 4.90 ± 0.62 b | 32.77 ± 0.09 b | 67.23 ± 0.09 b |
| 70% PM | 36.52 ± 0.01 a | 2.47 ± 1.66 ab | 61.01 ± 1.68 c | 38.99 ± 1.68 a |
| Control | 71.60 ± 0.52 d | 0.72 ± 0.02 a | 27.68 ± 0.55 a | 72.32 ± 0.55 c |
| Control | 34% PM 2 | 50% PM | 70% PM | ||
|---|---|---|---|---|---|
| TAC (mg C3-G/100 g) | BREAD 3 | nd | 23.14 ± 0.97 a | 25.54 ± 2.63 a | 48.08 ± 2.05 b |
| B | nd | nd | nd | nd | |
| PB | nd | nd | nd | nd | |
| TPC (mg AG/100 g) | BREAD | 17.39 ± 0.66 Ba | 49.96 ± 2.25 Cb | 78.68 ± 1.85 Cc | 119.29 ± 5.30 Cd |
| B | 22.16 ± 0.73 Ca | 24.93 ± 0.84 Bab | 27.31 ± 0.84 Bb | 50.16 ± 2.80 Bc | |
| PB | 4.72 ± 0.13 Aa | 5.33 ± 0.20 Aab | 6.39 ± 0.52 Abc | 6.78 ± 0.56 Ac | |
| FRAP (μmol TR/g) | BREAD | 1.05 ± 0.10 Ba | 3.56 ± 0.07 Bb | 5.45 ± 0.13 Cc | 8.17 ± 0.33 Cd |
| B | 3.27 ± 0.43 Ca | 3.88 ± 0.21 Ca | 3.85 ± 0.12 Ba | 7.55 ± 0.09 Bb | |
| PB | 0.28 ± 0.04 Aa | 0.37 ± 0.03 Aa | 0.49 ± 0.20 Aa | 0.53 ± 0.10 Aa | |
| TEAC (μmol TR/g) | BREAD | 0.55 ± 0.02 Aa | 5.12 ± 0.29 Bb | 5.53 ± 0.60 Bb | 9.87 ± 0.23 Bc |
| B | 10.20 ± 0.10 Ca | 10.85 ± 0.11 Cb | 11.00 ± 0.01 Cbc | 11.13 ± 0.04 Cc | |
| PB | 3.46 ± 0.01 Ba | 3.68 ± 0.03 Ab | 3.70 ± 0.01 Ab | 3.76 ± 0.01 Ac |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Monsierra, L.; Mansilla, P.S.; Pérez, G.T. Whole Flour of Purple Maize as a Functional Ingredient of Gluten-Free Bread: Effect of In Vitro Digestion on Starch and Bioaccessibility of Bioactive Compounds. Foods 2024, 13, 194. https://doi.org/10.3390/foods13020194
Monsierra L, Mansilla PS, Pérez GT. Whole Flour of Purple Maize as a Functional Ingredient of Gluten-Free Bread: Effect of In Vitro Digestion on Starch and Bioaccessibility of Bioactive Compounds. Foods. 2024; 13(2):194. https://doi.org/10.3390/foods13020194
Chicago/Turabian StyleMonsierra, Luisina, Pablo Sebastián Mansilla, and Gabriela Teresa Pérez. 2024. "Whole Flour of Purple Maize as a Functional Ingredient of Gluten-Free Bread: Effect of In Vitro Digestion on Starch and Bioaccessibility of Bioactive Compounds" Foods 13, no. 2: 194. https://doi.org/10.3390/foods13020194





