Formulation of Antioxidant Gummies Based on Gelatin Enriched with Citrus Fruit Peels Extract
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of the Citrus Extracts
2.3. Chemical Characterization of Citrus Extracts
2.4. Synthesis of the Antioxidant Gelatin Conjugate by Grafting Reaction
2.5. Differential Calorimetric Analysis of the Functionalized Gelatins
2.6. Preparation of Gummies Based on Functionalized Gelatins
2.7. Antioxidant Properties of Gummies
2.8. Total Phenolic Content
2.9. Phenolic Acid Content
2.10. Flavonoid Content
2.11. Scavenging Activity against DPPH Radical
2.12. Scavenging Activity against ABTS Radical
2.13. Rheological Characterization of Gummies
2.14. Statistical Analysis
3. Results and Discussion
3.1. Preparation and Chemical Characterization of the Citrus Extracts
3.2. Antioxidant Performances of the Extracts
3.3. Synthesis of Functionalized Gelatins
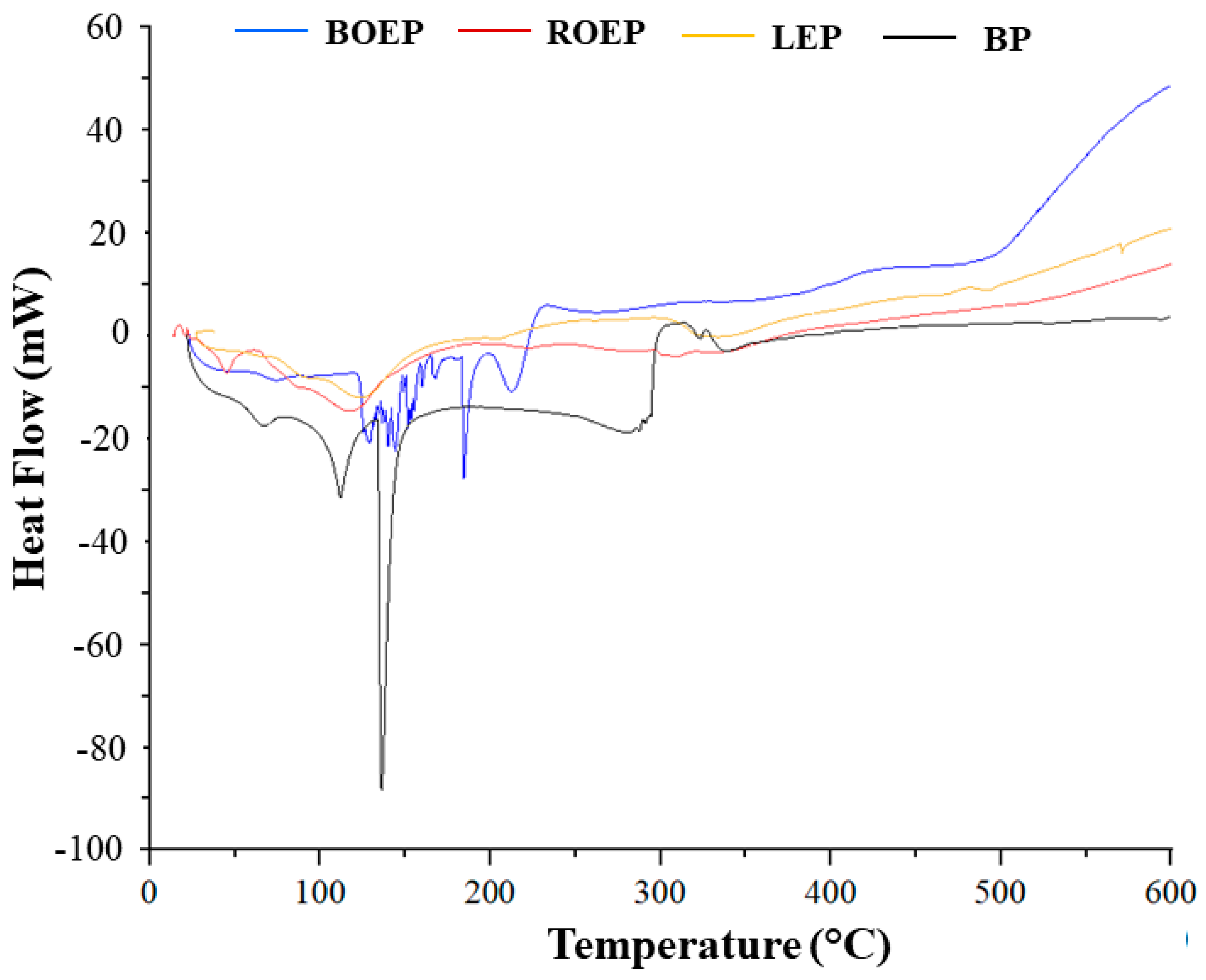
3.4. Characterization of Functionalized Gelatins
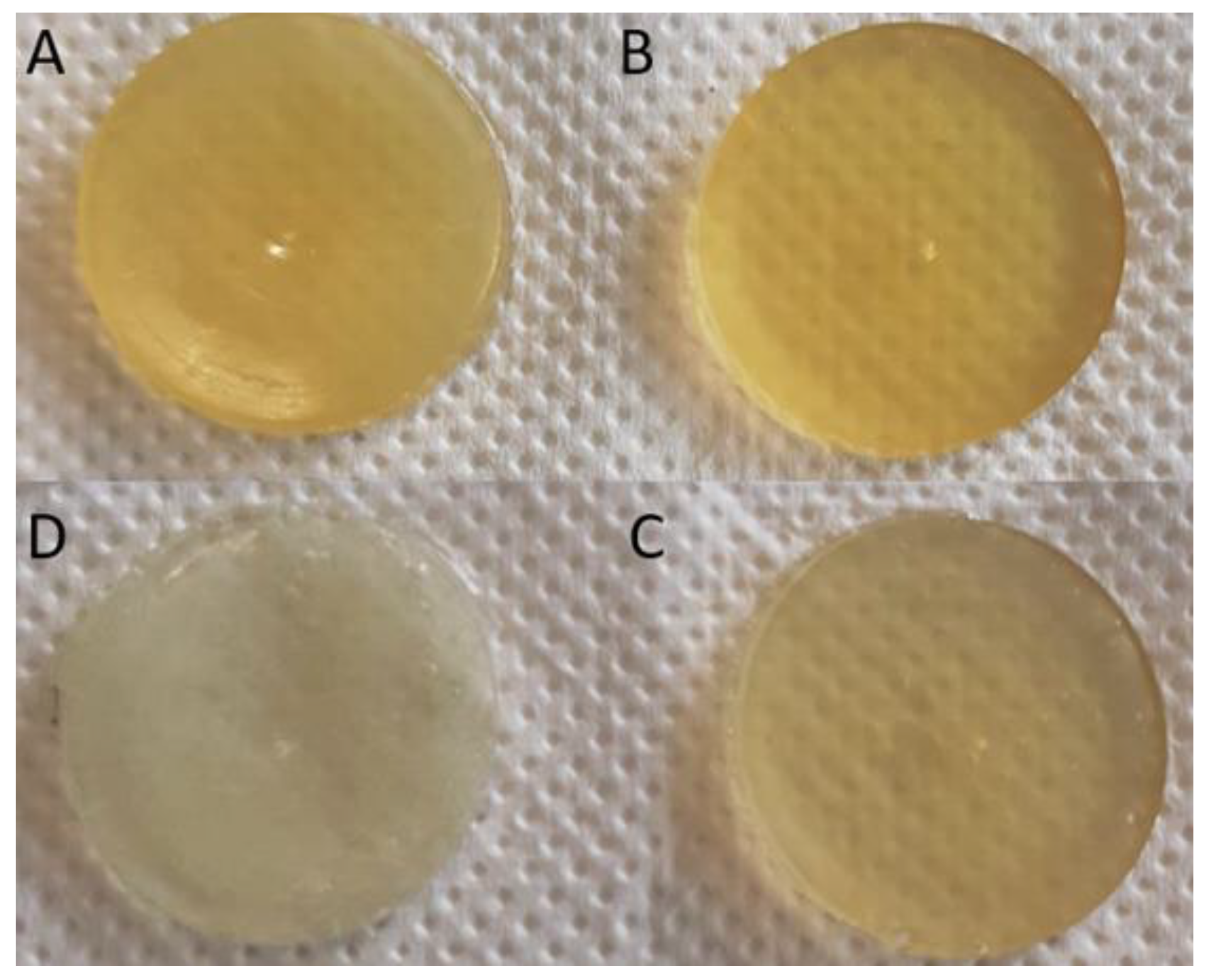
3.5. Preparation and Characterization of Functionalized Gummies
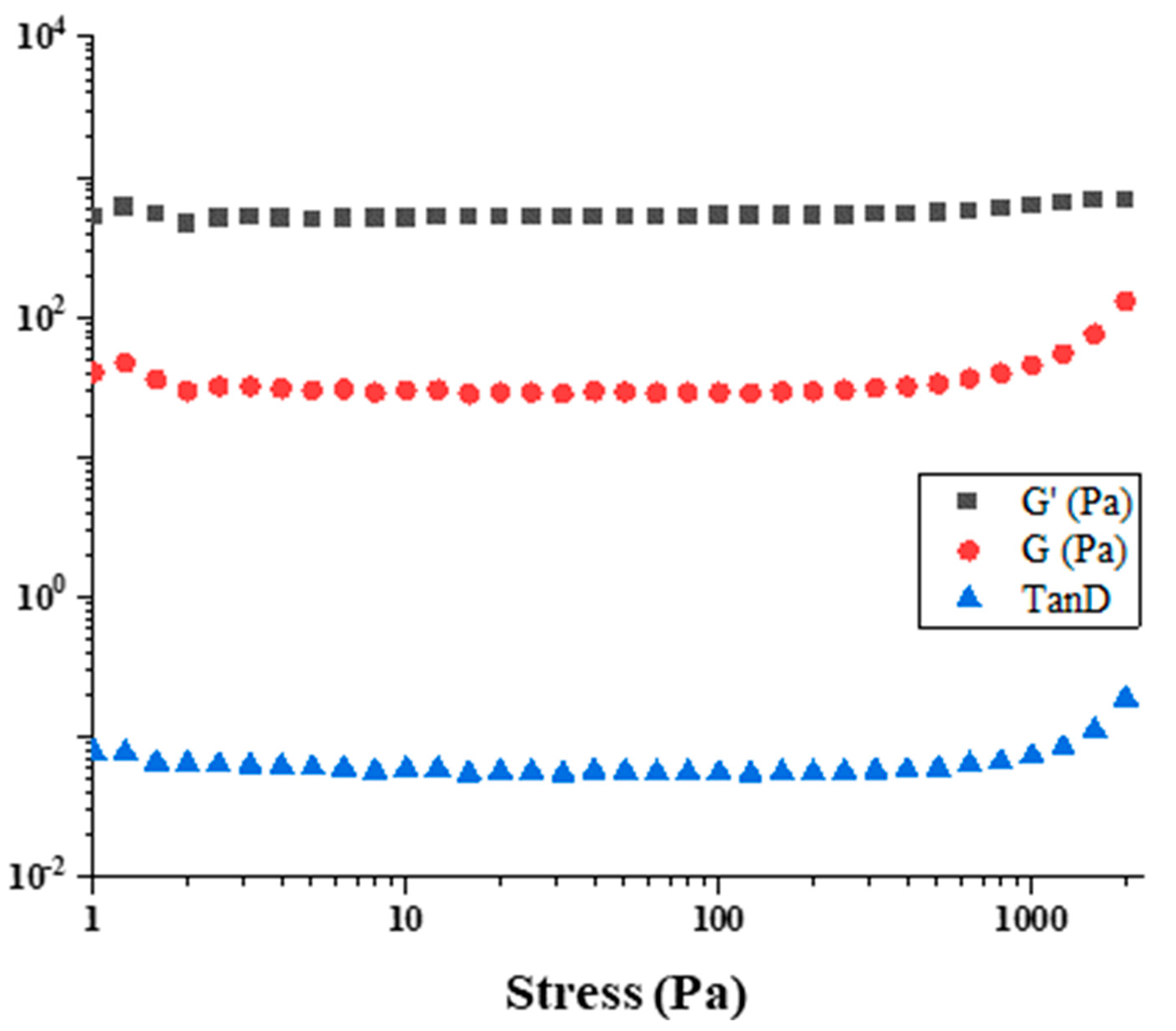
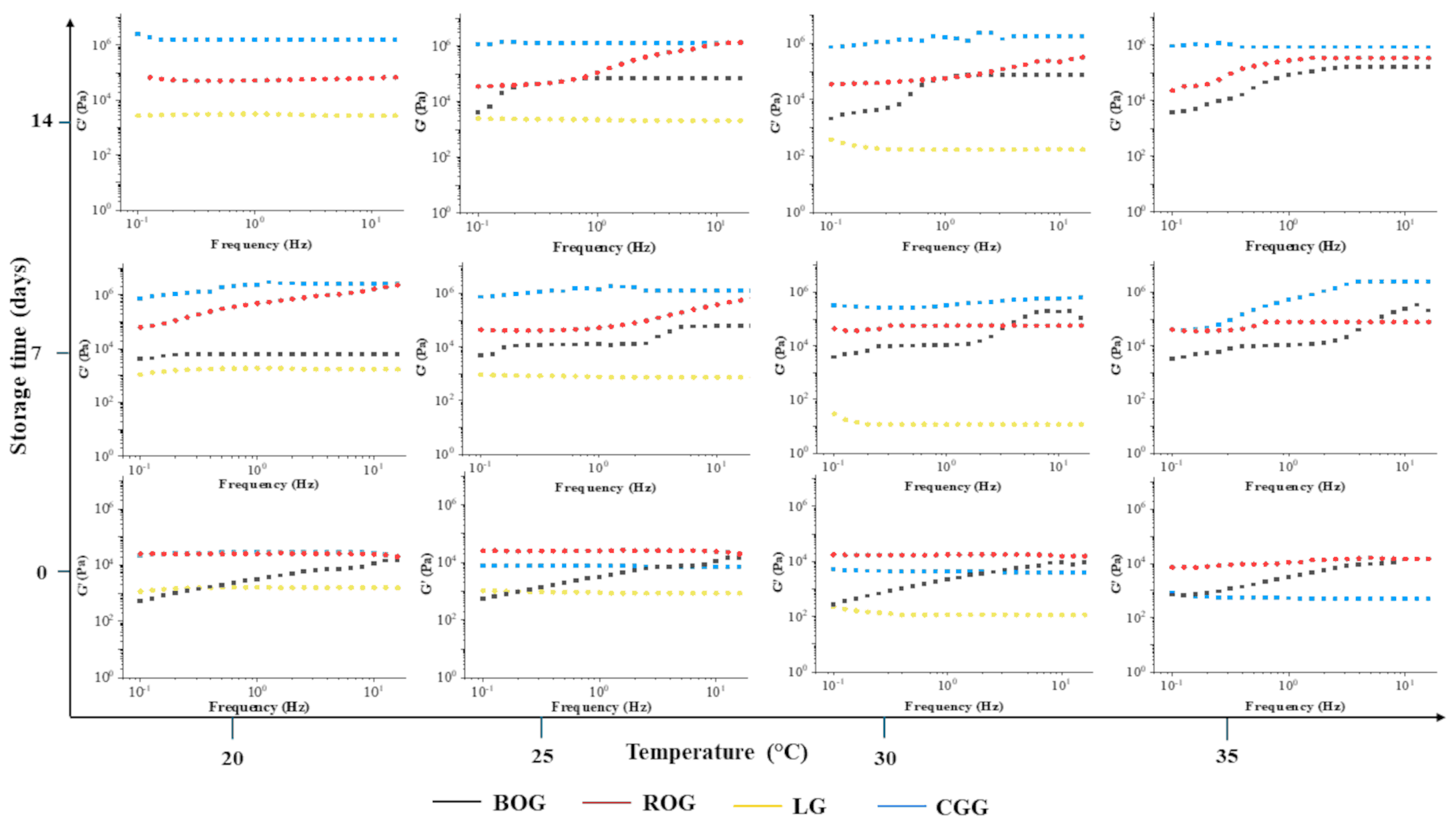
3.6. Rheological Properties of the Functionalized Gummies
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cognitive Market Research. Global Jellies and Gummies Market Report 2022, Market Size S2hare, Growth, CAGR, Forecast, Revenue. Report ID: CMR493857. 2022. Available online: https://www.cognitivemarketresearch.com/ (accessed on 30 September 2023).
- Future Market Insights. Jellies and Gummies Market—Global Industry Analysis, Share, Size and Forecast, 2018 to 2028. Report ID: GB-8156. 2022. Available online: https://www.futuremarketinsights.com/ (accessed on 30 September 2023).
- Report of United States Department of Agriculture Foreign Agricultural Service (USDA); “Citrus: World Markets and Trade”. Approved by the World Agricultural Outlook Board, 27 July 2023. Developed & Supported by Albert R. Mann Library, Cornell University, Ithaca, NY, USA. Available online: https://usda.library.cornell.edu/concern/publications/w66343603?locale=en (accessed on 30 September 2023).
- FAO Report. Citrus Fruit Fresh and Processed Statistical Bulletin. In Citrus Fruit Statistical Compendium 2020; FAO: Rome, Italy, 2021.
- Suri, S.; Singh, A.; Nema, P.K. Current applications of citrus fruit processing waste: A scientific outlook. Appl. Food Res. 2022, 2, 100050. [Google Scholar] [CrossRef]
- Kandemir, K.; Piskin, E.; Xiao, J.; Tomas, M.; Capanoglu, E. Fruit Juice Industry Wastes as a Source of Bioactives. J. Agric. Food Chem. 2022, 70, 6805–6832. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Vishvakarma, R.; Gautam, K.; Vimal, A. Valorization of citrus peel waste for the sustainable production of value-added products. Bioresour. Technol. 2022, 351, 127064. [Google Scholar] [CrossRef]
- Hsouna, A.B.; Sadaka, C.; Generalić Mekinić, I.; Garzoli, S.; Švarc-Gajić, J.; Rodrigues, F.; Morais, S.; Moreira, M.M.; Ferreira, E.; Spigno, G.; et al. The Chemical Variability, Nutraceutical Value, and Food-Industry and Cosmetic Applications of Citrus Plants: A Critical Review. Antioxidants 2023, 12, 481. [Google Scholar] [CrossRef] [PubMed]
- Alaqeel, N.K. Antioxidants from different citrus peels provide protection against cancer. Braz. J. Biol. 2024, 84, e271619. [Google Scholar] [CrossRef] [PubMed]
- M’hiri, N.; Ioannou, I.; Ghoul, M.; Mihoubi Boudhrioua, N. Extraction Methods of Citrus Peel Phenolic Compounds. Food Rev. Int. 2014, 30, 265–290. [Google Scholar] [CrossRef]
- Chávez, M.L.; Lopez, L.; Rodriguez, R.; Contreras-Esquivel, J.C. Enzyme-assisted extraction of citrus essential oil. Chem. Pap. 2015, 70, 412–417. [Google Scholar]
- Gil-Martín, E.; Forbes-Hernández, T.; Romero, A.; Cianciosi, D.; Giampieri, F.; Battino, M. Influence of the extraction method on the recovery of bioactive phenolic compounds from food industry by-products. Food Chem. 2022, 378, 131918. [Google Scholar] [CrossRef]
- Chavez-Gonzalez, M.L.; Sepúlveda, L.; Verma, D.K.; Luna-García, H.A.; RodríguezDuran, L.V.; Ilina, A.; Aguilar, C.N. Conventional and emerging extraction processes of flavonoids. Processes 2020, 8, 434. [Google Scholar] [CrossRef]
- Sharma, K.; Mahato, N.; Cho, M.H.; Lee, Y.R. Converting citrus wastes into value-added products: Economic and environmently friendly approaches. Nutrition 2017, 34, 29–46. [Google Scholar] [CrossRef]
- Panwar, D.; Panesar, P.S.; Chopra, H.K. Recent trends on the valorization strategies for the management of citrus by-products. Food Rev. Int. 2021, 37, 91–120. [Google Scholar] [CrossRef]
- Zarate Vilet, N.; Guè, E.; Servent, A.; Delalonde, M.; Wisniewski, C. Filtration-compression step as downstream process for flavonoids extraction from citrus peels: Performances and flavonoids dispersion state in the filtrate. Food Bioprod. Process. 2020, 120, 104–113. [Google Scholar] [CrossRef]
- Dougherty, P.G.; Sahni, A.; Pei, D. Understanding Cell Penetration of Cyclic Peptides. Chem. Rev. 2019, 119, 10241–10287. [Google Scholar] [CrossRef]
- Garcìa-Catsello, E.M.; Rodriguez-Lopez, A.D.; Mayor, L.; Ballesteros, R.; Conidi, C.; Cassano, A. Optimization of conventional and ultrasound assisted extraction of flavonoids from grapefruit (Citrus paradisi L.) solid wastes. Food Sci. Technol. 2015, 64, 1114–1122. [Google Scholar]
- Papoutsis, K.; Pristijono, P.; Golding, J.B.; Stathopopulus, C.E.; Scarlett, C.J.; Bowyer, M.C.; Vuong, Q. Impact of different solvents on the recovery of bioactive compounds and antioxidant properties from lemon (Citrus limon L.) pomace waste. Food Sci. Biotechnol. 2016, 25, 971–977. [Google Scholar] [CrossRef]
- Safdar, M.N.; Kausar, T.; Jabbar, S.; Mumtaz, A.; Ahad, K.; Saddozai, A.A. Extraction and quantification of polyphenols from kinnow (Citrus reticulate L.) peel using ultrasound and maceration techniques. J. Food Drug Anal. 2017, 25, 488–500. [Google Scholar] [CrossRef]
- Bitwell, C.; Indra, S.S.; Luke, C.; Kakoma, M.K. A review of modern and conventional extraction techniques and their applications for extracting phytochemicals from plants. Sci. Afr. 2023, 19, e01585. [Google Scholar] [CrossRef]
- Rudrapal, M.; Khairnar, S.J.; Khan, J.; Dukhyil, A.B.; Azam Ansari, M.; Alomary, M.N.; Alshabrmi, F.M.; Palai, S.; Kumar Deb, P.; Devi, R. Dietary Polyphenols and Their Role in Oxidative Stress-Induced Human Diseases: Insights Into Protective Effects, Antioxidant Potentials and Mechanism(s) of Action. Front. Pharmacol. 2022, 13, 806470. [Google Scholar] [CrossRef]
- Singh, B.; Singh, J.P.; Kaur, A.; Singh, N. Phenolic composition, antioxidant potential and health benefits of citrus peel. Food Res. Int. 2020, 132, 109114. [Google Scholar] [CrossRef]
- Carullo, G.; Ramunno, A.; Sommella, E.M.; De Luca, M.; Belsito, E.L.; Frattaruolo, L.; Brindisi, M.; Campiglia, P.; Cappello, A.R.; Aiello, F. Ultrasound-Assisted Extraction, Chemical Characterization, and Impact on Cell Viability of Food Wastes Derived from Southern Italy Autochthonous Citrus Fruits. Antioxidants 2022, 11, 285. [Google Scholar] [CrossRef]
- Mahato, N.; Sinha, M.; Sharma, K.; Koteswararao, R.; Cho, M.H. Modern Extraction and Purification Techniques for Obtaining High Purity Food-Grade Bioactive Compounds and Value-Added Co-Products from Citrus Wastes. Foods 2019, 8, 523. [Google Scholar] [CrossRef] [PubMed]
- Šafranko, S.; Corkovic, I.; Jerkovic, I.; Jakovljevic, M.; Aladic, K.; Šubaric, D.; Jokic, S. Green Extraction Techniques for Obtaining Bioactive Compounds from Mandarin Peel (Citrus unshiu var. Kuno): Phytochemical Analysis and Process Optimization. Foods 2021, 10, 1043. [Google Scholar] [CrossRef] [PubMed]
- Arun, K.B.; Madhavan, A.; Sindhu, R.; Binod, P.; Pandey, A.; Reshmy, R.; Sirohi, R. Remodeling agro-industrial and food wastes into value-added bioactives and biopolymers. Ind. Crops Prod. 2020, 154, 112–621. [Google Scholar] [CrossRef]
- Luque-Garcıa, J.L.; De Castro, M.L. Ultrasound: A powerful tool for leaching. Trends Anal. Chem. 2003, 22, 41–47. [Google Scholar] [CrossRef]
- Dalmau, E.; Rosselló, C.; Eim, V.; Ratti, C.; Simal, S. Ultrasound-Assisted Aqueous Extraction of Biocompounds from Orange Byproduct: Experimental Kinetics and Modeling. Antioxidants 2020, 9, 352. [Google Scholar] [CrossRef]
- Spizzirri, U.G.; Caputo, P.; Nicoletti, R.; Crupi, P.; D’Ascenzo, F.; Oliverio Rossi, C.; Clodoveo, M.L.; Aiello, F.; Restuccia, D. Unripe carob pods: An innovative source of antioxidant molecules for the preparation of high-added value gummies. Br. Food J. 2023, 56, 5497–5505. [Google Scholar] [CrossRef]
- Geremias-Andrade, I.M.; Souki, N.P.B.G.; Moraes, I.C.F.; Pinho, S.C. Rheology of Emulsion-Filled Gels Applied to the Development of Food Materials. Gels 2016, 2, 22. [Google Scholar] [CrossRef]
- Haug, I.J.; Draget, K.I. Gelatin. In Handbook of Food Proteins; Woodhead Publishing: Sawston, UK, 2011; pp. 92–115. [Google Scholar]
- Grand View Research. Jellies & Gummies Market Size, Share j Industry Report, 2025. Report ID: GVR-3-68038-647-9. 2022. Available online: https://www.grandviewresearch.com/ (accessed on 30 September 2023).
- Ramırez-Pulido, B.; Bas-Bellver, C.; Betoret, N.; Barrera, C.; Seguı, L. Valorization of vegetable fresh-processing residues as functional powdered ingredients. A review on the potential impact of pretreatments and drying methods on bioactive compounds and their bioaccessibility. Front. Sustain. Food Syst. 2021, 5, 654313. [Google Scholar] [CrossRef]
- Restuccia, D.; Giorgi, G.; Spizzirri, U.G.; Sciubba, F.; Capuani, G.; Rago, V.; Carullo, G.; Aiello, F. Autochthonous white grape pomaces as bioactive source for functional jams. Int. J. Food Sci. Technol. 2019, 54, 1313–1320. [Google Scholar] [CrossRef]
- Caputo, P.; Oliviero Rossi, C. Differential Scanning Calorimetry as a New Method to Evaluate the Effectiveness of Rejuvenating Agents in Bitumens. Appl. Sci. 2021, 11, 6528. [Google Scholar] [CrossRef]
- Sabri, L.A.; Khasraghi, A.H.; Sulaiman, H.T. Preparation and evaluation of oral soft chewable jelly containing flurbiprofen. J. Adv. Pharm. Technol. Res. 2022, 13, 306–311. [Google Scholar] [PubMed]
- Gohel, M.C.; Parikh, R.K.; Nagori, S.A.; Shah, S.N.; Dabhi, M.R. Preparation and evaluation of soft gellan gum gel containing paracetamol. Indian J. Pharm. Sci. 2009, 71, 120–124. [Google Scholar] [CrossRef] [PubMed]
- Cano-Lamadrid, M.; Calín-Sánchez, Á.; Clemente-Villalba, J.; Hernández, F.; Carbonell-Barrachina, Á.A.; Sendra, E.; Wojdyło, A. Quality parameters and consumer acceptance of caramella candies based on pomegranate juice “Mollar de Elche”. Foods 2020, 9, 516. [Google Scholar] [CrossRef]
- Spizzirri, U.G.; Carullo, G.; Aiello, F.; Paolino, D.; Restuccia, D. Valorisation of olive oil pomace extracts for a functional pear beverage formulation. Int. J. Food Sci. Technol. 2021, 56, 5497–5505. [Google Scholar] [CrossRef]
- De Luca, M.; Restuccia, D.; Spizzirri, U.G.; Crupi, P.; Ioele, G.; Gorelli, B.; Clodoveo, M.L.; Saponara, S.; Aiello, F. Wine Lees as Source of Antioxidant Molecules: Green Extraction Procedure and Biological Activity. Antioxidants 2023, 12, 622. [Google Scholar] [CrossRef]
- Spizzirri, U.G.; Caputo, P.; Oliviero Rossi, C.; Crupi, P.; Muraglia, M.; Rago, V.; Malivindi, R.; Clodoveo, M.L.; Restuccia, D.; Aiello, F. A Tara Gum/Olive Mill Wastewaters Phytochemicals Conjugate as a New Ingredient for the Formulation of an Antioxidant-Enriched Pudding. Foods 2022, 11, 158. [Google Scholar] [CrossRef] [PubMed]
- Spizzirri, U.G.; Carullo, G.; De Cicco, L.; Crispini, A.; Scarpelli, F.; Restuccia, D.; Aiello, F. Synthesis and characterization of a (+)-catechin and L-(+)-ascorbic acid cocrystal as a new functional ingredient for tea drinks. Heliyon 2019, 5, e02291. [Google Scholar] [CrossRef] [PubMed]
- Carullo, G.; Spizzirri, U.G.; Montopoli, M.; Cocetta, V.; Armentano, B.; Tinazzi, M.; Sciubba, F.; Giorgi, G.; Di Cocco, M.E.; Bohn, T.; et al. Milk kefir enriched with inulin-grafted seed extract from white wine pomace: Chemical characterization, antioxidant profile and in vitro gastrointestinal digestion. Int. J. Food Sci. Technol. 2022, 57, 4086–4095. [Google Scholar] [CrossRef]
- Oliviero Rossi, C.; Caputo, P.; Loise, V.; Ashimova, S.; Teltayev, B.; Sangiorgi, C. A New Green Rejuvenator: Evaluation of Structural Changes of Aged and Recycled Bitumens by Means of Rheology and NMR. In RILEM 252-CMB 2018, RILEM Bookseries; Poulikakos, L.D., Falchetto, A.C., Wistuba, M.P., Hofko, B., Porot, L., Di Benedetto, H., Eds.; Springer International Publishing: Berlin/Heidelberg, Germany, 2019; Volume 20, pp. 177–182. [Google Scholar]
- Caputo, P.; Miriello, D.; Bloise, A.; Baldino, N.; Mileti, O.; Ranieri, G.A. A comparison and correlation between bitumen adhesion evaluation test methods, boiling and contact angle tests. Int. J. Adhes. Adhes. 2020, 102, 102680. [Google Scholar] [CrossRef]
- Gómez-Mejía, E.; Sacristán, I.; Rosales-Conrado, N.; León-González, M.E.; Madrid, Y. Effect of Storage and Drying Treatments on Antioxidant Activity and Phenolic Composition of Lemon and Clementine Peel Extracts. Molecules 2023, 28, 1624. [Google Scholar] [CrossRef]
- Li, B.B.; Smith, B.; Hossain, M. Extraction of phenolics from citrus peels: I. Solvent extraction method. Sep. Purif. Technol. 2006, 48, 182–188. [Google Scholar] [CrossRef]
- Wang, Z.; Chen, X.; Guo, Z.; Feng, X.; Huang, X.; Huang, P.; Du, M.; Zalan, Z.; Kan, J. Distribution and natural variation of free, esterified, glycosylated, and insoluble-bound phenolic compounds in brocade orange (Citrus sinensis L. Osbeck) peel. Food Res. Int. 2022, 153, 110–958. [Google Scholar] [CrossRef] [PubMed]
- Gryko, K.; Kalinowska, M.; Ofman, P.; Choinska, R.; Swiderski, G.; Swisłocka, R.; Lewandowski, W. Natural Cinnamic Acid Derivatives: A Comprehensive Study on Structural, Anti/Pro-Oxidant, and Environmental Impacts. Materials 2021, 14, 6098. [Google Scholar] [CrossRef]
- Laura, A.; Moreno-Escamilla, J.O.; Rodrigo-García, J.; Alvarez-Parrilla, E. Phenolic compounds. In Postharvest Physiology and Biochemistry of Fruits and Vegetables; Woodhead Publishing: Sawston, UK, 2019; pp. 253–271. [Google Scholar]
- Liu, Y.; Benohoud, M.; Galani Yamdeu, J.H.; Gong, Y.Y.; Orfila, C. Green extraction of polyphenols from citrus peel by-products and their antifungal activity against Aspergillus flavus. Food Chem. X 2021, 12, 100144. [Google Scholar] [CrossRef] [PubMed]
- Saleem, M.; Durani, A.I.; Asari, A.; Ahmed, M.; Ahmad, M.; Yousaf, N.; Muddassar, M. Investigation of antioxidant and antibacterial effects of citrus fruits peels extracts using different extracting agents: Phytochemical analysis within silico studies. Heliyon 2023, 9, e15433. [Google Scholar] [CrossRef] [PubMed]
- Floegel, A.; Kim, D.O.; Chung, S.J.; Koo, S.I.; Chun, O.K. Comparison of ABTS/DPPH assays to measure antioxidant capacity in popular antioxidant rich US foods. J. Food Compos. Anal. 2011, 24, 1043–1048. [Google Scholar] [CrossRef]
- Azima, A.M.S.; Noriham, A.; Manshoor, N. Anthocyanin content in relation to the antioxidant activity and colour properties of Garcinia mangostana peel, Syzigium cumini and Clitoria ternatea extracts. Int. Food Res. J. 2014, 21, 2369–2375. [Google Scholar]
- El-aal, H.A.; Halaweish, F.T. Food preservative activity of phenolic compounds in orange peel extracts (Citrus sinensis L.). Lucr. Ştiinţ. 2010, 53, 233–240. [Google Scholar]
- Chen, M.L.; Yang, D.J.; Liu, S.C. Effects of drying temperature on the flavonoid, phenolic acid and antioxidative capacities of the methanol extract of citrus fruit (Citrus sinensis (L.) Osbeck) peels. Int. J. Food Sci. Technol. 2011, 46, 1179–1185. [Google Scholar] [CrossRef]
- Lagha-Benamrouche, S.; Madani, K. Phenolic contents and antioxidant activity of orange varieties (Citrus sinensis L. and Citrus aurantium L.) cultivated in Algeria: Peels and leaves. Ind. Crops Prod. 2013, 50, 723–730. [Google Scholar] [CrossRef]
- Far, B.F.; Naimi-Jamal, M.R.; Safaei, M.; Zarei, K.; Moradi, M.; Nezhad, H.Y. A Review on Biomedical Application of Polysaccharide-Based Hydrogels with a Focus on Drug Delivery Systems. Polymers 2022, 14, 5432–5457. [Google Scholar]
- Carullo, G.; Scarpelli, F.; Belsito, E.L.; Caputo, P.; Rossi, C.O.; Mincione, A.; Leggio, A.; Crispini, A.; Restuccia, D.; Spizzirri, U.G.; et al. Formulation of New Baking (+)-Catechin Based Leavening Agents: Effects on Rheology, Sensory and Antioxidant Features during Muffin Preparation. Foods 2020, 9, 1569. [Google Scholar] [CrossRef] [PubMed]
- Purohit, P.; Bhatt, A.; Mittal, R.K.; Abdellattif, M.H.; Farghaly, T.A. Polymer Grafting and its chemical reactions. Front. Bioeng. Biotechnol. 2022, 10, 1044927. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, R.; Qin, W.; Dai, J.; Zhang, Q.; Lee, K.; Liu, Y. Physicochemical properties of gelatin films containing tea polyphenol-loaded chitosan nanoparticles generated by electrospray. Mater. Des. 2020, 185, 108277. [Google Scholar] [CrossRef]
- Charoen, R. Development of Antioxidant Gummy Jelly Candy Supplemented with Psidium guajava Leaf Extract. Int. J. Appl. Sci. Technol. 2015, 8, 145–151. [Google Scholar] [CrossRef]
- Piechowiak, T.; Balawejder, M.; Grzelak-Błaszczyk, K.; Oracz, J.; Matłok, N. Antioxidant activity of fruit jellies enriched with phytochemicals from Pinus sylvestris L. LWT Food Sci. Technol. 2023, 173, 114262. [Google Scholar] [CrossRef]
- Cedeño-Pinos, C.; Martínez-Tomé, M.; Murcia, M.A.; Jordán, M.J.; Bañón, S. Assessment of Rosemary (Rosmarinus officinalis L.) Extract as Antioxidant in Jelly Candies Made with Fructan Fibres and Stevia. Antioxidants 2020, 9, 1289. [Google Scholar] [CrossRef] [PubMed]
- Soares Mateus, A.R.; Barros, S.; Pena, A.; Sanches-Silva, A. The potential of citrus by-products in the development of functional food and active packaging. Adv. Food Nutr. Res. 2023, 107, 41–90. [Google Scholar]
- Bohlin, L. A theory of flow as a cooperative phenomenon. J. Colloid Interface Sci. 1980, 74, 423–434. [Google Scholar] [CrossRef]
- Winter, H.H. Can the gel point of a cross-linking polymer be detected by the G-G” crossover? Polym. Eng. Sci. 1987, 27, 1698–1702. [Google Scholar] [CrossRef]
- Gabriele, D.; De Cindio, B.; D’Antona, P. A weak gel model for foods. Rheol. Acta 2001, 40, 120–127. [Google Scholar] [CrossRef]
- Garrido, J.I.; Lozano, J.E.; Genovese, D.B. Effect of formulation variables on rheology, texture, colour, and acceptability of apple jelly: Modelling and optimization. LWT Food Sci. Technol. 2015, 62, 325–332. [Google Scholar] [CrossRef]
- Endress, H.U.; Mattes, F. Rheological Characterization of Gum and Jelly Products. In Advances in Pectin and Pectinase Research; Voragen, F., Schols, H., Visser, R., Eds.; Springer: Dordrecht, The Netherlands, 2003. [Google Scholar]
- Ross-Murphy, S.B. Rheology of Biopolymer Solutions and Gels. J. Rheol. 1995, 39, 1451–1460. [Google Scholar] [CrossRef]
- Antunes, F.; Gentile, L.; Oliviero Rossi, C.; Tavano, L.; Ranieri, G.A. Gels of Pluronic F127 and nonionic surfactants from rheological characterization to controlled drug permeation. Colloids Surf. B Biointerfaces 2011, 87, 42–48. [Google Scholar] [CrossRef]
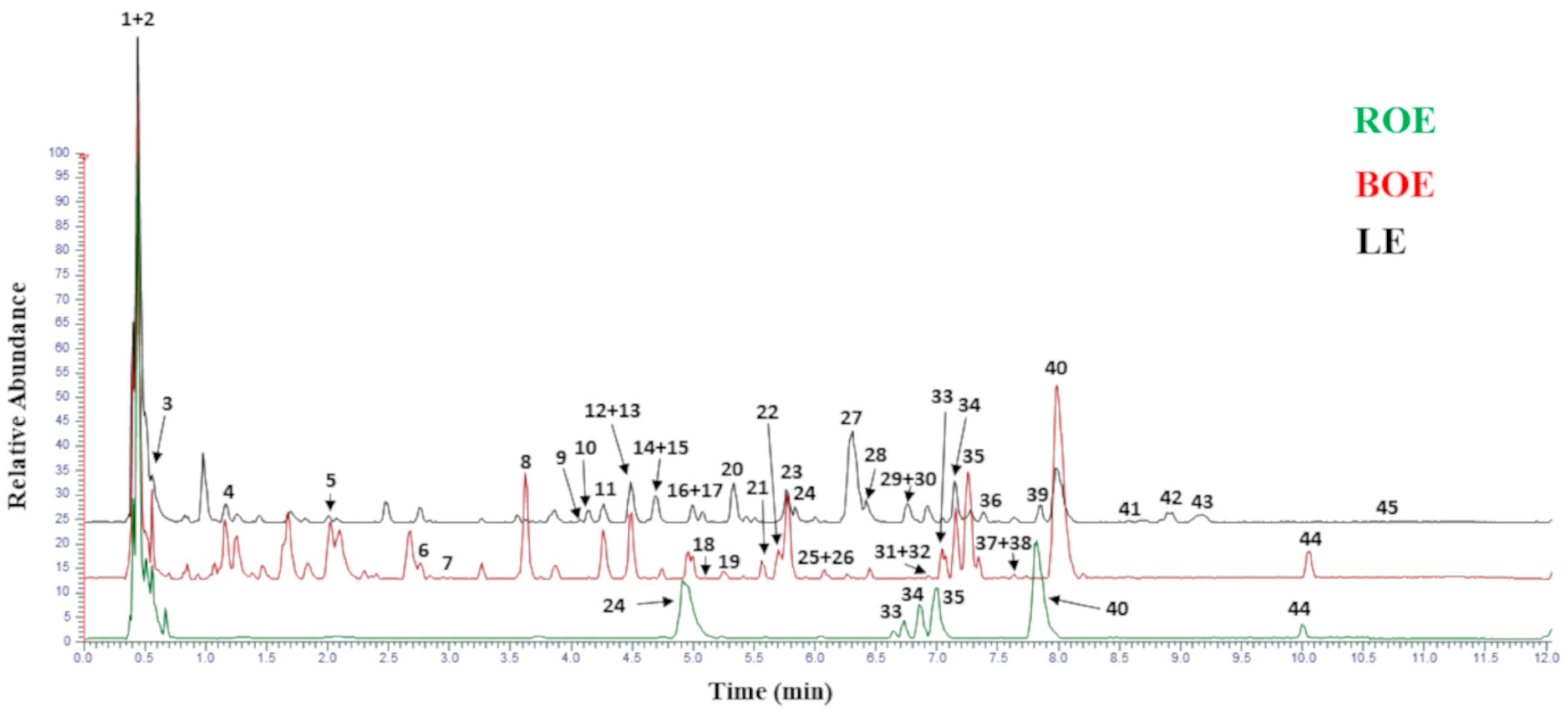
| Tag | Nome | m/z | Formula | RT (min) | Annot. Delta Mass (ppm) | ROE | BOE | LE |
|---|---|---|---|---|---|---|---|---|
| 1 | Gluconic acid | 195.05013 | C6H12O7 | 0.394 | −4.59 | Yes | Yes | Yes |
| 2 | Citric acid | 191.01894 | C6H8O7 | 0.441 | −4.11 | Yes | Yes | Yes |
| 3 | Gallic acid | 169.01344 | C7H6O5 | 0.711 | −4.73 | Yes | Yes | No |
| 4 | D-Saccharic acid | 209.02982 | C6H10O8 | 1.084 | −2.24 | Yes | Yes | No |
| 5 | Geniposidic acid | 373.11401 | C16H22O10 | 2.023 | −0.02 | Yes | Yes | No |
| 6 | Caffeic acid | 179.03426 | C9H8O4 | 2.789 | −4.03 | No | Yes | No |
| 7 | Fraxin | 369.08279 | C16H18O10 | 3.051 | 0.21 | No | Yes | No |
| 8 | Quercetin 3-(2G-glucosylrutinoside) | 771.19916 | C33H40O21 | 3.634 | 0.29 | Yes | Yes | No |
| 9 | Caffeic acid | 179.03429 | C9H8O4 | 3.736 | −1.60 | Yes | Yes | No |
| 10 | Tetrahydroxyflavanone 7-O-rutinoside | 595.16748 | C27H32O15 | 4.237 | 1.07 | Yes | No | No |
| 11 | Quercetin 3-O-arabinofuranoside. | 433.07748 | C20H18O11 | 4.27 | −0.35 | Yes | Yes | No |
| 12 | Quercetin 3-O-arabinofuranoside. | 433.07748 | C20H18O11 | 4.47 | −0.35 | Yes | Yes | No |
| 13 | Vicenin 2 | 593.15173 | C27H30O15 | 4.49 | 0.91 | Yes | Yes | No |
| 14 | Quercetin-3-hexoside | 463.08838 | C21H20O12 | 4.684 | 0.39 | Yes | Yes | No |
| 15 | Unknown | 715.2457 | C32H44O18 | 4.69 | 1.78 | Yes | Yes | No |
| 16 | Rhamnetin 3-neohesperidoside | 623.16187 | C28H32O16 | 4.99 | 0.17 | Yes | Yes | No |
| 17 | Kaempferol-7-O-hexoside | 447.09357 | C21H20O11 | 5.04 | 0.63 | Yes | Yes | No |
| 18 | Quercetin 3-(2Gal-rhamnosyl-robinobioside) | 755.20483 | C33H40O20 | 5.151 | 1.08 | No | Yes | No |
| 19 | Kaempferol-7-O-hexoside | 447.09357 | C21H20O11 | 5.25 | 0.63 | No | Yes | No |
| 20 | Rhamnetin 3-neohesperidoside | 623.16187 | C28H32O16 | 5.34 | 0.17 | Yes | No | No |
| 21 | Narirutin 4′-hexoside | 741.2254 | C33H42O19 | 5.56 | 0.88 | No | Yes | No |
| 22 | Sinapinic acid | 223.06079 | C11H12O5 | 5.703 | −1.81 | No | Yes | No |
| 23 | Limonin hexoside | 649.25451 | C32H42O14 | 5.769 | −0.28 | Yes | Yes | Yes |
| 24 | Rutin | 609.14612 | C27H30O16 | 5.789 | −0.18 | Yes | No | No |
| 25 | Quercetin-3-hexoside | 463.08878 | C21H20O12 | 5.95 | 1.24 | Yes | Yes | No |
| 26 | Quercetin-3-hexoside | 463.08878 | C21H20O12 | 6.034 | 1.24 | Yes | Yes | No |
| 27 | Eriocitrin | 595.16705 | C27H32O15 | 6.31 | 0.35 | Yes | No | No |
| 28 | Vicenin 2 isomer | 593.15173 | C27H30O15 | 6.42 | 0.91 | Yes | Yes | No |
| 29 | Rhamnocitrin 3-hexoside | 461.10962 | C22H22O11 | 6.76 | 1.48 | Yes | No | No |
| 30 | Luteolin 3′-methyl ether 7-malonylhexoside | 547.1095 | C25H24O14 | 6.76 | 0.31 | Yes | No | No |
| 31 | Rhamnocitrin 3-hexoside | 461.10962 | C22H22O11 | 6.92 | 1.48 | No | Yes | No |
| 32 | Luteolin 3′-methyl ether 7-malonylhexoside | 547.1095 | C25H24O14 | 6.92 | 0.31 | No | Yes | No |
| 33 | Deacetyl nomilin hexoside | 651.266 | C32H44O14 | 7.05 | 1.92 | No | Yes | Yes |
| 34 | Nomilinic acid hexoside | 711.287 | C34H48O16 | 7.15 | 1.67 | Yes | Yes | Yes |
| 35 | Unknown Limonoid hexoside | 843.2223 | C36H44O23 | 7.25 | 1.27 | Yes | Yes | Yes |
| 36 | Unknown Limonoid hexoside | 843.2223 | C36H44O23 | 7.34 | 1.27 | Yes | Yes | No |
| 37 | Rhamnetin 3-galactoside | 477.10446 | C22H22O12 | 7.63 | 1.28 | No | Yes | No |
| 38 | Neodiosmin | 607.1699 | C26H32O15 | 7.68 | −1.14 | No | Yes | No |
| 39 | Hesperetin-O-hex.-O-rhamn.-O-hexoside | 771.2355 | C34H44O20 | 7.85 | 1.24 | Yes | No | No |
| 40 | Hesperidin | 609.18219 | C28H34O15 | 7.983 | −0.5 | Yes | Yes | Yes |
| 41 | Obocunone hexoside | 633.2556 | C32H42O13 | 8.59 | 1.04 | Yes | No | No |
| 42 | Hesperidin derivative | 977.29511 | C45H54O24 | 8.952 | 1.92 | Yes | No | No |
| 43 | Homoesperetin 7-rutinoside | 623.19983 | C29H36O15 | 9.791 | 2.7 | Yes | No | No |
| 44 | Didymin | 593.18805 | C28H34O14 | 10.049 | 0.79 | No | Yes | Yes |
| 45 | Catechin 7-O-gallate | 441.08292 | C22H18O10 | 10.626 | 0.44 | Yes | No | No |
| Code | TPC (mg GAE/g) | PAC (mg GAE/g) | FC (mg di CTE/g) | ABTS IC50 (mg mL−1) | DPPH IC50 (mg mL−1) |
|---|---|---|---|---|---|
| ROE | 28.33 ± 1.35 c | 6.37 ± 0.26 c | 2.41 ± 0.11 c | 0.154 ± 0.007 c | 0.861 ± 0.041 c |
| BOE | 33.33 ± 1.44 b | 13.05 ± 0.52 b | 5.07 ± 0.20 b | 0.130 ± 0.005 b | 0.782 ± 0.032 b |
| LE | 39.80 ± 1.53 a | 15.91 ± 0.70 a | 7.06 ± 0.31 a | 0.081 ± 0.003 a | 0.634 ± 0.023 a |
| Sample | T Center Peak (°C) | Enthalpy (J g−1) |
|---|---|---|
| BP | 66.0 | 16.8 |
| 111.8 | 70.9 | |
| 136.1 | 144.8 | |
| 287.6 | 144.0 | |
| 323.6 | 2.9 | |
| 338.1 | 37.0 | |
| BOEP | 73.9 | 4.0 |
| 128.1 | 16.0 | |
| 139.9 | 3.8 | |
| 144.4 | 6.5 | |
| 157.6 | 11.1 | |
| 167.5 | 3.9 | |
| 184.4 | 22.1 | |
| 213.5 | 34.1 | |
| ROEP | 45.0 | 37.0 |
| 117.7 | 400.8 | |
| 223.0 | 8.1 | |
| 306.0 | 8.5 | |
| LEP | 64.4 | 0.9 |
| 124.5 | 247.8 | |
| 338.5 | 170.3 | |
| 466.3 | 5.0 | |
| 494.6 | 4.1 |
| Code | TPC (mg GAE/g) | PAC (mg GAE/g) | FC (mg di CTE/g) | ABTS IC50 (mg mL−1) | DPPH IC50 (mg mL−1) |
|---|---|---|---|---|---|
| ROEP | 16.81 ± 0.71 c | 4.32 ± 0.16 c | 2.12 ± 0.08 c | 0.454 ± 0.018 c | 2.453 ± 0.117 c |
| BOEP | 27.19 ± 0.94 b | 11.82 ± 0.48 b | 3.21 ± 0.12 b | 0.310 ± 0.012 b | 1.468 ± 0.061 b |
| LEP | 31.41 ± 1.12 a | 14.06 ± 0.59 a | 6.42 ± 0.21 a | 0.201 ± 0.008 a | 1.341 ± 0.054 a |
| BP | 5.53 ± 0.13 e | - | - | - | - |
| CG | 12.36 ± 0.49 d | - | - | - | - |
| Time (Days) | Code | TPC (mg GAE g−1) | ABTS IC30 (mg mL−1) |
|---|---|---|---|
| 0 | ROG | 2.45 ± 0.09 c | 6.34 ± 0.24 a |
| BOG | 3.41 ± 0.12 b | 5.20 ± 0.21 b | |
| LG | 5.19 ± 0.14 a | 1.22 ± 0.04 c | |
| CGG | 1.71 ± 0.05 d | - | |
| 7 | ROG | 2.29 ± 0.05 c | 6.83 ± 0.28 a |
| BOG | 2.97 ± 0.12 b | 5.80 ± 0.21 b | |
| LG | 4.73 ± 0.17 a | 1.56 ± 0.05 c | |
| CGG | 1.46 ± 0.05 d | - | |
| 14 | ROG | 1.33 ± 0.04 c | 8.28 ± 0.34 a |
| BOG | 2.54 ± 0.09 b | 7.01 ± 0.26 b | |
| LG | 4.19 ± 0.15 a | 2.93 ± 0.11 c | |
| CGG | 0.99 ± 0.03 d | - |
| A Pa × s1/z (±100) | BOG | LG | ROG | CGG |
|---|---|---|---|---|
| 20 °C t0 | 520 | 1150 | 25,420 | 21,260 |
| 20 °C t1 | 4040 | 1070 | 62,080 | 716,230 |
| 20 °C t2 | 2710 | 67,370 | 2,400,000 | |
| 25 °C t0 | 520 | 1050 | 25,400 | 7250 |
| 25 °C t1 | 4730 | 940 | 44,370 | 722,800 |
| 25 °C t2 | 4070 | 2460 | 35,550 | 1,100,000 |
| 30 °C t0 | 260 | 230 | 17,600 | 4940 |
| 30 °C t1 | 3700 | 30 | 44,310 | 312,000 |
| 30 °C t2 | 2100 | 384 | 35,600 | 716,900 |
| 35 °C t0 | 700 | 7300 | 8000 | |
| 35 °C t1 | 3200 | 40,950 | 38,300 | |
| 35 °C t2 | 3600 | 23,000 | 890,000 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aiello, F.; Caputo, P.; Oliviero Rossi, C.; Restuccia, D.; Spizzirri, U.G. Formulation of Antioxidant Gummies Based on Gelatin Enriched with Citrus Fruit Peels Extract. Foods 2024, 13, 320. https://doi.org/10.3390/foods13020320
Aiello F, Caputo P, Oliviero Rossi C, Restuccia D, Spizzirri UG. Formulation of Antioxidant Gummies Based on Gelatin Enriched with Citrus Fruit Peels Extract. Foods. 2024; 13(2):320. https://doi.org/10.3390/foods13020320
Chicago/Turabian StyleAiello, Francesca, Paolino Caputo, Cesare Oliviero Rossi, Donatella Restuccia, and Umile Gianfranco Spizzirri. 2024. "Formulation of Antioxidant Gummies Based on Gelatin Enriched with Citrus Fruit Peels Extract" Foods 13, no. 2: 320. https://doi.org/10.3390/foods13020320






