Evaluation of the Emulsifying Property and Oxidative Stability of Myofibrillar Protein-Diacylglycerol Emulsions Containing Catechin Subjected to Different pH Values
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Extraction of the Myofibrillar Proteins
2.3. Preparation of Diacylglycerol
2.4. Preparation of the Emulsions at Different pH Values
2.5. Emulsifying Activity Indices and Emulsifying Stability Indices
2.6. Particle Size and Absolute Value of the ζ-Potential
2.7. Confocal Laser Scanning Microscopy
2.8. Rheological Property
2.9. Lipid Oxidation
2.10. Carbonyl Content
2.11. Total Sulfydryl Content
2.12. Statistical Analysis
3. Results and Discussion
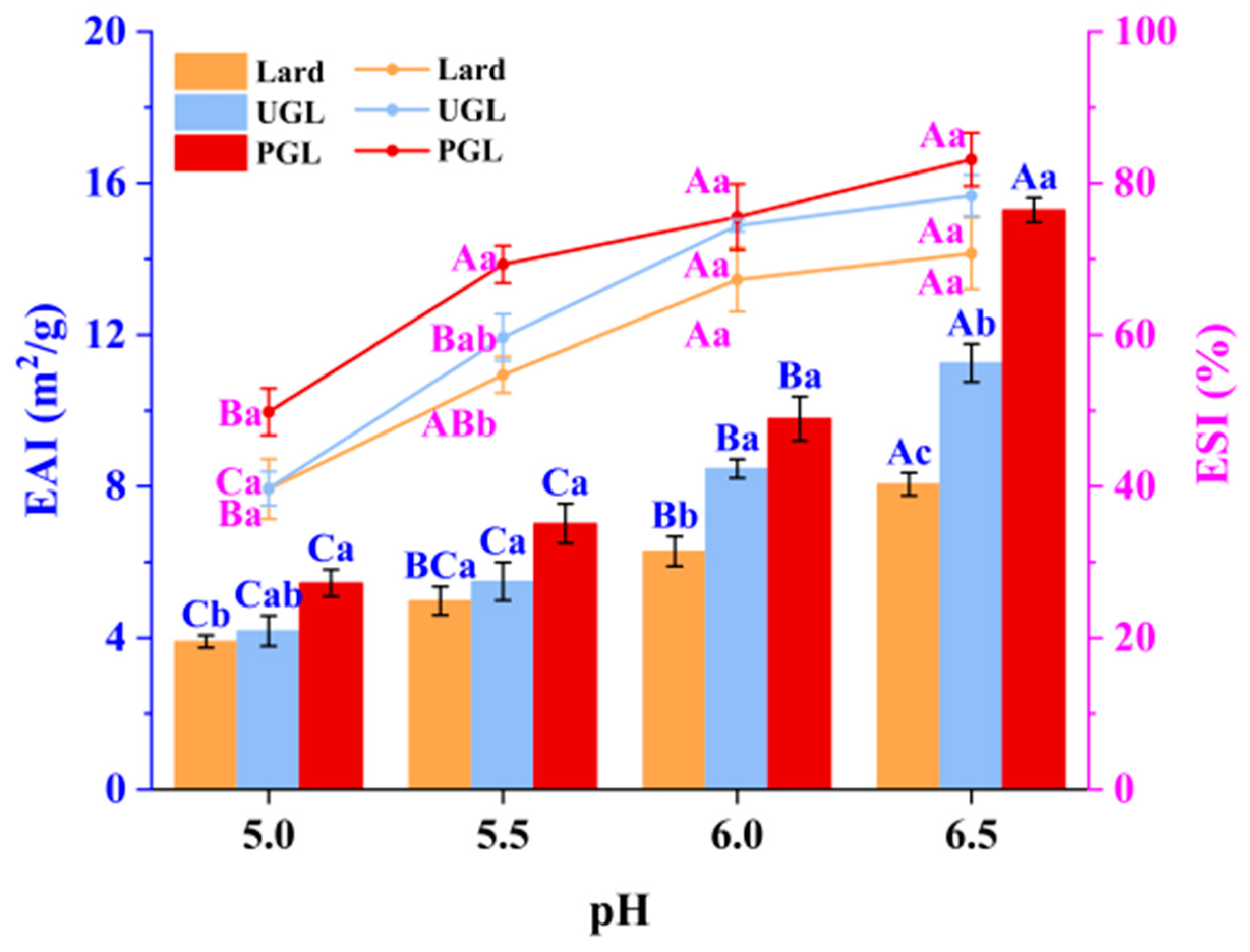
3.1. Emulsifying Activity Indices and Emulsifying Stability Indices
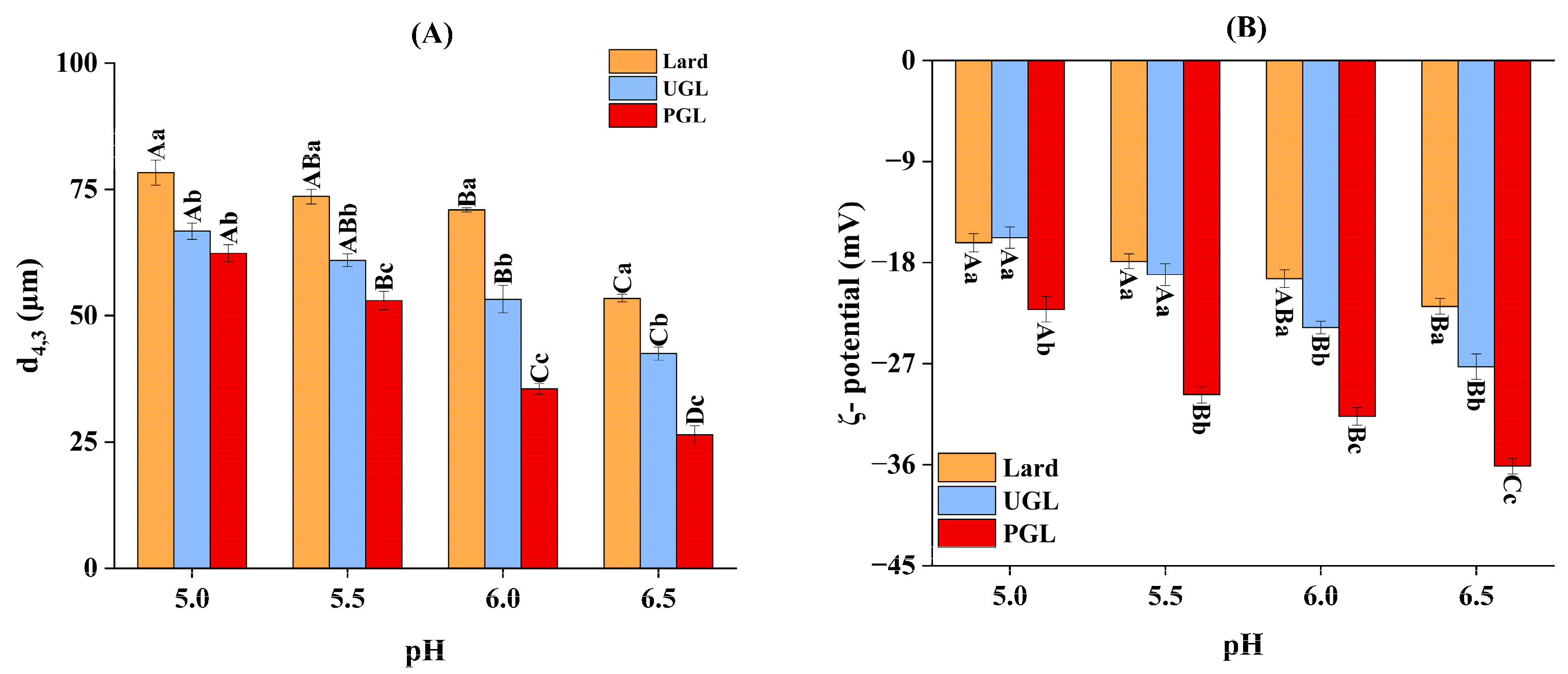
3.2. Particle Size and Absolute Value of ζ-Potential
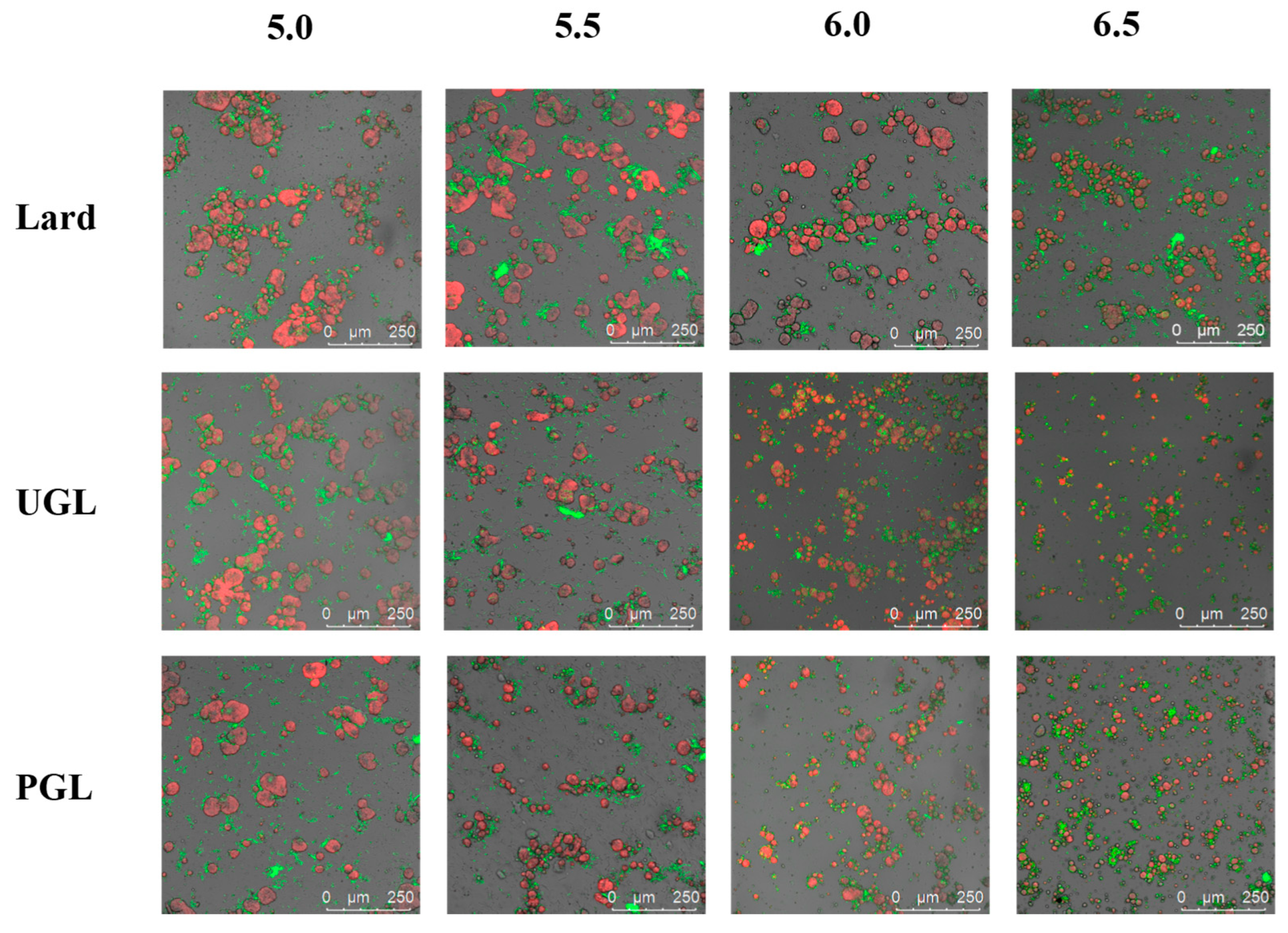
3.3. Microstructure
3.4. Rheological Property
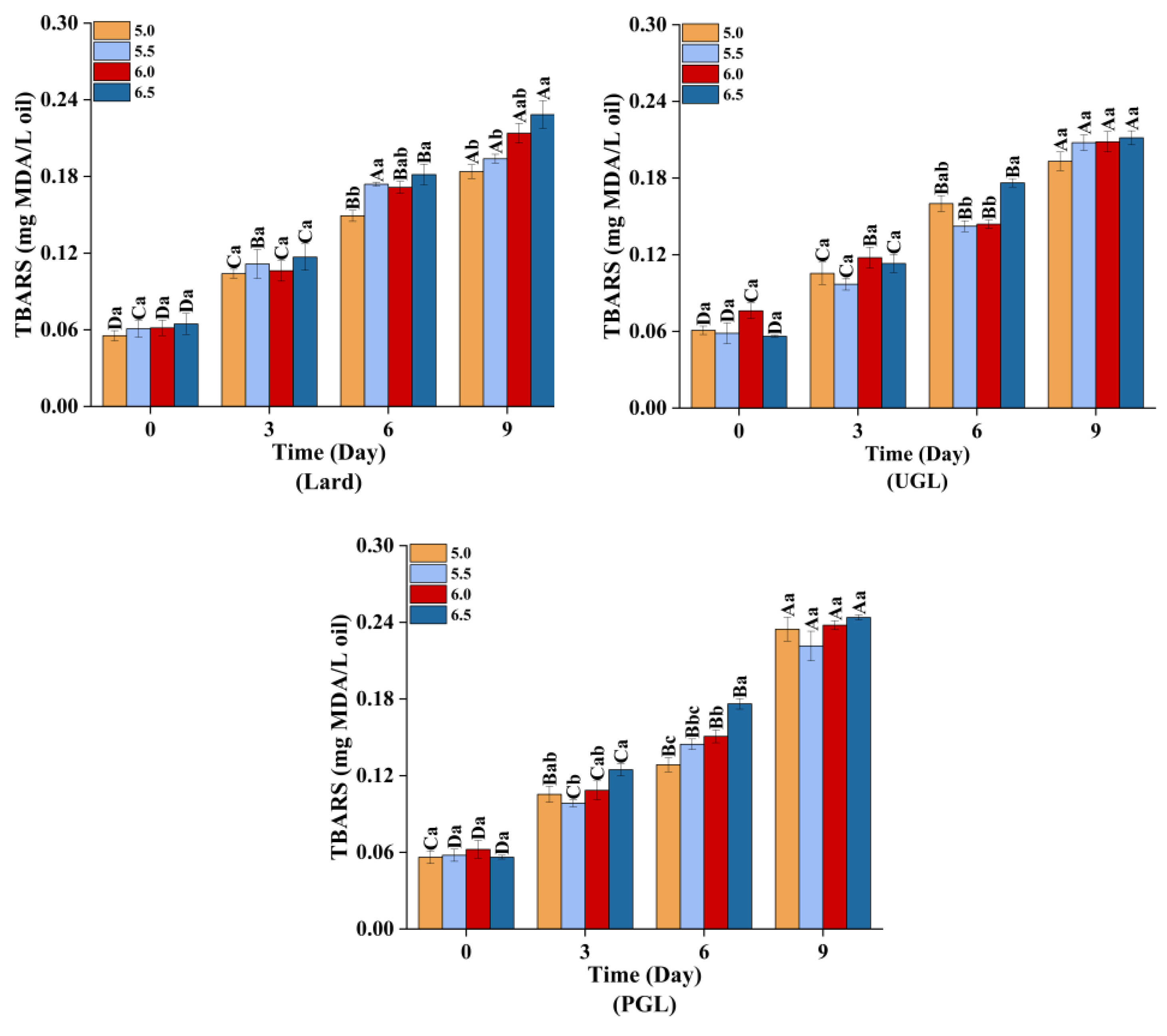
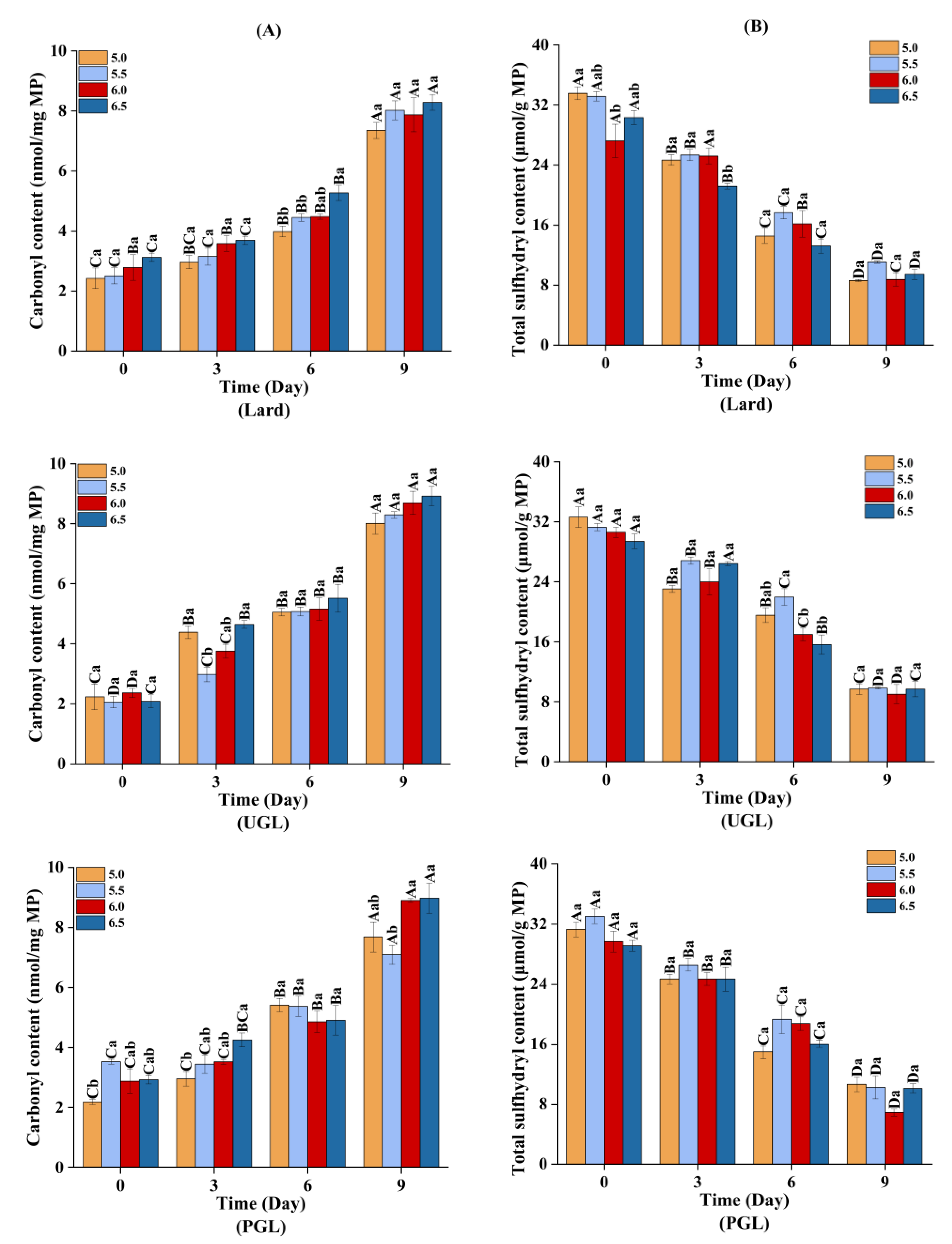
3.5. Oxidation Stability of the Emulsions during Storage
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Bohrer, B.M. Review: Nutrient density and nutritional value of meat products and non-meat foods high in protein. Trends Food Sci. Technol. 2017, 65, 103–112. [Google Scholar] [CrossRef]
- Zhou, X.; Chen, H.; Lyu, F.; Lin, H.; Zhang, Q.; Ding, Y. Physicochemical properties and microstructure of fish myofibrillar protein-lipid composite gels: Effects of fat type and concentration. Food Hydrocoll. 2019, 90, 433–442. [Google Scholar] [CrossRef]
- Jia, B.; Chen, J.; Yang, G.; Bi, J.; Guo, J.; Shang, K.; Zhang, K. Improvement of solubility, gelation and emulsifying properties of myofibrillar protein from mantis shrimp (Oratosquilla oratoria) by phosphorylation modification under low ionic strength of KCl. Food Chem. 2023, 403, 134497. [Google Scholar] [CrossRef]
- Li, Y.; Xu, Y.; Xu, X.; Zeng, X.; Zhou, G. Explore the mechanism of continuous cyclic glycation in affecting the stability of myofibrillar protein emulsion: The influence of pH. Food Res. Int. 2022, 161, 111834. [Google Scholar] [CrossRef]
- Diao, X.; Guan, H.; Zhao, X.; Chen, Q.; Kong, B. Properties and oxidative stability of emulsions prepared with myofibrillar protein and lard diacylglycerols. Meat Sci. 2016, 115, 16–23. [Google Scholar] [CrossRef]
- Zhao, X.; Han, G.; Sun, Q.; Liu, H.; Liu, Q.; Kong, B. Influence of lard-based diacylglycerol on the rheological and physicochemical properties of thermally induced pork myofibrillar protein gels at different pH levels. LWT 2020, 117, 108708. [Google Scholar] [CrossRef]
- Zhang, H.; Luo, Y.; Lu, D.L.; Jiao, J.G.; Li, L.Y.; Qin, J.G.; Chen, L.Q. Diacylglycerol oil reduces fat accumulation and increases protein content by inducing lipid catabolism and protein metabolism in Nile tilapia (Oreochromis niloticus). Aquaculture. 2019, 510, 90–99. [Google Scholar] [CrossRef]
- Devi, B.L.A.P.; Gangadhar, K.N.; Prasad, R.B.N.; Sugasini, D.; Rao, Y.P.; Lokesh, B.R. Nutritionally enriched 1,3-diacylglycerol-rich oil: Low calorie fat with hypolipidemic effects in rats. Food Chem. 2018, 248, 210–216. [Google Scholar] [CrossRef]
- Ahmed, M.J.P.T.; Ahmad, M.L.A.; Farid, M.J. Oxidation of lipids in foods. Sarhad J. Agric. 2016, 32, 230–238. [Google Scholar] [CrossRef]
- Sallam, K.I.; Abd-Elghany, S.M.; Imre, K.; Morar, A.; Herman, V.; Hussein, M.A.; Mahros, M.A. Ensuring safety and improving keeping quality of meatballs by addition of sesame oil and sesamol as natural antimicrobial and antioxidant agents. Food Microbiol. 2021, 99, 103834. [Google Scholar] [CrossRef] [PubMed]
- Noon, J.; Mills, T.B.; Norton, I.T. The use of natural antioxidants to combat lipid oxidation in O/W emulsions. J. Food Eng. 2020, 281, 110006. [Google Scholar] [CrossRef]
- Xiong, Y.L.; Guo, A. Animal and Plant Protein Oxidation. Chemical and Functional Property Significance. Foods 2020, 10, 40. [Google Scholar] [CrossRef]
- Stadtman, E.R.; Levine, R.L. Free radical-mediated oxidation of free amino acids and amino acid residues in proteins. Amino Acids. 2003, 25, 207–218. [Google Scholar] [CrossRef]
- Jiang, J.; Xiong, Y.L. Natural antioxidants as food and feed additives to promote health benefits and quality of meat products: A review. Meat Sci. 2016, 120, 107–117. [Google Scholar] [CrossRef]
- Wang, C.; Sun, C.; Lu, W.; Gul, K.; Mata, A.; Fang, Y. Emulsion structure design for improving the oxidative stability of polyunsaturated fatty acids. Compr. Rev. Food Sci. Food Saf. 2020, 19, 2955–2971. [Google Scholar] [CrossRef]
- Dalluge, J.; Nelson, B.; Thomas, J.; Sander, L. Selection of column and gradient elution system for the separation of catechins in green tea using high performance liquid chromatography. J. Chromatogr. A 1998, 793, 265–274. [Google Scholar] [CrossRef]
- Zhao, M.; Jiao, M.; Zhou, F.; Lin, L.; Sun, W. Interaction of beta-conglycinin with catechin-impact on physical and oxidative stability of safflower oil-in-water emulsion. Food Chem. 2018, 268, 315–323. [Google Scholar] [CrossRef]
- Zhou, S.D.; Lin, Y.F.; Xu, X.; Meng, L.; Dong, M.S. Effect of non-covalent and covalent complexation of (-)-epigallocatechin gallate with soybean protein isolate on protein structure and in vitro digestion characteristics. Food Chem. 2020, 309, 125718. [Google Scholar] [CrossRef]
- Wang, Y.D.; Zhuang, Y.; Yan, H.; Lu, Y.X.; Deng, X.Q.; Hu, Y.; Xiong, S.B.; Yang, H. The influence of pH and monovalent/divalent cations on the structural and physicochemical properties of myofibrillar protein from silver carp. Food chem. 2023, 404, 134519. [Google Scholar] [CrossRef]
- Dumetz, A.C.; Chockla, A.M.; Kaler, E.W.; Lenhoff, A.M. Effects of pH on protein–protein interactions and implications for protein phase behavior. Biochim. Biophys. Acta. 2008, 1784, 600–610. [Google Scholar] [CrossRef] [PubMed]
- Tang, M.; Sun, Y.; Feng, X.; Ma, L.; Dai, H.; Fu, Y.; Zhang, Y. Regulation mechanism of ionic strength on the ultra-high freeze-thaw stability of myofibrillar protein microgel emulsions. Food Chem. 2023, 419, 136044. [Google Scholar] [CrossRef]
- Li, S.; Jiao, B.; Faisal, S.; Zhang, Y.; Wu, B.; Li, W.; Wang, Q. 50/50 oil/water emulsion stabilized by pea protein isolate microgel particles/xanthan gum complexes and co-emulsifiers. Food Hydrocoll. 2023, 134, 108078. [Google Scholar] [CrossRef]
- Xia, X.F.; Kong, B.H.; Liu, Q.; Liu, J. Physicochemical change and protein oxidation in porcine longissimus dorsi as influenced by different freeze-thaw cycles. Meat Sci. 2009, 83, 239–245. [Google Scholar] [CrossRef]
- Cho, D.Y.; Jo, K.; Cho, S.Y.; Kim, J.M.; Lim, K.; Suh, H.J.; Oh, S. Antioxidant effect and functional properties of hydrolysates derived from egg-white protein. Korean J. Food Sci. Anim. Resour. 2014, 34, 362–371. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhou, F.; Zhao, M.; Lin, L.; Ning, Z.; Sun, B. Soy peptide nanoparticles by ultrasound-induced self-assembly of large peptide aggregates and their role on emulsion stability. Food Hydrocoll. 2018, 74, 62–71. [Google Scholar] [CrossRef]
- Mei, L.; Mcclements, D.J.; Wu, J.; Decker, E.A. Iron-catalyzed lipid oxidation in emulsion as affected by surfactant, ph and nacl. Food Chem. 1998, 61, 307–312. [Google Scholar] [CrossRef]
- Oliver, C.N.; Ahn, B.W.; Moerman, E.J.; Goldstein, S.; Stadtman, E.R. Age-related changes in oxidized proteins. J. Biol. Chem. 1987, 262, 5488–5491. [Google Scholar] [CrossRef]
- Benjakul, S.; Bauer, F. Physicochemical and enzymatic changes of cod muscle proteins subjected to different freeze-thaw cycles. J. Sci. Food Agr. 2000, 80, 1143–1150. [Google Scholar] [CrossRef]
- Shimida, A.; Ohashi, K. Interfacial and emulsifying properties of diacylglycerol. Food Sci. Technol. Res. 2003, 9, 142–147. [Google Scholar] [CrossRef]
- Liu, N.; Li, N.; Faiza, M.; Li, D.; Yao, X.; Zhao, M. Stability and in vitro digestion of high purity diacylglycerol oil-in-water emulsions. LWT 2021, 148, 111744. [Google Scholar] [CrossRef]
- Long, Z.; Zhao, M.M.; Liu, N.; Liu, D.L.; Sun-Waterhouse, D.; Zhao, Q.Z. Physicochemical properties of peanut oil-based diacylglycerol and their derived oil-in-water emulsions stabilized by sodium caseinate. Food Chem. 2015, 184, 105–113. [Google Scholar] [CrossRef]
- Zhou, L.; Feng, F.; Zhang, W. Effects of pH on the emulsifying, gelation and curcumin delivery properties of myofibrillar protein and carboxymethyl cellulose emulsions. Food Biosci. 2023, 53, 102744. [Google Scholar] [CrossRef]
- Du, X.; Zhao, M.; Pan, N.; Wang, S.; Xia, X.; Zhang, D. Tracking aggregation behaviour and gel properties induced by structural alterations in myofibrillar protein in mirror carp (Cyprinus carpio) under the synergistic effects of pH and heating. Food Chem. 2021, 362, 130222. [Google Scholar] [CrossRef]
- Liu, R.; Zhao, S.M.; Xie, B.J.; Xiong, S.B. Contribution of protein conformation and intermolecular bonds to fish and pork gelation properties. Food Hydrocoll. 2011, 25, 898–906. [Google Scholar] [CrossRef]
- Guo, X.Y.; Peng, Z.Q.; Zhang, Y.W.; Liu, B.; Cui, Y.Q. The solubility and conformational characteristics of porcine myosin as affected by the presence of l-lysine and l-histidine. Food Chem. 2015, 170, 212–217. [Google Scholar] [CrossRef]
- Jiang, J.; Chen, J.; Xiong, Y.L. Structural and emulsifying properties of soy protein isolate subjected to acid and alkaline ph-shifting processes. J. Agric. Food Chem. 2009, 57, 7576–7583. [Google Scholar] [CrossRef]
- Wang, M.; Li, Y.; Ma, C.; Zhang, Z.; Guo, L.; Huang, M.; Sun, J. Stability of native/thermally denatured myofibrillar protein particles: Improvement with decreasing pH. Food Hydrocoll. 2023, 140, 108628. [Google Scholar] [CrossRef]
- McClements, D.J. Advances in fabrication of emulsions with enhanced functionality using structural design principles. Curr. Opin. Colloid Interface Sci. 2012, 17, 235–245. [Google Scholar] [CrossRef]
- Wang, K.; Zhang, Y.; Sun, J. Synergistic effect of high-intensity ultrasound and pH-shifting on the functionalities of chicken wooden breast myofibrillar protein: Reveal the mechanism of protein structure change. LWT 2023, 181, 114743. [Google Scholar] [CrossRef]
- Destribats, M.; Rouvet, M.; Gehin-Delval, C.; Schmitt, C.; Binks, B.P. Emulsions stabilised by whey protein microgel particles: Towards food-grade Pickering emulsions. Soft Matter. 2014, 10, 6941–6954. [Google Scholar] [CrossRef]
- Owens, C.; Griffin, K.; Khouryieh, H.; Williams, K. Creaming and oxidative stability of fish oil-in-water emulsions stabilized by whey protein-xanthan-locust bean complexes: Impact of pH. Food Chem. 2018, 239, 314–322. [Google Scholar] [CrossRef]
- Jiang, Y.; Zhu, Y.; Zheng, Y.; Liu, Z.; Zhong, Y.; Deng, Y.; Zhao, Y. Effects of salting-in/out-assisted extractions on structural, physicochemical and functional properties of Tenebrio molitor larvae protein isolates. Food Chem. 2021, 338, 128158. [Google Scholar] [CrossRef]
- Kuhn, K.R.; Cunha, R.L. Flaxseed oil-Whey protein isolate emulsions: Effect of high pressure homogenization. J. Food Eng. 2012, 111, 449–457. [Google Scholar] [CrossRef]
- Tan, M.; Ye, J.; Xie, J. Freezing-induced myofibrillar protein denaturation: Role of pH change and freezing rate. LWT 2021, 152, 112381. [Google Scholar] [CrossRef]
- Lu, J.; Zhang, W.; Zhao, X.; Xu, X. Comparison of the interfacial properties of native and refolded myofibrillar proteins subjected to pH-shifting. Food Chem. 2022, 380, 131734. [Google Scholar] [CrossRef] [PubMed]
- Sui, X.; Sun, H.; Qi, B.; Zhang, M.; Li, Y.; Jiang, L. Functional and conformational changes to soy proteins accompanying anthocyanins: Focus on covalent and non-covalent interactions. Food Chem. 2018, 245, 871–878. [Google Scholar] [CrossRef]
- Færgemand, M.; Otte, J.; Qvist, K.B. Emulsifying properties of milk proteins cross-linked with microbial transglutaminase. Int. Dairy J. 1998, 8, 715–723. [Google Scholar] [CrossRef]
- Xu, Q.Q.; Qin, X.L.; Lan, D.M.; Liu, X.; Yang, B.; Liao, S.T.; Wang, W.F.; Wang, Y.H. Water-in-oil emulsions enriched with alpha-linolenic acid in diacylglycerol form: Stability, formation mechanism and in vitro digestion analysis. Food Chem. 2022, 391, 133201. [Google Scholar] [CrossRef]
- Loi, C.C.; Eyres, G.T.; Birch, E.J. Effect of mono- and diglycerides on physical properties and stability of a protein-stabilised oil-in-water emulsion. J. Food Eng. 2019, 240, 56–64. [Google Scholar] [CrossRef]
- Wang, S.; Zhang, Y.; Chen, L.; Xu, X.; Zhou, G.; Li, Z.; Feng, X. Dose-dependent effects of rosmarinic acid on formation of oxidatively stressed myofibrillar protein emulsion gel at different NaCl concentrations. Food Chem. 2018, 243, 50–57. [Google Scholar] [CrossRef]
- Zhou, F.B.; Zhao, M.M.; Cui, C.; Sun, W.Z. Influence of linoleic acid-induced oxidative modifications on physicochemical changes and in vitro digestibility of porcine myofibrillar proteins. LWT-Food Sci. Technol. 2015, 61, 414–421. [Google Scholar] [CrossRef]
- Yoon, E.S.; Singh, R.A.; Oh, H.J.; Kong, H. The effect of contact area on nano/micro-scale friction. Wear 2005, 259, 1424–1431. [Google Scholar] [CrossRef]
- Wei, Z.H.; Huang, Q.R. Edible Pickering emulsions stabilized by ovotransferrin–gum arabic particles. Food Hydrocoll. 2019, 89, 590–601. [Google Scholar] [CrossRef]
- Yan, S.Z.; Yao, Y.X.; Zhang, S.; Huang, Y.Y.; Zhu, H.P.; Li, Y.; Qi, B.K. Comparison of the physical stabilities and oxidation of lipids and proteins in natural and polyphenol-modified soybean protein isolate-stabilized emulsions. Food Res. Int. 2022, 162, 112066. [Google Scholar] [CrossRef]
- Waraho, T.; McClements, D.J.; Decker, E.A. Mechanisms of lipid oxidation in food dispersions. Trends Food Sci. Technol. 2011, 22, 3–13. [Google Scholar] [CrossRef]
- Mancuso, J.R.; Mcclements, D.J.; Decker, E.A. The effects of surfactant type, ph, and chelators on the oxidation of salmon oil-in-water emulsions. J. Agric. Food Chem. 1999, 47, 4112–4116. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Wang, H.; Zhao, Y.; Chen, Q.; Xia, X.; Liu, Q.; Kong, B. Evaluation of the Emulsifying Property and Oxidative Stability of Myofibrillar Protein-Diacylglycerol Emulsions Containing Catechin Subjected to Different pH Values. Foods 2024, 13, 253. https://doi.org/10.3390/foods13020253
Li Y, Wang H, Zhao Y, Chen Q, Xia X, Liu Q, Kong B. Evaluation of the Emulsifying Property and Oxidative Stability of Myofibrillar Protein-Diacylglycerol Emulsions Containing Catechin Subjected to Different pH Values. Foods. 2024; 13(2):253. https://doi.org/10.3390/foods13020253
Chicago/Turabian StyleLi, Yuexin, Hui Wang, Yubo Zhao, Qian Chen, Xiufang Xia, Qian Liu, and Baohua Kong. 2024. "Evaluation of the Emulsifying Property and Oxidative Stability of Myofibrillar Protein-Diacylglycerol Emulsions Containing Catechin Subjected to Different pH Values" Foods 13, no. 2: 253. https://doi.org/10.3390/foods13020253





