Determination of Eugenol Residues in Fish Tissue, Transport, and Temporary Water of Aquatic Product by Gas Chromatography–Tandem Mass Spectrometry with Application of the Electrospun Nanofibrous Membrane
Abstract
:1. Introduction
2. Experimental
2.1. Reagents and Chemicals
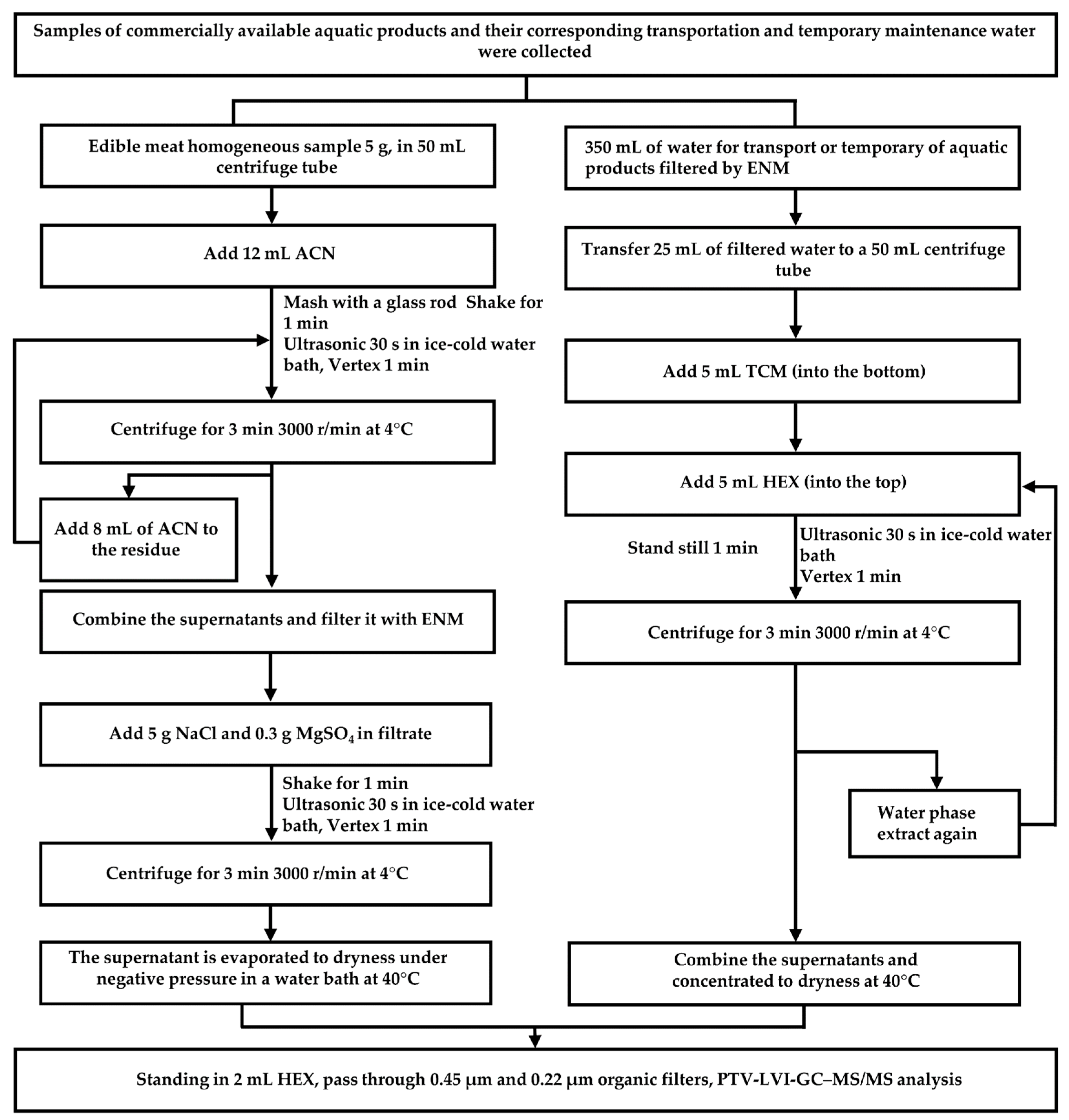
2.2. Sample Collection
2.3. Apparatus
2.3.1. GC Operation Conditions
2.3.2. Electrostatic Spinning Machine Operating Conditions
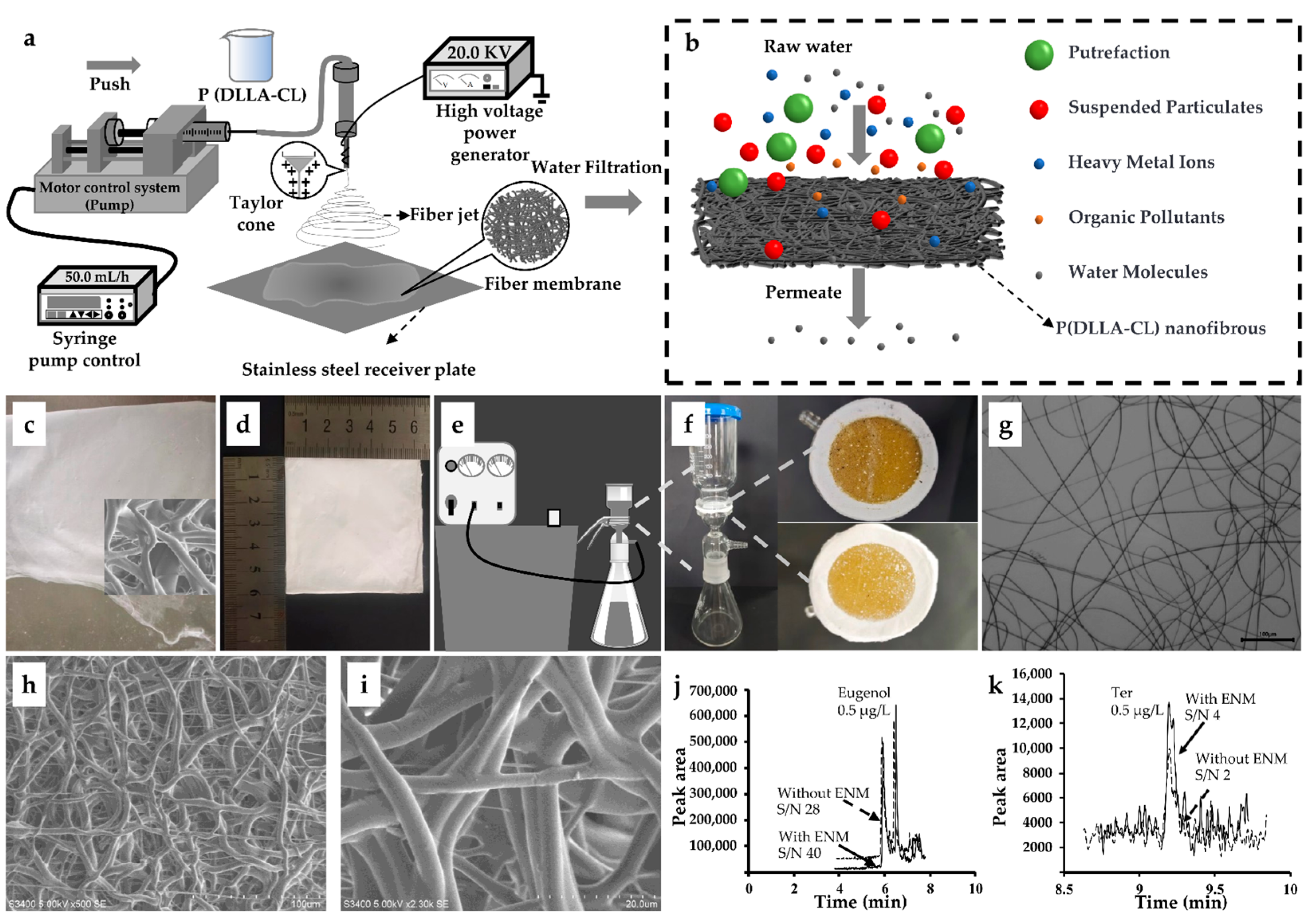
2.4. Preparation of ENM
2.5. Procedure
2.6. Method Validation
3. Results and Discussion
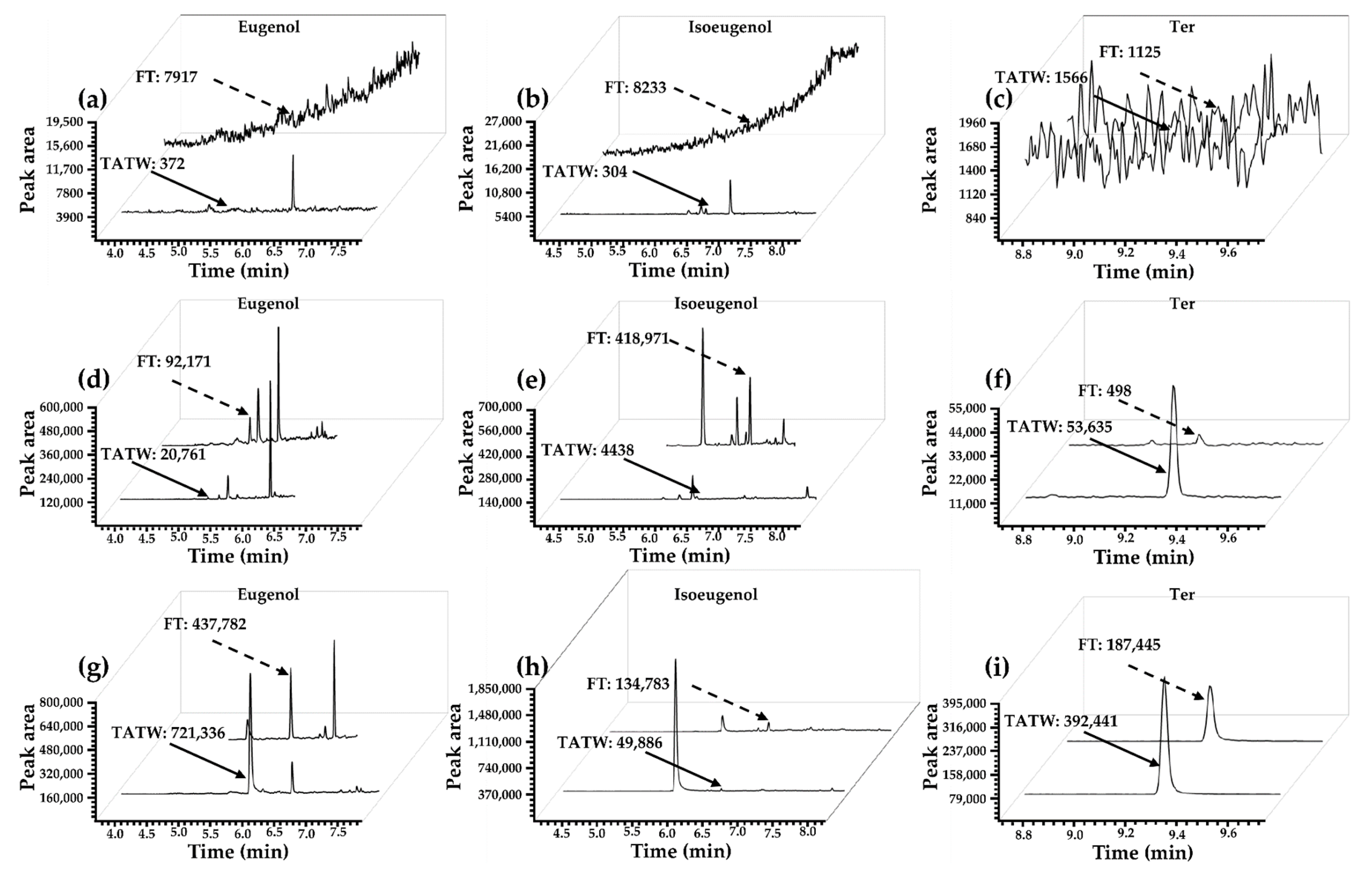
3.1. Optimization of the Extraction of Eugenol
3.2. Preparation and Characterization of ENM
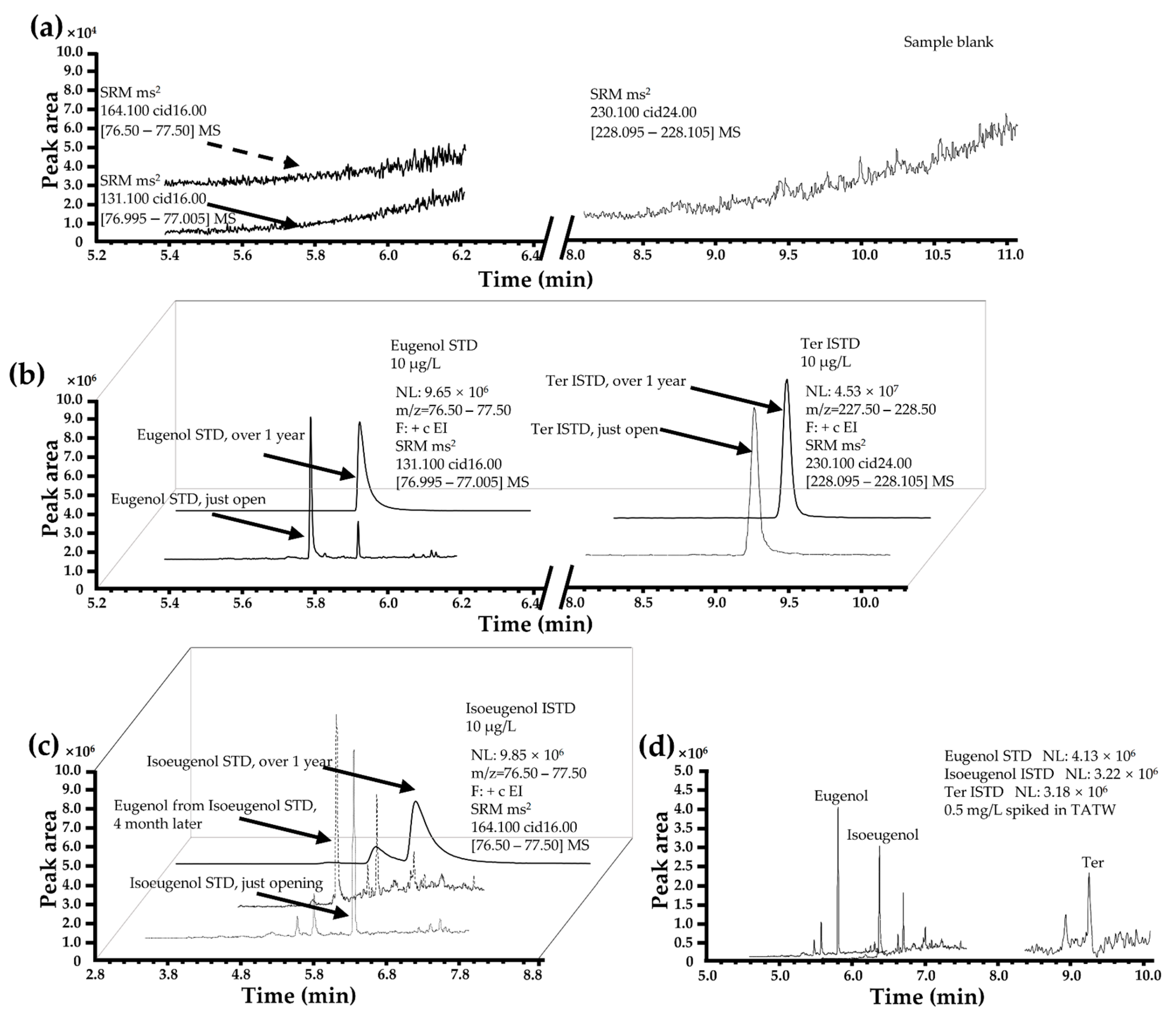
3.3. Optimization of ISTD
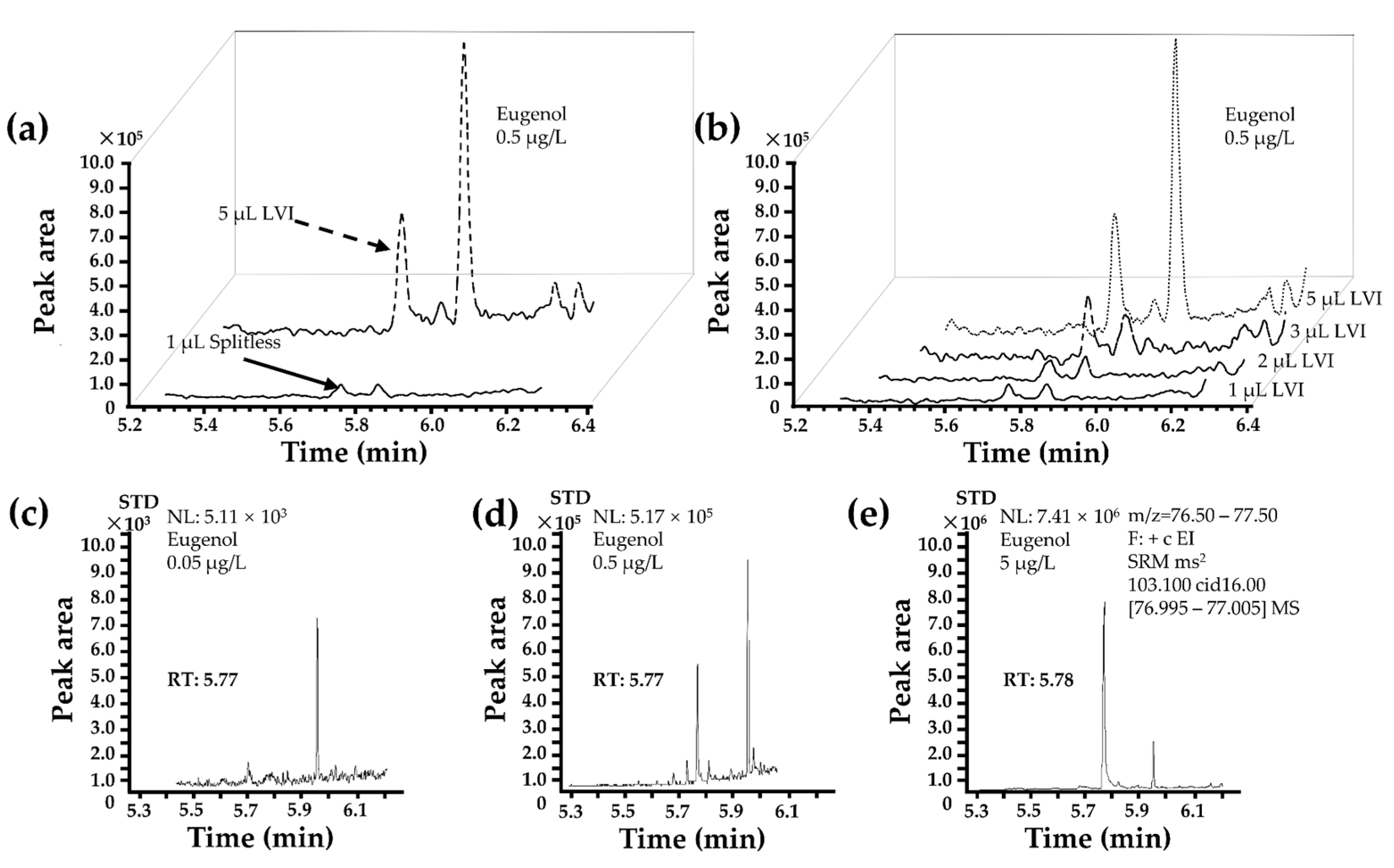
3.4. Optimization of Injection Mode
3.5. Matrix Effect
3.6. Inspection of Eugenol in Real Samples
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lei, X.; Xu, X.; Wang, L.; Liu, L.; Kuang, H.; Xu, L.; Xu, C. Fluorescent microsphere-based lateral-flow immunoassay for rapid and sensitive determination of eugenols. Food Chem. 2023, 411, 135475. [Google Scholar] [CrossRef]
- Jiang, L.; Huang, B.; Tang, J.; Jiang, P.; Chen, D.; Zhang, C. Comprehensive Analysis of Physiological, Biochemical and Flavor Characteristics Changes in Crucian Carp (Carassius auratus) under Different Concentrations of Eugenol. Foods 2023, 12, 2820. [Google Scholar] [CrossRef]
- Abuohashish, H.M.; Khairy, D.A.; Abdelsalam, M.M.; Alsayyah, A.; Ahmed, M.M.; Al-Rejaie, S.S. In-vivo assessment of the osteo-protective effects of eugenol in alveolar bone tissues. Biomed. Pharmacother. 2018, 97, 1303–1310. [Google Scholar] [CrossRef]
- Purbosari, N.; Warsiki, E.; Syamsu, K.; Santoso, J. Natural versus synthetic anesthetic for transport of live fish: A review. Aquac. Fish. 2019, 4, 129–133. [Google Scholar] [CrossRef]
- Cetin-Karaca, H.; Newman, M.C. Antimicrobial efficacy of plant phenolic compounds against Salmonella and Escherichia coli. Food Biosci. 2015, 11, 8–16. [Google Scholar] [CrossRef]
- Cava-Roda, R.; Taboada-Rodriguez, A.; Lopez-Gomez, A.; Martinez-Hernandez, G.B.; Marin-Iniesta, F. Synergistic Antimicrobial Activities of Combinations of Vanillin and Essential Oils of Cinnamon Bark, Cinnamon Leaves, and Cloves. Foods 2021, 10, 1406. [Google Scholar] [CrossRef]
- Endo, T.; Ogishima, K.; Tanaka, H.; Ohshima, S. Studies on the Anesthetic Effect of Eugenol in Some Fresh Water Fishes. Nippon Suisan Gakkaishi 1972, 38, 761–767. [Google Scholar] [CrossRef]
- Wang, W.; Dong, H.; Sun, Y.; Sun, C.; Duan, Y.; Gu, Q.; Li, Y.; Xie, M.; Zhang, J. Immune and physiological responses of juvenile Chinese sea bass (Lateolabrax maculatus) to eugenol and tricaine methanesulfonate (MS-222) in gills. Aquac. Rep. 2020, 18, 100554. [Google Scholar] [CrossRef]
- Wang, J.; Xiong, G.; Bai, C.; Liao, T. Anesthetic efficacy of two plant phenolics and the physiological response of juvenile Ictalurus punctatus to simulated transport. Aquaculture 2021, 538, 736566. [Google Scholar] [CrossRef]
- Ke, C.; Liu, Q.; Li, L.; Chen, J.; Zhao, C.; Xu, J.; Huang, K.; Mengsong, M.; Li, L. Residual levels and risk assessment of eugenol and its isomers in fish from China markets. Aquaculture 2018, 484, 338–342. [Google Scholar] [CrossRef]
- Zeppenfeld, C.C.; Toni, C.; Becker, A.G.; Miron, D.d.S.; Parodi, T.V.; Heinzmann, B.M.; Barcellos, L.J.G.; Koakoski, G.; Rosa, J.G.S.d.; Loro, V.L.; et al. Physiological and biochemical responses of silver catfish, Rhamdia quelen, after transport in water with essential oil of Aloysia triphylla (L’Herit) Britton. Aquaculture 2014, 418–419, 101–107. [Google Scholar] [CrossRef]
- Taheri Mirghaed, A.; Ghelichpour, M. Effects of anesthesia and salt treatment on stress responses, and immunological and hydromineral characteristics of common carp (Cyprinus carpio, Linnaeus, 1758) subjected to transportation. Aquaculture 2019, 501, 1–6. [Google Scholar] [CrossRef]
- Tang, Y.; Zhang, H.; Yang, G.; Fang, C.; Kong, C.; Tian, L.; Huang, X. Pharmacokinetics studies of eugenol in Pacific white shrimp (Litopenaeus vannamei) after immersion bath. BMC Vet. Res. 2022, 18, 122. [Google Scholar] [CrossRef]
- Meinertz, J.R.; Porcher, S.T.; Smerud, J.R.; Gaikowski, M.P. Determination of the exposure parameters that maximise the concentrations of the anaesthetic/sedative eugenol in rainbow trout (Oncorhynchus mykiss) skin-on fillet tissue. Food Addit. Contam. Part A Chem. Anal Control. Exp. Risk Assess 2014, 31, 1522–1528. [Google Scholar] [CrossRef]
- Cupp, A.R.; Hartleb, C.F.; Fredricks, K.T.; Gaikowski, M.P. Effectiveness of eugenol sedation to reduce the metabolic rates of cool and warm water fish at high loading densities. Aquac. Res. 2014, 47, 234–242. [Google Scholar] [CrossRef]
- Cupp, A.R.; Fredricks, K.T.; Porcher, S.T.; Smerud, J.R.; Hartleb, C.F.; Gaikowski, M.P. Survival and behavioural responses of cool and warm water fish sedated with AQUI-S®20E (10% eugenol) at high loading densities. Aquac. Res. 2017, 48, 1576–1589. [Google Scholar] [CrossRef]
- Barbas, L.A.L.; Maltez, L.C.; Stringhetta, G.R.; Garcia, L.d.O.; Monserrat, J.M.; da Silva, D.T.; Heinzmann, B.M.; Sampaio, L.A. Properties of two plant extractives as anaesthetics and antioxidants for juvenile tambaqui Colossoma macropomum. Aquaculture 2017, 469, 79–87. [Google Scholar] [CrossRef]
- Meinertz, J.R.; Schreier, T.M.; Porcher, S.T.; Smerud, J.R.; Gaikowski, M.P. Depletion of eugenol residues from the skin-on fillet tissue of rainbow trout exposed to 14C-labeled eugenol. Aquaculture 2014, 430, 74–78. [Google Scholar] [CrossRef]
- Meinertz, J.R.; Greseth, S.L.; Schreier, T.M.; Bernardy, J.A.; Gingerich, W.H. Isoeugenol concentrations in rainbow trout (Oncorhynchus mykiss) skin-on fillet tissue after exposure to AQUI-S™ at different temperatures, durations, and concentrations. Aquaculture 2006, 254, 347–354. [Google Scholar] [CrossRef]
- Saran, S.; Menon, S.; Shailajan, S.; Pokharna, P. Validated RP-HPLC method to estimate eugenol from commercial formulations like Caturjata Churna, Lavangadi Vati, Jatiphaladi Churna, Sitopaladi Churna and clove oil. J. Pharm. Res. 2013, 6, 53–60. [Google Scholar] [CrossRef]
- Boyer, S.E.; White, J.S.; Stier, A.C.; Osenberg, C.W. Effects of the fish anesthetic, clove oil (eugenol), on coral health and growth. J. Exp. Mar. Biol. Ecol. 2009, 369, 53–57. [Google Scholar] [CrossRef]
- European Food Safety Authority (EFSA). Conclusion on the peer review of the pesticide risk assessment of the active substance eugenol. EFSA J. 2012, 10, 2914. [Google Scholar] [CrossRef]
- Li, J.; Liu, H.; Wang, C.; Wu, L.; Liu, D. Determination of eugenol in fish and shrimp muscle tissue by stable isotope dilution assay and solid-phase extraction coupled gas chromatography-triple quadrupole mass spectrometry. Anal. Bioanal. Chem. 2016, 408, 6537–6544. [Google Scholar] [CrossRef]
- Meinertz, J.R.; Schreier, T.M.; Porcher, S.T.; Smerud, J.R. Evaluation of a Method for Quantifying Eugenol Concentrations in the Fillet Tissue from Freshwater Fish Species. J. AOAC Int. 2016, 99, 558–564. [Google Scholar] [CrossRef] [PubMed]
- Rivera-Perez, A.; Lopez-Ruiz, R.; Romero-Gonzalez, R.; Garrido Frenich, A. A new strategy based on gas chromatography-high resolution mass spectrometry (GC-HRMS-Q-Orbitrap) for the determination of alkenylbenzenes in pepper and its varieties. Food Chem. 2020, 321, 126727. [Google Scholar] [CrossRef]
- Li, J.; Zhang, J.; Liu, Y. Optimization of solid-phase-extraction cleanup and validation of quantitative determination of eugenol in fish samples by gas chromatography-tandem mass spectrometry. Anal. Bioanal. Chem. 2015, 407, 6563–6568. [Google Scholar] [CrossRef]
- Ke, C.; Liu, Q.; Li, L.; Chen, J.; Wang, X.; Huang, K. Simultaneous determination of eugenol, isoeugenol and methyleugenol in fish fillet using gas chromatography coupled to tandem mass spectrometry. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2016, 1031, 189–194. [Google Scholar] [CrossRef]
- Meinertz, J.R.; Hess, K.R. Evaluation of analytical techniques to determine AQUI-S® 20E (eugenol) concentrations in water. Aquaculture 2014, 418–419, 62–66. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, Q.; Yang, B.; Li, J.; Yang, C.; Meng, Y.; Kuang, H. GC-MS method for determination and pharmacokinetic study of four phenylpropanoids in rat plasma after oral administration of the essential oil of Acorus tatarinowii Schott rhizomes. J. Ethnopharmacol. 2014, 155, 1134–1140. [Google Scholar] [CrossRef] [PubMed]
- Schulz, K.; Schlenz, K.; Malt, S.; Metasch, R.; Romhild, W.; Dressler, J.; Lachenmeier, D.W. Headspace solid-phase microextraction-gas chromatography-mass spectrometry for the quantitative determination of the characteristic flavouring agent eugenol in serum samples after enzymatic cleavage to validate post-offence alcohol drinking claims. J. Chromatogr. A 2008, 1211, 113–119. [Google Scholar] [CrossRef]
- Beaudry, F.; Guenette, S.A.; Vachon, P. Determination of eugenol in rat plasma by liquid chromatography-quadrupole ion trap mass spectrometry using a simple off-line dansyl chloride derivatization reaction to enhance signal intensity. Biomed. Chromatogr. 2006, 20, 1216–1222. [Google Scholar] [CrossRef] [PubMed]
- Shishov, A.; Gagarionova, S.; Bulatov, A. Deep eutectic mixture membrane-based microextraction: HPLC-FLD determination of phenols in smoked food samples. Food Chem. 2020, 314, 126097. [Google Scholar] [CrossRef] [PubMed]
- Kreutz, T.; Lucca, L.G.; Loureiro-Paes, O.A.R.; Teixeira, H.F.; Veiga, V.F., Jr.; Limberger, R.P.; Ortega, G.G.; Koester, L.S. Optimization, validation and application of headspace solid-phase microextraction gas chromatography for the determination of 1-nitro-2-phenylethane and methyleugenol from Aniba canelilla (H.B.K.) Mez essential oil in skin permeation samples. J. Chromatogr. A 2018, 1564, 163–175. [Google Scholar] [CrossRef]
- Fadillah, G.; Wicaksono, W.P.; Fatimah, I.; Saleh, T.A. A sensitive electrochemical sensor based on functionalized graphene oxide/SnO2 for the determination of eugenol. Microchem. J. 2020, 159, 105353. [Google Scholar] [CrossRef]
- Liu, B.L.; Wang, D.Q.; Yang, Z.H.; Zhao, Y.; Xie, J.; Zhong, J.; Wang, Z.Q. Fabrication and characterization of 4-aminoantipyrine electrospun nanofiber polymer membranes for rapid visualization of pentachlorophenol in water samples. Mater. Express 2022, 12, 1126–1134. [Google Scholar] [CrossRef]
- Yurova, N.S.; Danchuk, A.; Mobarez, S.N.; Wongkaew, N.; Rusanova, T.; Baeumner, A.J.; Duerkop, A. Functional electrospun nanofibers for multimodal sensitive detection of biogenic amines in food via a simple dipstick assay. Anal. Bioanal. Chem. 2018, 410, 1111–1121. [Google Scholar] [CrossRef]
- Behroozi, A.H.; Al-Shaeli, M.; Vatanpour, V. Fabrication and modification of nanofiltration membranes by solution electrospinning technique: A review of influential factors and applications in water treatment. Desalination 2023, 558, 116638. [Google Scholar] [CrossRef]
- Amin, N.A.A.M.; Mokhter, M.A.; Salamun, N.; Mohamad, M.F.b.; Mahmood, W.M.A.W. Anti-fouling electrospun organic and inorganic nanofiber membranes for wastewater treatment. S. Afr. J. Chem. Eng. 2023, 44, 302–317. [Google Scholar] [CrossRef]
- Malara, A.; Fotia, A.; Paone, E.; Serrano, G. Electrospun Nanofibers and Electrochemical Techniques for the Detection of Heavy Metal Ions. Materials 2021, 14, 3000. [Google Scholar] [CrossRef]
- Daels, N.; Radoicic, M.; Radetic, M.; De Clerck, K.; Van Hulle, S.W.H. Electrospun nanofibre membranes functionalised with TiO2 nanoparticles: Evaluation of humic acid and bacterial removal from polluted water. Sep. Purif. Technol. 2015, 149, 488–494. [Google Scholar] [CrossRef]
- Zheng, Y.; Yao, F.; Chen, F. Curcumin-loaded electrospun peanut protein isolate/ poly-l-lactic acid nanofibre membranes: Preparation and characterisation and release behaviour. LWT 2022, 169, 113978. [Google Scholar] [CrossRef]
- Cui, Y.; Wang, Z.; Cong, J.; Wang, L.; Liu, Y.; Wang, X.; Xie, J. Determination of Polychlorinated Biphenyls in Fish Tissues from Shanghai Seafood Markets Using a Modified QuEChERS Method. Anal. Sci. 2017, 33, 973–977. [Google Scholar] [CrossRef]
- Liu, Z.; Li, K.; Wang, Q.; Liu, C.; Wang, B.; Hou, R.; Diao, Y.; Wang, J.; Du, X.-D.; Qu, L. Isotopic internal standard correction for the determination of diludine residue in animal-derived matrix samples using enhanced matrix removal-lipid clean-up combined with ultra-performance liquid chromatography-tandem mass spectrometry. Microchem. J. 2023, 195, 109395. [Google Scholar] [CrossRef]
- Sun, G.; Wei, D.; Liu, X.; Chen, Y.; Li, M.; He, D.; Zhong, J. Novel biodegradable electrospun nanofibrous P(DLLA-CL) balloons for the treatment of vertebral compression fractures. Nanomedicine 2013, 9, 829–838. [Google Scholar] [CrossRef]
- Li, F.; Li, X.; He, R.; Cheng, J.; Ni, Z.; Zhao, G. Preparation and evaluation of poly(d,l-lactic acid)/poly(L-lactide-co-ε-caprolactone) blends for tunable sirolimus release. Colloids Surf. A Physicochem. Eng. Asp. 2020, 590, 124518. [Google Scholar] [CrossRef]
- Galle, T.; Bayerle, M.; Pittois, D. Geochemical matrix differently affects the response of internal standards and target analytes for pesticide transformation products measured in groundwater samples. Chemosphere 2022, 307, 135815. [Google Scholar] [CrossRef]
| Sample Amount | Compounds | Internal Standard a | Technique b | Sorbent c | Solvent | Equipment | LODs | LOQs | Recovery (%) | Comments | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Clove oil/1 g | Eugenol | a - | LLE | - | MT | HPLC-UV | 25.0 μg/L | 50.0 μg/L | >85.0 | From formulation | [20] |
| Fish Shrimp tissue/2 g | Eugenol | Eugenol-d3 | SIDA+SPE +UE | - | ACN, EA | GC-MS/MS | 2.5 μg/kg | 5.0 μg/kg | 94.7–109.8 | Method development | [23] |
| Fish/2 g | Eugenol | - | SPE | - | MT/H2O (1:9, v/v) | GC-MS/MS | 2.5 μg/kg | 5.0 μg/kg | 94.8–103.6 | Method optimization for fish | [26] |
| Fish fillet/2 g | Eugenol +isoeugenol methyl-eugenol | - | SPE | - | EA; HEX | GC-MS | 0.4, 1.2, 0.2 μg/kg | 1.2, 4.0, 0.7 μg/kg | 76.4–99.9 | Eugenol in fish | [27] |
| Fish/5 g | Eugenol+ isoeugenol | - | LLE+SPE | Dry ice | AC | HPLC-UV | 4.0 μg/kg | 12.0 μg/kg | 80.0–105.0 | Stability of eugenol | [24] |
| Rainbow trout fillet/0.5 g | Eugenol | 14C-eugenol | SPE+UE | Dry ice | AC, MT/H2O (9:1, v/v) | GC-MS | - | 20.0 mg/kg | 87.3–95.1 | Not for residue analysis | [18] |
| Smoked sausages, Smoked fish/0.5 g | Eugenol +isoeugenol, | - | Mb ME | chloride acts | HEX | HPLC-UV | 0.6 μg/kg | 2.0 μg/kg | 70.0–80.0 | Smoked food | [32] |
| Water/10 mL; Fish fillet/5 g | Isoeugenol | - | SPE | - | MT/H2O (9:1, v/v) | HPLC-UV | 30.0 μg/kg | 90.0 μg/kg | 80.0–105.0 | Water and fish both | [19] |
| Water/5 mL | Eugenol | - | SP; SPE | - | AC | GC-MS | 2.3 μg/L | 7.7 μg/L | 87.0–104.0 | Compare SP and SPE | [28] |
| Rat plasma/200 μL | cis-methyl isoeugenol | 1-naphthol | SPME; LLE | - | MT; AC | HSSPME-GC/MS | - | 7.6 μg/L | 80.1–89.0 | Application in plasma | [29] |
| Serum/200 μL | Eugenol | DCHM | SPME | - | ET | HPLC-MS/MS | 3.2 μg/L | 4.8 μg/L | - | HS used | [30] |
| Rat plasma/50 μL | Eugenol | Thymol | LLE | Formic acid | AC | GC-FID/MS | - | 100.0 μg/L | - | Method for plasma | [31] |
| NP and ME from EOAC/1 mL | Methyl-eugenol | - | HS-SPME | NaCl +Na2SO4 | DCM, HEX, MeOH | GC-FID/MS | 0.16 μg/mL | 0.4 mg/L | >90.0 | On skin samples | [33] |
| Eugenol Standard/0.1 mM | Eugenol | - | APTMS- GO@SnO2/CPE | - | - | FTIR, SEM-EDX, XRD, BET | - | - | >95.0 | Standard eugenol | [34] |
| Pepper samples/1 g | Eugenol, methyl-eugenol | DCHM | LLE+UE | Citrate salts +NaCl+MgSO4 | AcOEt, ACN | GC-HRMS-Q-Orbitrap | 0.01 mg/kg | 0.2 mg/kg | 88.0–102.0 | Alkenylbenzenes in pepper and its varieties | [25] |
| Water/25 g | Eugenol | Ter | LLE+UE | NaCl+MgSO4 | TCM, HEX | LVI-GC-MS/MS | 0.5 μg/L | 1.5 μg/L | 97.6–104.9 | This study | |
| Fish fillet/5 g | Eugenol | Ter | LLE+UE | NaCl+MgSO4 | ACN | LVI-GC-MS/MS | 0.1 μg/kg | 0.5 μg/kg | 92.3–111.2 | This study |
| (a) | ||||||
| Compound | tR (min) | Precursor Ion (m/z) | Product Ion (m/z) | Collision Energy (eV) | Dwell Time (s) | |
| Eugenol | 5.8 | 164.1 | 77.1 | 26 | 4.0 | |
| 131.1 | 77.0 | 24 | 4.0 | |||
| 103.1 | 77.0 | 16 | 4.0 | |||
| Isoeugenol | 6.4 | 164.1 | 149.1 | 12 | 4.0 | |
| 149.1 | 77.0 | 20 | 4.0 | |||
| 103.1 | 77.0 | 12 | 4.0 | |||
| p-Terphenyl | 9.3 | 230.1 | 228.1 | 24 | 1.0 | |
| 229.1 | 228.1 | 10 | 1.0 | |||
| 228.1 | 226.1 | 26 | 1.0 | |||
| (b) | ||||||
| Name | Splitless Ter as ISTD a | LVI Ter as ISTD | ||||
| Equation | Y (peak area) = −0.2605 + 0.1769X (concentration) | Y = −0.0617 + 0.1208X | ||||
| r2 b | 0.9918 | 0.9988 | ||||
| LOD c | 4 µg/L | 0.5 µg/L | ||||
| LOQ d | 13 µg/L | 1.5 µg/L | ||||
| S/N e | 26.9 | 2.3 | ||||
| Response f | Low | High | ||||
| (c) | ||||||
| Spiking Level (µg/L) g | Ter as ISTD | |||||
| Value ± SD h (µg/L) | ||||||
| 5.0 | 5.2 ± 0.3 | |||||
| 20.0 | 20.0 ± 0.9 | |||||
| 50.0 | 48.8 ± 1.1 | |||||
| 100.0 | 104.9 ± 3.8 | |||||
| 150.0 | 151.8 ± 8.0 | |||||
| 200.0 | 198.0 ± 14.4 | |||||
| (d) | ||||||
| Matrix TATW/FT | RSD (%) (Intra-Day Variation, n = 6) | RSD (%) (Inter-Day Variation, n = 6) | ||||
| 10 i | 20 | 50 | 10 | 20 | 50 | |
| Channa argus | 3.6/3.4 | 6.7/2.8 | 1.5/1.6 | 3.1/6.2 | 7.6/5.5 | 0.4/3.2 |
| Pelteobagrus fulvidraco | 10.0/2.8 | 7.6/3.1 | 5.3/1.9 | 6.3/5.9 | 3.7/6.2 | 1.9/3.7 |
| Penaeus vannamei | 13.5/4.9 | 11.1/8.8 | 2.0/0.9 | 8.3/10.5 | 1.0/9.3 | 7.5/1.7 |
| Macrobrachium rosenbergii | 7.4/6.8 | 10.6/1.3 | 5.9/4.2 | 8.3/4.2 | 9.9/5.6 | 1.8/3.1 |
| Scallop | 4.5/1.5 | 8.6/1.9 | 9.0/1.1 | 7.7/2.4 | 9.2/3.5 | 2.0/3.0 |
| Procambarus clarkii | 8.3/3.3 | 14.9/5.0 | 0.3/0.9 | 5.1/4.9 | 5.2/8.5 | 8.7/0.7 |
| Mean Recovery ± Standard Deviation (n = 3) | |||||||
|---|---|---|---|---|---|---|---|
| Spiked with Analyte (µg/L) | Channa argus. | Pelteobagrus. fulvidraco | Penaeus. vannamei | Macrobrachium. rosenbergii | Scallop. | Procambarus. clarkii | |
| Matrix water | 10 a | 97.2 ± 4.3 | 92.3 ± 2.1 | 99.0 ± 4.3 | 97.5 ± 4.3 | 99.1 ± 3.3 | 97.1 ± 7.4 |
| (µg/L) | 20 | 96.3 ± 3.2 | 97.5 ± 3.3 | 97.5 ± 4.6 | 97.6 ± 3.6 | 100.3 ± 3.5 | 98.2 ± 3.9 |
| 50 | 100.0 ± 4.6 | 97.6 ± 5.8 | 95.7 ± 5.2 | 95.3 ± 4.9 | 100.2 ± 2.1 | 99.4 ± 4.4 | |
| ME (%) | 7.3 | 21.3 | 9.2 | 18.4 | 17.3 | 17.0 | |
| Fish tissue (µg/kg) | 10 | 103.1 ± 6.0 | 98.2 ± 5.9 | 98.1 ± 2.6 | 102.1 ± 3.1 | 99.6 ± 5.9 | 97.5 ± 7.6 |
| 20 | 96.4 ± 4.6 | 95.1 ± 3.8 | 98.1 ± 3.4 | 97.6 ± 6.3 | 97.7 ± 3.5 | 97.3 ± 2.2 | |
| 50 | 102.7 ± 7.1 | 99.0 ± 4.6 | 97.4 ± 5.3 | 97.3 ± 4.5 | 97.2 ± 4.2 | 97.8 ± 3.1 | |
| ME (%) | 10.23 | 0.4 | 9.7 | 8.0 | 3.4 | 0.7 | |
| (a) | ||||||||||||||||
| Market | Type | Samples, n | Positive Samples, n (%) | Concentration (μg/L) | Concentration Distribution of Samples (μg/kg/μg/L), n (%) | |||||||||||
| Mean | ± | SEM | Range | 5–50 | 50–100 | 100–1000 | >1000 | |||||||||
| Zhejiang | AQS (μg/kg) | 74 | 8(10.8) | 7271.6 | ± | 4190.1 | 1.1 | – | 32,668.8 | 2(2.7) | 1(1.4) | 2(2.7) | 3(4.1) | |||
| TRWS (μg/L) | 48 | 40(8.3) | 352.8 | ± | 337.8 | 11.8 | – | 1366.3 | 3(6.3) | 0(0.0) | 0(0.0) | 1(2.1) | ||||
| TEWS (μg/L) | 32 | 30(9.4) | 12.3 | ± | 6.2 | 0.1 | – | 20.0 | 3(9.4) | 0(0.0) | 0(0.0) | 0(0.0) | ||||
| Jiangsu | AQS | 49 | 40(8.1) | 10,318.0 | ± | 8648.1 | 243.0 | – | 36,090.3 | 0(0.0) | 0(0.0) | 2(4.1) | 2(4.1) | |||
| TRWS | 40 | 5(12.5) | 84.0 | ± | 64.1 | 0.0 | – | 337.0 | 3(7.5) | 1(2.5) | 1(2.5) | 0(0.0) | ||||
| Shandong | AQS | 30 | 10(3.3) | 4678.4 | ± | 0.0 | 4678.4 | 0(0.0) | 0(0.0) | 0(0.0) | 1(3.3) | |||||
| TEWS | 9 | 1(11.1) | 13.2 | ± | 0.0 | 13.2 | 1(11.1) | 0(0.0) | 0(0.0) | 0(0.0) | ||||||
| Guangdong | AQS | 36 | 4(11.1) | 3107.6 | ± | 3011.4 | 53.1 | – | 12,141.6 | 0(0.0) | 2(5.6) | 1(2.8) | 1(2.8) | |||
| TRWS | 16 | 10(6.3) | 648.0 | ± | 0.0 | – | 648.0 | 0(0.0) | 0(0.0) | 1(6.3) | 0(0.0) | |||||
| Fujian | AQS | 81 | 15(18.5) | 4692.1 | ± | 2196.0 | 0.4 | – | 30593.6 | 4(4.9) | 1(1.2) | 3(3.7) | 7(8.6) | |||
| TRWS | 67 | 13(19.4) | 20.8 | ± | 6.8 | 0.0 | – | 89.2 | 12(17.9) | 1(1.5) | 0(0.0) | 0(0.0) | ||||
| TEWS | 36 | 10(2.8) | 25.4 | ± | 0.0 | 25.4 | 1(2.8) | 0(0.0) | 0(0.0) | 0(0.0) | ||||||
| Anhui | AQS | 9 | 1(11.1) | 163.0 | ± | 0.0 | 163.0 | 0(0.0) | 0(0.0) | 1(11.1) | 0(0.0) | |||||
| TRWS | 11 | 10(9.1) | 993.0 | ± | 0.0 | 993.0 | 0(0.0) | 0(0.0) | 1(9.1) | 0(0.0) | ||||||
| Shanghai | AQS | 30 | 10(3.3) | 109.0 | ± | 0.0 | 109.0 | 0(0.0) | 0(0.0) | 1(3.3) | 0(0.0) | |||||
| TRWS | 43 | 20(4.7) | 177.0 | ± | 177.0 | 0.0 | – | 354.0 | 1(2.3) | 0(0.0) | 1(2.3) | 0(0.0) | ||||
| TEWS | 57 | 40(7.0) | 10.0 | ± | 4.5 | 0.0 | – | 18.1 | 4(7.0) | 0(0.0) | 0(0.0) | 0(0.0) | ||||
| Jiangxi | AQS | 6 | 2(33.3) | 145.9 | ± | 78.2 | 67.7 | – | 224.0 | 0(0.0) | 1(16.7) | 1(16.7) | 0(0.0) | |||
| TRWS | 7 | 1(14.3) | 19.6 | ± | 0.0 | – | 19.6 | 1(14.3) | 0(0.0) | 0(0.0) | 0(0.0) | |||||
| Total | AQS | 315 | 36(11.4) | 5208.3 | ± | 1609.8 | 0.4 | – | 36,090.3 | 6(1.9) | 5(1.6) | 11(3.5) | 14(4.4) | |||
| TRWS | 247 | 27(10.9) | 152.5 | ± | 64.3 | 152.5 | – | 64.3 | 20(8.1) | 2(0.8) | 4(1.6) | 1(0.4) | ||||
| TEWS | 165 | 90(5.5) | 12.8 | ± | 3.0 | 0.0 | – | 25.4 | 9(5.5) | 0(0.0) | 0(0.0) | 0(0.0) | ||||
| (b) | ||||||||||||||||
| Market | Type | Samples, n | Positive Samples, n(%) | Concentration (μg/L) | Concentration Distribution of Samples (μg/kg/μg/L), n (%) | |||||||||||
| Mean | ± | SEM | Range | 5–50 | 50–100 | 100–1000 | >1000 | |||||||||
| Shanghai | AQS | 116 | 0(0.0) | 0.0 | 0.0 | 0(0.0) | 0(0.0) | 0(0.0) | 0(0.0) | |||||||
| TRWS | 83 | 7(8.4) | 19.1 | ± | 7.9 | 0.0 | – | 59.0 | 6(7.2) | 1(1.2) | 0(0.0) | 0(0.0) | ||||
| TEWS | 69 | 3(4.3) | 13.3 | ± | 4.4 | 4.6 | – | 18.1 | 3(4.3) | 0(0.0) | 0(0.0) | 0(0.0) | ||||
| Zhejiang | AQS | 104 | 14(13.5) | 7272.9 | ± | 3312.0 | 1.1 | – | 36,090.3 | 2(1.9) | 3(2.9) | 3(2.9) | 6(5.8) | |||
| TRWS | 85 | 5(5.9) | 18.5 | ± | 2.6 | 11.8 | – | 24.2 | 5(5.9) | 0(0.0) | 0(0.0) | 0(0.0) | ||||
| TEWS | 41 | 1(2.4) | 13.2 | ± | 0.0 | – | 13.2 | 1(2.4) | 0(0.0) | 0(0.0) | 0(0.0) | |||||
| Fujian | AQS | 95 | 22(23.2) | 3894.4 | ± | 1592.1 | 0.4 | – | 30,593.6 | 4(4.2) | 2(2.1) | 8(8.4) | 8(8.4) | |||
| TRWS | 79 | 15(19.0) | 259.3 | ± | 109.4 | 259.3 | – | 109.4 | 9(11.4) | 1(1.3) | 3(3.8) | 2(2.5) | ||||
| TEWS | 55 | 5(9.1) | 12.4 | ± | 5.2 | 0.0 | – | 25.4 | 5(9.1) | 0(0.0) | 0(0.0) | 0(0.0) | ||||
| Total | AQS | 315 | 36(11.4) | 5208.3 | ± | 1609.8 | 0.4 | – | 36,090.3 | 6(1.9) | 5(1.6) | 11(3.5) | 14(4.4) | |||
| TRWS | 247 | 27(10.9) | 152.5 | ± | 64.3 | 152.5 | – | 64.3 | 20(8.1) | 2(0.8) | 3(1.2) | 2(0.8) | ||||
| TEWS | 165 | 9(5.5) | 12.8 | ± | 3.0 | 0.0 | – | 25.4 | 9(5.5) | 0(0.0) | 0(0.0) | 0(0.0) | ||||
| (c) | ||||||||||||||||
| Market | Type | Samples, n | Positive Samples, n (%) | Concentration (μg/L) | Distribution in Different Months of a Year (μg/kg/μg/L), n (%) | |||||||||||
| Mean | ± | SEM | Range | Apr. | May. | Jun. | Jul. | Aug. | Sep. | Oct. | ||||||
| Eastern | AQS | 279 | 32(11.5) | 5470.9 | ± | 1778.2 | 0.4 | – | 36,090.3 | 0(0.0) | 2(0.7) | 7(2.5) | 2(0.7) | 11(3.9) | 4(1.4) | 6(2.2) |
| TRWS | 231 | 26(11.3) | 133.4 | ± | 63.8 | 0.0 | – | 1366.3 | 2(0.9) | 1(0.4) | 2(0.9) | 3(1.3) | 12(5.2) | 2(0.9) | 4(1.7) | |
| TEWS | 165 | 9(5.5) | 12.8 | ± | 3.0 | 0.0 | – | 25.4 | 0(0.0) | 0(0.0) | 6(3.6) | 2(1.2) | 1(0.6) | 0(0.0) | 0(0.0) | |
| Southern | AQS | 36 | 4(11.1) | 3107.6 | ± | 3011.4 | 53.1 | – | 12,141.6 | 1(2.8) | 0(0.0) | 1(2.8) | 0(0.0) | 0(0.0) | 1(2.8) | 1(2.8) |
| TRWS | 16 | 1(6.3) | 648.0 | ± | 0.0 | 648.0 | 0(0.0) | 0(0.0) | 0(0.0) | 0(0.0) | 0(0.0) | 1(6.3) | 0(0.0) | |||
| Total | AQS | 315 | 36(11.4) | 5208.3 | ± | 1609.8 | 0.4 | – | 36,090.3 | 1(0.3) | 2(0.6) | 8(2.5) | 2(0.6) | 11(3.5) | 5(1.6) | 7(2.2) |
| TRWS | 247 | 27(10.9) | 152.5 | ± | 64.3 | 0.0 | – | 1366.3 | 2(0.8) | 1(0.4) | 2(0.8) | 3(1.2) | 12(4.9) | 3(1.2) | 4(1.6) | |
| TEWS | 165 | 9(5.5) | 12.8 | ± | 3.0 | 0.0 | – | 25.4 | 0(0.0) | 0(0.0) | 6(3.6) | 2(1.2) | 1(0.6) | 0(0.0) | 0(0.0) | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, D.; Wang, Y.; Liu, B.; Ni, L.; Zhong, J.; Xie, J.; Wang, Z. Determination of Eugenol Residues in Fish Tissue, Transport, and Temporary Water of Aquatic Product by Gas Chromatography–Tandem Mass Spectrometry with Application of the Electrospun Nanofibrous Membrane. Foods 2024, 13, 238. https://doi.org/10.3390/foods13020238
Wang D, Wang Y, Liu B, Ni L, Zhong J, Xie J, Wang Z. Determination of Eugenol Residues in Fish Tissue, Transport, and Temporary Water of Aquatic Product by Gas Chromatography–Tandem Mass Spectrometry with Application of the Electrospun Nanofibrous Membrane. Foods. 2024; 13(2):238. https://doi.org/10.3390/foods13020238
Chicago/Turabian StyleWang, Deqian, Yunning Wang, Bolin Liu, Ling Ni, Jian Zhong, Jing Xie, and Zhengquan Wang. 2024. "Determination of Eugenol Residues in Fish Tissue, Transport, and Temporary Water of Aquatic Product by Gas Chromatography–Tandem Mass Spectrometry with Application of the Electrospun Nanofibrous Membrane" Foods 13, no. 2: 238. https://doi.org/10.3390/foods13020238





