Co-Occurrence of Aflatoxin B1, Zearalenone and Ochratoxin A in Feed and Feed Materials in Central Italy from 2018 to 2022
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Collection
2.2. Standards and Reagents
2.3. Sample Preparation
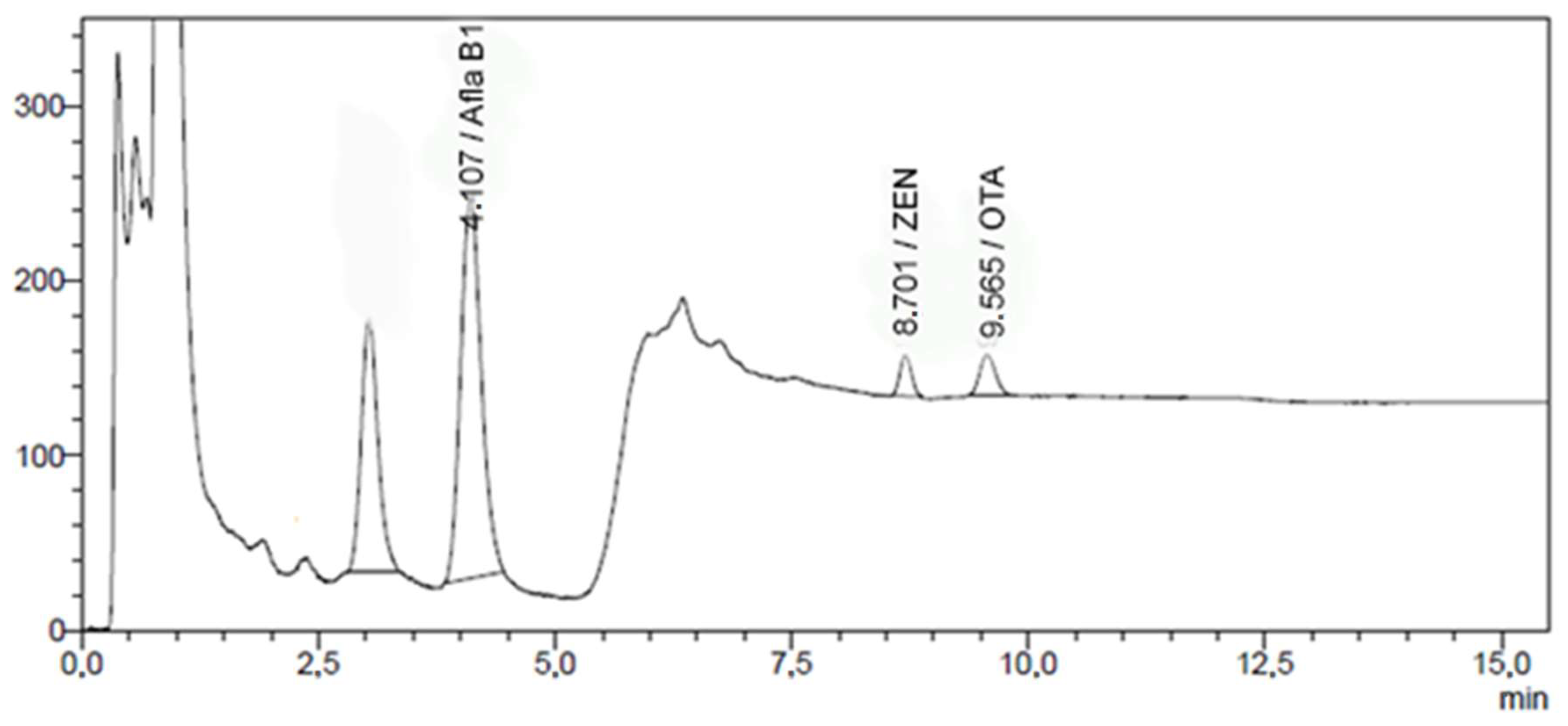
2.4. UPLC-FLD Method
2.5. Method Validation
3. Results
3.1. Method Validation
3.2. Occurrence in Feed and Feed Materials Samples
4. Discussion
4.1. Aflatoxin B1
4.2. Zearalenone
4.3. Ochratoxin A
4.4. General Considerations and Future Perspectives
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Bennett, J.W. Mycotoxins, Mycotoxicoses, Mycotoxicology and Mycopathologia. Mycopathologia 1987, 100, 3–5. [Google Scholar] [CrossRef]
- Miller, J.D. Fungi and Mycotoxins in Grain: Implications for Stored Product Research. J. Stored Prod. Res. 1995, 31, 1–16. [Google Scholar] [CrossRef]
- Ferreira, R.G.; Cardoso, M.V.; de Souza Furtado, K.M.; Espíndola, K.M.M.; Amorim, R.P.; Monteiro, M.C. Epigenetic Alterations Caused by Aflatoxin B1: A Public Health Risk in the Induction of Hepatocellular Carcinoma. Transl. Res. 2019, 204, 51–71. [Google Scholar] [CrossRef] [PubMed]
- Kőszegi, T.; Poór, M. Ochratoxin A: Molecular Interactions, Mechanisms of Toxicity and Prevention at the Molecular Level. Toxins 2016, 8, 111. [Google Scholar] [CrossRef] [PubMed]
- Newberne, P.M.; Butler, W.H. Acute and Chronic Effects of Aflatoxin on the Liver of Domestic and Laboratory Animals: A Review. Cancer Res. 1969, 29, 236–250. [Google Scholar] [PubMed]
- Yang, C.; Song, G.; Lim, W. Effects of Mycotoxin-Contaminated Feed on Farm Animals. J. Hazard. Mater. 2020, 389, 122087. [Google Scholar] [CrossRef]
- Martinez-Martinez, L.; Valdivia-Flores, A.; Guerrero-Barrera, A.L.; Quezada-Tristán, T.; Rangel-Muñoz, E.J.; Ortiz-Martínez, R. Toxic Effect of Aflatoxins in Dogs Fed Contaminated Commercial Dry Feed: A Review. Toxins 2021, 13, 65. [Google Scholar] [CrossRef]
- Bastianello, S.S.; Nesbit, J.W.; Williams, M.C.; Lange, A.L. Pathological Findings in a Natural Outbreak of Aflatoxicosis in Dogs. Onderstepoort J. Vet. 1987, 54, 635–640. [Google Scholar]
- Arnot, L.; Duncan, N.; Coetzer, H.; Botha, C.P. An Outbreak of Canine Aflatoxicosis in Gauteng Province, South Africa. J. S. Afr. Vet. Assoc. 2012, 83, 4. [Google Scholar] [CrossRef] [PubMed]
- Newman, S.J.; Smith, J.R.; Stenske, K.A.; Newman, L.B.; Dunlap, J.R.; Imerman, P.M.; Kirk, C.A. Aflatoxicosis in Nine Dogs after Exposure to Contaminated Commercial Dog Food. J. Vet. Diagn. Investig. 2007, 19, 168–175. [Google Scholar] [CrossRef] [PubMed]
- Wouters, A.T.B.; Casagrande, R.A.; Wouters, F.; Watanabe, T.T.N.; Boabaid, F.M.; Cruz, C.E.F.; Driemeier, D. An Outbreak of Aflatoxin Poisoning in Dogs Associated with Aflatoxin B1–Contaminated Maize Products. J. Vet. Diagn. Investig. 2013, 25, 282–287. [Google Scholar] [CrossRef]
- Tan, Y.Q.; Li, Q.; Wang, L.; Chiu-Leung, L.C.; Leung, L.K. The Livestock Growth-Promoter Zeranol Facilitates GLUT4 Translocation in 3T3 L1 Adipocytes. Chemosphere 2020, 253, 126772. [Google Scholar] [CrossRef] [PubMed]
- Hald, B. Porcine Nephropathy in Europe; IARC Scientific Publications: Lyon, France, 1991; pp. 49–56. [Google Scholar]
- Battacone, G.; Nudda, A.; Pulina, G. Effects of Ochratoxin A on Livestock Production. Toxins 2010, 2, 1796–1824. [Google Scholar] [CrossRef] [PubMed]
- Marquardt, R.R. Effects of Molds and Their Toxins on Livestock Performance: A Western Canadian Perspective. Anim. Feed. Sci. Technol. 1996, 58, 77–89. [Google Scholar] [CrossRef]
- Muñoz-Solano, B.; González-Peñas, E. Co-Occurrence of Mycotoxins in Feed for Cattle, Pigs, Poultry, and Sheep in Navarra, a Region of Northern Spain. Toxins 2023, 15, 172. [Google Scholar] [CrossRef] [PubMed]
- Daou, R.; Joubrane, K.; Maroun, R.G.; Khabbaz, L.R.; Ismail, A.; Khoury, A.E. Mycotoxins: Factors Influencing Production and Control Strategies. AIMS Agr. Food 2021, 6, 416–447. [Google Scholar] [CrossRef]
- Kos, J.; Anić, M.; Radić, B.; Zadravec, M.; Hajnal, E.J.; Pleadin, J. Climate Change—A Global Threat Resulting in Increasing Mycotoxin Occurrence. Foods 2023, 12, 2704. [Google Scholar] [CrossRef] [PubMed]
- Bhat, R.; Ramakrishna, Y.; Beedu, S.; Munshi, K.L. Outbreak of trichothecene mycotoxicosis associated with consumption of mould-damaged wheat products in Kashmir Valley, India. Lancet 1989, 333, 35–37. [Google Scholar] [CrossRef]
- Reddy, B.; Raghavender, C. Outbreaks of Fusarial-Toxicoses in India. Cereal Res. Commun. 2008, 36, 321–325. [Google Scholar] [CrossRef]
- Kamala, A.; Shirima, C.; Jani, B.; Bakari, M.; Sillo, H.; Rusibamayila, N.; De Saeger, S.; Kimanya, M.; Gong, Y.Y.; Simba, A. Outbreak of an Acute Aflatoxicosis in Tanzania during 2016. World Mycotoxin J. 2018, 11, 311–320. [Google Scholar] [CrossRef]
- Sypecka, Z.; Kelly, M.; Brereton, P. Deoxynivalenol and Zearalenone Residues in Eggs of Laying Hens Fed with a Naturally Contaminated Diet: Effects on Egg Production and Estimation of Transmission Rates from Feed to Eggs. J. Agric. Food Chem. 2004, 52, 5463–5471. [Google Scholar] [CrossRef] [PubMed]
- Bozzo, G.; Bonerba, E.; Ceci, E.; Colao, V.; Tantillo, G. Determination of Ochratoxin A in Eggs and Target Tissues of Experimentally Drugged Hens Using HPLC–FLD. Food Chem. 2011, 126, 1278–1282. [Google Scholar] [CrossRef]
- Puga-Torres, B.; Cáceres-Chicó, M.; Alarcón-Vásconez, D.; Gómez, C.A. Determination of Zearalenone in Raw Milk from Different Provinces of Ecuador. Vet. World 2021, 14, 2048–2054. [Google Scholar] [CrossRef] [PubMed]
- Mehta, R.; Shetty, S.A.; Young, M.F.; Ryan, P.B.; Rangiah, K. Quantification of Aflatoxin and Ochratoxin Contamination in Animal Milk Using UHPLC-MS/SRM Method: A Small-Scale Study. J. Food Technol. 2021, 58, 3453–3464. [Google Scholar] [CrossRef]
- Altafini, A.; Roncada, P.; Guerrini, A.; Minkoumba Sonfack, G.; Fedrizzi, G.; Caprai, E. Occurrence of Ochratoxin A in Different Types of Cheese Offered for Sale in Italy. Toxins 2021, 13, 540. [Google Scholar] [CrossRef] [PubMed]
- Mahmudiono, T.; Mazaheri, Y.; Sadighara, P.; Akbarlou, Z.; Hoseinvandtabar, S.; Fakhri, Y. Prevalence and Concentration of Aflatoxin M1 and Ochratoxin A in Cheese: A Global Systematic Review and Meta-Analysis and Probabilistic Risk Assessment. Rev. Environ. Health, 2023; online ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Canela, R.; Viladrich, R.; Velázquez, C.; Sanchis, V. A Survey of Porcine Kidneys and Chicken Liver for Ochratoxin A in Spain. Mycopathologia 1994, 125, 29–32. [Google Scholar] [CrossRef]
- Gareis, M.; Scheuer, R. Ochratoxin A in Meat and Meat Products. Archiv Lebensmittelhyg. 2000, 51, 102–104. [Google Scholar]
- Paoloni, A.; Solfrizzo, M.; Bibi, R.; Pecorelli, I. Development and Validation of LC-MS/MS Method for the Determination of Ochratoxin A and Its Metabolite Ochratoxin α in Poultry Tissues and Eggs. J. Environ. Sci. Health B 2018, 53, 327–333. [Google Scholar] [CrossRef]
- Prandini, A.; Tansini, G.; Sigolo, S.; Filippi, L.; Laporta, M.; Piva, G. On the Occurrence of Aflatoxin M1 in Milk and Dairy Products. Food Chem. Toxicol. 2009, 47, 984–991. [Google Scholar] [CrossRef]
- Roila, R.; Branciari, R.; Verdini, E.; Ranucci, D.; Valiani, A.; Pelliccia, A.; Fioroni, L.; Pecorelli, I. A Study of the Occurrence of Aflatoxin M1 in Milk Supply Chain over a Seven-Year Period (2014–2020): Human Exposure Assessment and Risk Characterization in the Population of Central Italy. Foods 2021, 10, 1529. [Google Scholar] [CrossRef] [PubMed]
- Wexler, P. Encyclopedia of Toxicology. 2, Dep—Iso; Elsevier/Academic Press: Amsterdam, The Netherlands, 2014; ISBN 9780127999388. [Google Scholar]
- IARC Working Group on the Evaluation of the Carcinogenic Risk of Chemicals to Humans; International Agency for Research on Cancer. Chemical Agents and Related Occupations; International Agency for Research on Cancer: Lyon, France, 2012; ISBN 9789283213239. [Google Scholar]
- European Commission Commission Regulation (EC), No. 401/2006 of 23 February 2006 Laying Down the Methods of Sampling and Analysis for the Official Control of the Levels of Mycotoxins in Foodstuffs. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A02006R0401-20140701 (accessed on 14 December 2023).
- European Commission Commission Regulation, No. 691/2013 of 19 July 2013 Amending Regulation (EC) No. 152/2009 as Regards Methods of Sampling and Analysis. Available online: https://eur-lex.europa.eu/eli/reg/2013/691/oj (accessed on 14 December 2023).
- European Commission Commission Regulation (EU), No. 915/2023 of 25 April 2023 on Maximum Levels for Certain Contaminants in Food and Repealing Regulation (EC) No. 1881/2006. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A02023R0915-20230810 (accessed on 14 December 2023).
- European Commission Directive 2002/32/EC of the European Parliament and of the Council of 7 May 2002 on Undesirable Substances in Animal Feed. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A02002L0032-20191128 (accessed on 14 December 2023).
- European Commission Commission Recommendation (EU), No. 1319/2016 of 29 July 2016 Amending Recommendation 2006/576/EC as Regards Deoxynivalenol, Zearalenone and Ochratoxin A in Pet Food. Available online: https://eur-lex.europa.eu/eli/reco/2016/1319/oj (accessed on 14 December 2023).
- Goryacheva, I.Y.; Saeger, S.D.; Eremin, S.A.; Peteghem, C.V. Immunochemical Methods for Rapid Mycotoxin Detection: Evolution from Single to Multiple Analyte Screening: A Review. Food Addit. Contam. 2007, 24, 1169–1183. [Google Scholar] [CrossRef] [PubMed]
- Songsermsakul, P.; Razzazi-Fazeli, E. A Review of Recent Trends in Applications of Liquid Chromatography-Mass Spectrometry for Determination of Mycotoxins. J. Liq. Chromatogr. Relat. Technol. 2008, 31, 1641–1686. [Google Scholar] [CrossRef]
- Turner, N.W.; Subrahmanyam, S.; Piletsky, S.A. Analytical Methods for Determination of Mycotoxins: A Review. Anal. Chim. Acta 2009, 632, 168–180. [Google Scholar] [CrossRef]
- Shanakhat, H.; Sorrentino, A.; Raiola, A.; Romano, A.; Masi, P.; Cavella, S. Current Methods for Mycotoxins Analysis and Innovative Strategies for Their Reduction in Cereals: An Overview. J. Sci. Food Agric. 2018, 98, 4003–4013. [Google Scholar] [CrossRef]
- Locatelli, S.; Scarpino, V.; Lanzanova, C.; Romano, E.; Amedeo, R. Multi-Mycotoxin Long-Term Monitoring Survey on North-Italian Maize over an 11-Year Period (2011–2021): The Co-Occurrence of Regulated, Masked and Emerging Mycotoxins and Fungal Metabolites. Toxins 2022, 14, 520. [Google Scholar] [CrossRef] [PubMed]
- Arranz, I.; Baeyens, W.R.G.; Van der Weken, G.; De Saeger, S.; Van Peteghem, C. Review: HPLC Determination of Fumonisin Mycotoxins. Crit. Rev. Food Sci. 2004, 44, 195–203. [Google Scholar] [CrossRef]
- Shephard, G.S. Determination of Mycotoxins in Human Foods. Chem. Soc. Rev. 2008, 37, 2468–2477. [Google Scholar] [CrossRef]
- Chen, F.; Luan, C.; Wang, L.; Wang, S.; Shao, L. Simultaneous Determination of Six Mycotoxins in Peanut by High-Performance Liquid Chromatography with a Fluorescence Detector. J. Sci. Food Agric. 2016, 97, 1805–1810. [Google Scholar] [CrossRef]
- Lippolis, V.; Pascale, M.; Valenzano, S.; Visconti, A. Comparison of Slurry Mixing and Dry Milling in Laboratory Sample Preparation for Determination of Ochratoxin A and Deoxynivalenol in Wheat. J. AOAC Int. 2012, 95, 452–458. [Google Scholar] [CrossRef]
- Göbel, R.; Lusky, K. Simultaneous Determination of Aflatoxins, Ochratoxin A, and Zearalenone in Grains by New Immunoaffinity Column/Liquid Chromatography. J. AOAC Int. 2004, 87, 411–416. [Google Scholar] [CrossRef] [PubMed]
- European Commission Guidance Document SANTE/12089/2016 on Identification of Mycotoxins in Food and Feed. Available online: https://food.ec.europa.eu/system/files/2023-10/cs_contaminants_sampling_guid-doc-ident-mycotoxins.pdf (accessed on 15 January 2024).
- European Commission Guidance Document SANTE 11312/2021v2—Analytical Quality Control and Method Validation Procedures for Pesticide Residues Analysis in Food and Feed. Available online: https://food.ec.europa.eu/system/files/2023-11/pesticides_mrl_guidelines_wrkdoc_2021-11312.pdf (accessed on 15 January 2024).
- Borman, P.; Elder, D. Q2(R1) Validation of Analytical Procedures. In ICH Quality Guidelines: An Implementation Guide; Wiley: Hoboken, NJ, USA, 2017; pp. 127–166. [Google Scholar] [CrossRef]
- Ente Italiano di Normazione (UNI) Technical Report CEN/TR 16059:2010; Food Analysis–Performance Criteria for Single Laboratory Validated Methods of Analysis for the Determination of Mycotoxins. Ente Italiano di Normazione: Milan, Italy, 2010.
- ISO/IEC 17025; General Requirements for the Competence Of testing and Calibration Laboratories. International Organization for Standardization/International Electrotechnical Committee: Geneva, Switzerland, 2017.
- Dimitrieska-Stojković, E.; Stojanovska-Dimzoska, B.; Ilievska, G.; Uzunov, R.; Stojković, G.; Hajrulai-Musliu, Z.; Jankuloski, D. Assessment of Aflatoxin Contamination in Raw Milk and Feed in Macedonia during 2013. Food Control. 2016, 59, 201–206. [Google Scholar] [CrossRef]
- Iqbal, S.Z.; Asi, M.R.; Nisar, S.; Zia, K.M.; Jinap, S.; Malik, N. A Limited Survey of Aflatoxins and Zearalenone in Feed and Feed Ingredients from Pakistan. J. Food Protect. 2016, 79, 1798–1801. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Zhang, L.; Xu, Z.; Liu, X.-D.; Chen, L.; Dai, J.-F.; Karrow, N.A.; Sun, L. Occurrence of Aflatoxin B1, Deoxynivalenol and Zearalenone in Feeds in China during 2018–2020. J. Anim. Sci. Biotechnol. 2021, 12, 74. [Google Scholar] [CrossRef] [PubMed]
- Zinedine, A.; Juan, C.; Soriano, J.M.; Moltó, J.C.; Idrissi, L.; Mañes, J. Limited Survey for the Occurrence of Aflatoxins in Cereals and Poultry Feeds from Rabat, Morocco. Int. J. Food Microbiol. 2007, 115, 124–127. [Google Scholar] [CrossRef]
- Bervis, N.; Lorán, S.; Juan, T.; Carramiñana, J.J.; Herrera, A.; Agustín, A.; Herrera, M. Field Monitoring of Aflatoxins in Feed and Milk of High-Yielding Dairy Cows under Two Feeding Systems. Toxins 2021, 13, 201. [Google Scholar] [CrossRef] [PubMed]
- Mahato, D.K.; Devi, S.; Pandhi, S.; Sharma, B.; Maurya, K.K.; Mishra, S.; Dhawan, K.; Selvakumar, R.; Kamle, M.; Mishra, A.K.; et al. Occurrence, Impact on Agriculture, Human Health, and Management Strategies of Zearalenone in Food and Feed: A Review. Toxins 2021, 13, 92. [Google Scholar] [CrossRef]
- Chang, H.; Kim, W.; Park, J.-H.; Kim, D.; Kim, C.-R.; Chung, S.; Lee, C. The Occurrence of Zearalenone in South Korean Feedstuffs between 2009 and 2016. Toxins 2017, 9, 223. [Google Scholar] [CrossRef] [PubMed]
- Cavaglieri, L. Fungal and Mycotoxin Contamination of Cow Feeding Stuffs in Argentina. In Proceedings of the International Conference. Fifth Framework Program. European Union Myco-Globe Project, Accra, Ghana, 13–16 September 2005. [Google Scholar]
- Kosicki, R.; Błajet-Kosicka, A.; Grajewski, J.; Twarużek, M. Multiannual Mycotoxin Survey in Feed Materials and Feedingstuffs. Anim. Feed Sci. Technol. 2016, 215, 165–180. [Google Scholar] [CrossRef]
- Janić Hajnal, E.; Kos, J.; Krulj, J.; Krstović, S.; Jajić, I.; Pezo, L.; Šarić, B.; Nedeljković, N. Aflatoxins Contamination of Maize in Serbia: The Impact of Weather Conditions in 2015. Food Addit. Contam. A 2017, 34, 1999–2010. [Google Scholar] [CrossRef] [PubMed]
- Labuda, R.; Parich, A.; Berthiller, F.; Tančinová, D. Incidence of Trichothecenes and Zearalenone in Poultry Feed Mixtures from Slovakia. Int. J. Food Microbiol. 2005, 105, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.J.; Kim, H.J. Development of an Immunoaffinity Chromatography and LC-MS/MS Method for the Determination of 6 Zearalenones in Animal Feed. PLoS ONE 2018, 13, e0193584. [Google Scholar] [CrossRef]
- Nuryono, N.; Noviandi, C.T.; Böhm, J.; Razzazi-Fazeli, E. A Limited Survey of Zearalenone in Indonesian Maize-Based Food and Feed by ELISA and High Performance Liquid Chromatography. Food Control. 2005, 16, 65–71. [Google Scholar] [CrossRef]
- Liu, J.; Sun, L.; Zhang, J.; Guo, J.; Chen, L.; Qi, D.; Zhang, N. Aflatoxin B1, Zearalenone and Deoxynivalenol in Feed Ingredients and Complete Feed from Central China. Food Addit. Contam. B 2016, 9, 91–97. [Google Scholar] [CrossRef] [PubMed]
- Pozzo, L.; Cavallarin, L.; Nucera, D.; Antoniazzi, S.; Schiavone, A. A Survey of Ochratoxin A Contamination in Feeds and Sera from Organic and Standard Swine Farms in Northwest Italy. J. Sci. Food Agric. 2010, 90, 1467–1472. [Google Scholar] [CrossRef] [PubMed]
- Pietruszka, K.; Piątkowska, M.; Jedziniak, P. Occurrence of Ochratoxin A in Animal Tissues and Feeds in Poland in 2014–2016. J. Vet. Res. 2017, 61, 483–487. [Google Scholar] [CrossRef] [PubMed]
- Binder, E.M.; Tan, L.M.; Chin, L.J.; Handl, J.; Richard, J. Worldwide Occurrence of Mycotoxins in Commodities, Feeds and Feed Ingredients. Anim. Feed Sci. Technol. 2007, 137, 265–282. [Google Scholar] [CrossRef]
- Böhm, J.; Koinig, L.; Razzazi-Fazeli, E.; Blajet-Kosicka, A.; Twaruzek, M.; Grajewski, J.; Lang, C. Survey and Risk Assessment of the Mycotoxins Deoxynivalenol, Zearalenone, Fumonisins, Ochratoxin A, and Aflatoxins in Commercial Dry Dog Food. Mycotoxin Res. 2010, 26, 147–153. [Google Scholar] [CrossRef] [PubMed]
- Shao, M.; Li, L.; Gu, Z.; Yao, M.; Xu, D.; Fan, W.; Yan, L.; Song, S. Mycotoxins in Commercial Dry Pet Food in China. Food Addit. Contam. B 2018, 11, 237–245. [Google Scholar] [CrossRef]
- Gazzotti, T.; Biagi, G.; Pagliuca, G.; Pinna, C.; Scardilli, M.; Grandi, M.; Zaghini, G. Occurrence of Mycotoxins in Extruded Commercial Dog Food. Anim. Feed Sci. Technol. 2015, 202, 81–89. [Google Scholar] [CrossRef]
- Trocino, A.; Cotozzolo, E.; Zomeño, C.; Petracci, M.; Xiccato, G.; Castellini, C. Rabbit Production and Science: The World and Italian Scenarios from 1998 to 2018. Ital. J. Anim. Sci. 2019, 18, 1361–1371. [Google Scholar] [CrossRef]
- Li, P.; Yang, S.; Zhang, X.; Huang, S.; Wang, N.; Wang, M.; Long, M.; He, J. Zearalenone Changes the Diversity and Composition of Caecum Microbiota in Weaned Rabbit. BioMed Res. Int. 2018, 2018, 3623274. [Google Scholar] [CrossRef] [PubMed]
- Tsouloufi, T.K.; Tsakmakidis, Ι.; Tsousis, G.; Papaioannou, N.; Tzika, E.; Kritsepi-Konstantinou, M. The Effect of Subchronic Oral Exposure to Zearalenone on Hematologic and Biochemical Analytes, and the Blood Redox Status of Adult Rabbit Bucks. Vet. Clin. Pathol. 2019, 48, 328–334. [Google Scholar] [CrossRef] [PubMed]
- Panel, E.; Chain, F. European Food Safety Authority (EFSA) Scientific Opinion on the Risks for Human and Animal Health Related to the Presence of Modified Forms of Certain Mycotoxins in Food and Feed. EFSA J. 2014, 12, 3916. [Google Scholar] [CrossRef]
- Organisation for Economic Co-operation Development (OECD); Food and Agricultural Organization (FAO). OECD-FAO Agricultural Outlook 2021–2030; OECD: Paris, France, 2021; ISBN 9789264721388. [Google Scholar]
- Devegowda, G.; Ravikiran, D. Mycotoxins and Eggshell Quality: Cracking the Problem. World Mycotoxin J. 2008, 1, 203–208. [Google Scholar] [CrossRef]
- Rogers, G.M.; Poore, M.H.; Paschal, J.C. Feeding Cotton Products to Cattle. Vet. Clin. N. Am. Food Anim. Pr. 2002, 18, 267–294. [Google Scholar] [CrossRef] [PubMed]
- Jaime-Garcia, R.; Cotty, P.J. Aflatoxin Contamination of Commercial Cottonseed in South Texas. Phytopathology 2003, 93, 1190–1200. [Google Scholar] [CrossRef] [PubMed]
- Feizy, J.; Beheshti, H.R.; Asadi, M. A Survey of Aflatoxin in Cotton Seed in Iran by HPLC with On-Line Photochemical Derivatisation and Fluorescence Detection. Food Addit. Contam. B 2012, 5, 200–203. [Google Scholar] [CrossRef] [PubMed]
- Smith, M.-C.; Madec, S.; Coton, E.; Hymery, N. Natural Co-Occurrence of Mycotoxins in Foods and Feeds and Their in Vitro Combined Toxicological Effects. Toxins 2016, 8, 94. [Google Scholar] [CrossRef] [PubMed]
- Thapa, A.; Horgan, K.A.; White, B.; Walls, D. Deoxynivalenol and Zearalenone—Synergistic or Antagonistic Agri-Food Chain Co-Contaminants? Toxins 2021, 13, 561. [Google Scholar] [CrossRef]
- Hooker, D.C.; Schaafsma, A.W.; Tamburic-Ilincic, L. Using Weather Variables Pre- and Post-Heading to Predict Deoxynivalenol Content in Winter Wheat. Plant Dis. 2002, 86, 611–619. [Google Scholar] [CrossRef] [PubMed]
- Van der Fels-Klerx, H.J.; Vermeulen, L.C.; Gavai, A.K.; Liu, C. Climate Change Impacts on Aflatoxin B1 in Maize and Aflatoxin M1 in Milk: A Case Study of Maize Grown in Eastern Europe and Imported to the Netherlands. PLoS ONE 2019, 14, e0218956. [Google Scholar] [CrossRef] [PubMed]
- Liu, N.; Cheng, L.; Dudaš, T.; Loc, M.; Bagi, F.; Van Der Fels-Klerx, H.J. Improved Aflatoxins and Fumonisins Forecasting Models for Maize (PREMA and PREFUM), Using Combined Mechanistic and Bayesian Network Modeling—Serbia as a Case Study. Front. Microbiol. 2021, 12, 643604. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Liu, C.; van der Fels-Klerx, H.J. Regional Prediction of Multi-Mycotoxin Contamination of Wheat in Europe Using Machine Learning. Food Res. Int. 2022, 159, 111588. [Google Scholar] [CrossRef] [PubMed]
- Joint FAO/WHO Codex Alimentarius Commission; World Health Organization; Food and Agriculture Organization of the United Nations. Prevention and Reduction of Food and Feed Contamination; World Health Organization: Rome, Italy, 2012; ISBN 9789251071199. [Google Scholar]
- IARC. Practical Approaches to Control Mycotoxins; IARC Scientific Publications: Lyon, France, 2012; pp. 131–146. [Google Scholar]
| Products Intended for Animal Feed | Maximum Content 1 (mg/kg) | ||
|---|---|---|---|
| AFB1 | Feed materials | 0.02 | |
| Complementary and complete feed | 0.01 | ||
| Compound feed for dairy cattle and calves, dairy sheep and lambs, dairy goats and kids, piglets and young poultry animals | 0.005 | ||
| Compound feed for cattle (except dairy cattle and calves), sheep (except dairy sheep and lambs), goats (except dairy goats and kids), pigs (except piglets) and poultry (except young animals) | 0.02 | ||
| Products intended for animal feed | Guidance Value 2 (mg/kg) | ||
| ZEN | Feed materials | Cereals and cereal products with the exception of maize by-products | 2 |
| Maize by-products | 3 | ||
| Compound feed for: | Piglets, gilts (young sows), puppies, kittens, dogs and cats for reproduction | 0.1 | |
| Adult dogs and cats other than for reproduction | 0.2 | ||
| Sows and fattening pigs | 0.25 | ||
| Calves, dairy cattle, sheep (including lamb) and goats (including kids) | 0.5 | ||
| OTA | Feed materials | Cereals and cereal products | 0.25 |
| Compound feed for: | Pigs | 0.05 | |
| Poultry | 0.1 | ||
| Cats and dogs | 0.01 | ||
| LOQ (mg/kg) | Matrix | Level (mg/kg) | Recovery (%) | sr | RSDr | |
|---|---|---|---|---|---|---|
| AFB1 | 0.0020 | Compound feed (BCR 375) | 0.0020 | 103 | 5.8 | 5.7 |
| Cow feed | 0.0049 | 89 | 4.4 | 5.0 | ||
| Chicken feed | 0.0101 | 100 | 3.0 | 3.0 | ||
| Horse feed | 0.0115 | 91 | 11 | 12 | ||
| Swine feed | 0.0230 | 97 | 3.9 | 4.1 | ||
| Matrix | Level (mg/kg) | Recovery (%) | sWR | RSDwR | ||
| Compound feed (ERM-BE375) | 0.0020 | 92 | 15 | 16 | ||
| LOQ (mg/kg) | Matrix | Level (mg/kg) | Recovery (%) | sr | RSDr | |
| ZEN | 0.050 | Compound feed (BCR 375) | 0.050 | 100 | 9.5 | 9.5 |
| Swine feed | 0.106 | 106 | 7.2 | 6.8 | ||
| Chicken feed | 0.247 | 98 | 2.6 | 2.6 | ||
| Horse feed | 0.247 | 109 | 11 | 11 | ||
| Cow feed | 0.500 | 89 | 5.2 | 5.9 | ||
| Matrix | Level (mg/kg) | Recovery (%) | sWR | RSDwR | ||
| Compound feed (ERM-BE375) | 0.050 | 84 | 14 | 16 | ||
| LOQ (mg/kg) | Matrix | Level (mg/kg) | Recovery (%) | sr | RSDr | |
| OTA | 0.0040 | Dog feed | 0.0040 | 90 | 6.4 | 7.1 |
| 0.025 | Compound feed (BCR 375) | 0.025 | 72 | 3.5 | 4.9 | |
| Swine feed | 0.053 | 92 | 2.9 | 3.1 | ||
| Chicken feed | 0.101 | 76 | 2.1 | 2.8 | ||
| Horse feed | 0.105 | 85 | 10 | 12 | ||
| Cow feed | 0.103 | 84 | 2.4 | 3.9 | ||
| LOQ (mg/kg) | Matrix | Level (mg/kg) | Recovery (%) | sWR | RSDwR | |
| 0.0040 | Dog feed | 0.0040 | 81 | 9.4 | 11 | |
| 0.025 | Compound feed (ERM-BE375) | 0.025 | 79 | 10 | 13 |
| LOQ (mg/kg) | Matrix | Level (mg/kg) | Recovery (%) | sr | RSDr | |
|---|---|---|---|---|---|---|
| AFB1 | 0.0080 | Rapeseed | 0.0084 (LOQ) | 73 | 4.6 | 6.5 |
| Maize | 0.0201 | 66 | 3.8 | 5.7 | ||
| Maize | 0.0401 | 70 | 4.0 | 5.7 | ||
| Matrix | Level (mg/kg) | Recovery (%) | sWR | RSDwR | ||
| Maize | 0.0080 (LOQ) | 72 | 14 | 22 | ||
| Barley | 0.0080 (LOQ) | 84 | 14 | 16 | ||
| LOQ (mg/kg) | Matrix | Level (mg/kg) | Recovery (%) | sr | RSDr | |
| ZEN | 0.200 | Rapeseed | 0.200 (LOQ) | 76 | 13 | 17 |
| Maize | 3.030 | 89 | 1.7 | 1.9 | ||
| Maize | 5.998 | 86 | 2.6 | 3.1 | ||
| Matrix | Level (mg/kg) | Recovery (%) | sWR | RSDwR | ||
| Maize | 0.267 (LOQ) | 81 | 8.6 | 11 | ||
| Barley | 0.200 (LOQ) | 84 | 11 | 14 | ||
| LOQ (mg/kg) | Matrix | Level (mg/kg) | Recovery (%) | sr | RSDr | |
| OTA | 0.100 | Rapeseed | 0.102 (LOQ) | 83 | 3.7 | 4.5 |
| Maize | 0.247 | 89 | 2.0 | 2.2 | ||
| Maize | 0.509 | 90 | 2.4 | 2.7 | ||
| Matrix | Level (mg/kg) | Recovery (%) | sWR | RSDwR | ||
| Maize | 0.100 (LOQ) | 75 | 5.7 | 7.5 | ||
| Barley | 0.100 (LOQ) | 86 | 7.3 | 7.5 |
| Animal Species/ Feed Material | Samples | Detected Samples 1 (% of Samples) | Above Maximum Content/ Guidance Value (% of Samples) |
|---|---|---|---|
| Feed | |||
| Cattle | 194 | 48 (25%) | 4 (2.1%) |
| Cow | 54 | 15 (28%) | - |
| Dog | 26 | - | - |
| Poultry | 62 | 10 (16%) | 1 (1.6%) |
| Rabbit | 19 | 10 (53%) | - |
| Sheep | 261 | 58 (22%) | 10 (3.1%) |
| Swine | 179 | 62 (35%) | 1 (0.6%) |
| Other species | 31 | 9 (29%) | - |
| TOT | 826 | 211 (26%) | 16 (1.9%) |
| Feed materials | |||
| Barley | 186 | - | - |
| Maize | 240 | 35 (15%) | 14 (5.8%) |
| Oat | 44 | - | - |
| Triticale | 30 | - | - |
| Wheat | 61 | - | - |
| Other materials | 56 | 1 (0.2%) | - |
| TOT | 617 | 36 (5.8%) | 14 (2.2%) |
| Analyte | Species/Materials | Incidence (%) | Concentration (mg/kg) | ||
|---|---|---|---|---|---|
| Minimum | Maximum | Average | |||
| AFB1 | Cattle | 14 | 0.0025 | 0.1045 | 0.0133 |
| Cow | 11 | 0.0036 | 0.0179 | 0.0072 | |
| Poultry | 8 | 0.0030 | 0.0291 | 0.0107 | |
| Rabbit | 5 | 0.0022 | |||
| Sheep | 5 | 0.0021 | 0.0216 | 0.0061 | |
| Swine | 13 | 0.0021 | 0.0138 | 0.0045 | |
| Other species | 10 | 0.0024 | 0.0040 | 0.0031 | |
| Maize | 15 | 0.0085 | 0.1234 | 0.0349 | |
| Other feed materials | 0.2 | 0.0156 | |||
| ZEN | Cattle | 11 | 0.059 | 6.420 | 0.527 |
| Cow | 20 | 0.057 | 1.017 | 0.204 | |
| Poultry | 8 | 0.058 | 0.330 | 0.196 | |
| Rabbit | 47 | 0.058 | 5.723 | 0.765 | |
| Sheep | 17 | 0.056 | 5.387 | 0.602 | |
| Swine | 25 | 0.051 | 1.698 | 0.182 | |
| Other species | 16 | 0.054 | 0.189 | 0.114 | |
| Maize | 0.2 | 0.668 | |||
| OTA | Cattle | 1 | 0.034 | 0.042 | 0.039 |
| Cow | 2 | 0.036 | |||
| Sheep | 1 | 0.038 | 0.085 | 0.061 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sdogati, S.; Pacini, T.; Bibi, R.; Caporali, A.; Verdini, E.; Orsini, S.; Ortenzi, R.; Pecorelli, I. Co-Occurrence of Aflatoxin B1, Zearalenone and Ochratoxin A in Feed and Feed Materials in Central Italy from 2018 to 2022. Foods 2024, 13, 313. https://doi.org/10.3390/foods13020313
Sdogati S, Pacini T, Bibi R, Caporali A, Verdini E, Orsini S, Ortenzi R, Pecorelli I. Co-Occurrence of Aflatoxin B1, Zearalenone and Ochratoxin A in Feed and Feed Materials in Central Italy from 2018 to 2022. Foods. 2024; 13(2):313. https://doi.org/10.3390/foods13020313
Chicago/Turabian StyleSdogati, Stefano, Tommaso Pacini, Rita Bibi, Angela Caporali, Emanuela Verdini, Serenella Orsini, Roberta Ortenzi, and Ivan Pecorelli. 2024. "Co-Occurrence of Aflatoxin B1, Zearalenone and Ochratoxin A in Feed and Feed Materials in Central Italy from 2018 to 2022" Foods 13, no. 2: 313. https://doi.org/10.3390/foods13020313







