Agri-Food Waste Recycling for Healthy Remedies: Biomedical Potential of Nutraceuticals from Unripe Tomatoes (Solanum lycopersicum L.)
Abstract
:1. Introduction
2. Unripe Green Tomatoes: A Source of Glycoalkaloids
3. α-Tomatine Extraction from Green Tomatoes
4. α-Tomatine Analysis Methods
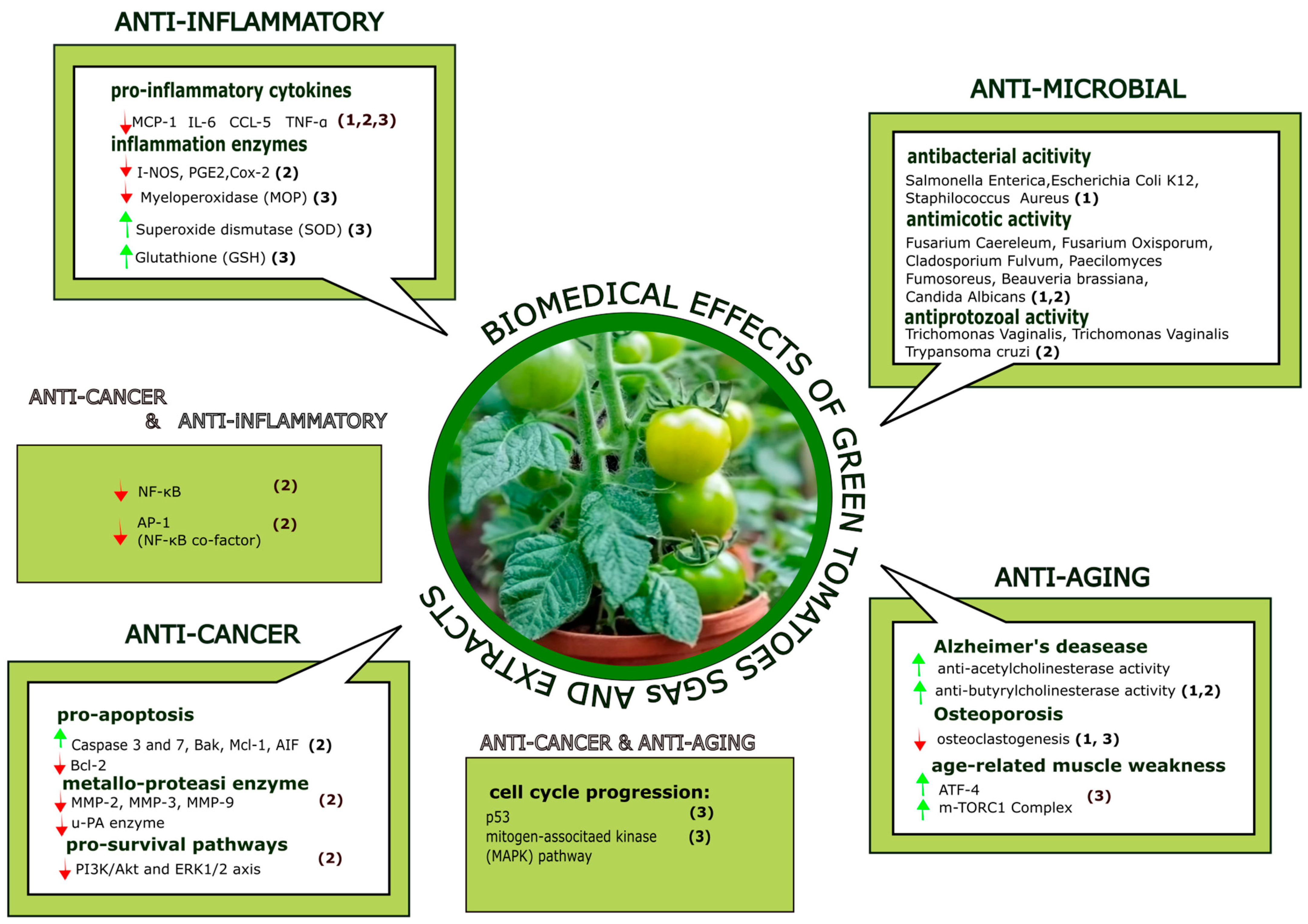
5. Nutraceutical Potential of Green Tomatoes
5.1. Antiviral, Antifungal, and Antibiotic Activity
5.2. Anti-Inflammatory Effects
5.3. Anti-Aging Effects
5.4. Anti-Tumoral Effects
5.5. Pharmacokinetics and Toxicological Aspects of Glycoalkaloids and Green Tomato Extracts
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Garcia-Marti, M.; Simal-Gandara, J. Chapter 5: Chemical and Biological Valorization of Tomato Waste. In Agri-Food Waste Valorisation; Royal Society of Chemistry: London, UK, 2023; Volume 78, p. 326. [Google Scholar]
- Unlu, N.Z.; Bohn, T.; Francis, D.M.; Nagaraja, H.N.; Clinton, S.K.; Schwartz, S.J. Lycopene from Heat-Induced Cis-Isomer-Rich Tomato Sauce Is More Bioavailable than from All-Trans-Rich Tomato Sauce in Human Subjects. Br. J. Nutr. 2007, 98, 140–146. [Google Scholar] [CrossRef] [PubMed]
- Carillo, P.; D’Amelia, L.; Dell’Aversana, E.; Faiella, D.; Cacace, D.; Giuliano, B.; Morrone, B. Eco-Friendly Use of Tomato Processing Residues for Lactic Acid Production in Campania. Chem. Eng. Trans. 2018, 64, 223–228. [Google Scholar] [CrossRef]
- Matei, E.; Râpă, M.; Predescu, A.M.; Țurcanu, A.A.; Vidu, R.; Predescu, C.; Bobirica, C.; Bobirica, L.; Orbeci, C. Valorization of Agri-Food Wastes as Sustainable Eco-Materials for Wastewater Treatment: Current State and New Perspectives. Materials 2021, 14, 4581. [Google Scholar] [CrossRef] [PubMed]
- Silva, Y.P.A.; Borba, B.C.; Pereira, V.A.; Reis, M.G.; Caliari, M.; Brooks, M.S.-L.; Ferreira, T.A.P.C. Characterization of Tomato Processing By-Product for Use as a Potential Functional Food Ingredient: Nutritional Composition, Antioxidant Activity and Bioactive Compounds. Int. J. Food Sci. Nutr. 2019, 70, 150–160. [Google Scholar] [CrossRef] [PubMed]
- Maisto, M.; Annunziata, G.; Schiano, E.; Piccolo, V.; Iannuzzo, F.; Santangelo, R.; Ciampaglia, R.; Tenore, G.C.; Novellino, E.; Grieco, P. Potential Functional Snacks: Date Fruit Bars Supplemented by Different Species of Lactobacillus spp. Foods 2021, 10, 1760. [Google Scholar] [CrossRef] [PubMed]
- Schiano, E.; Piccolo, V.; Novellino, E.; Maisto, M.; Iannuzzo, F.; Summa, V.; Tenore, G.C. Thinned Nectarines, an Agro-Food Waste with Antidiabetic Potential: HPLC-HESI-MS/MS Phenolic Characterization and In Vitro Evaluation of Their Beneficial Activities. Foods 2022, 11, 1010. [Google Scholar] [CrossRef] [PubMed]
- Patel, A.H.; Sharma, H.P. Vaishali Physiological Functions, Pharmacological Aspects and Nutritional Importance of Green Tomato- a Future Food. Crit. Rev. Food Sci. Nutr. 2023, 1–23. [Google Scholar] [CrossRef] [PubMed]
- El Mashad, H.M.; Zhao, L.; Zhang, R.; Pan, Z. Tomato. In Integrated Processing Technologies for Food and Agricultural By-Products; Elsevier: Amsterdam, The Netherlands, 2019; pp. 107–131. ISBN 9780128141397. [Google Scholar]
- Lu, S.; Chen, S.; Li, H.; Paengkoum, S.; Taethaisong, N.; Meethip, W.; Surakhunthod, J.; Sinpru, B.; Sroichak, T.; Archa, P.; et al. Sustainable Valorization of Tomato Pomace (Lycopersicon esculentum) in Animal Nutrition: A Review. Animals 2022, 12, 3294. [Google Scholar] [CrossRef]
- Faria-Silva, C.; de Sousa, M.; Carvalheiro, M.C.; Simões, P.; Simões, S. Alpha-Tomatine and the Two Sides of the Same Coin: An Anti-Nutritional Glycoalkaloid with Potential in Human Health. Food Chem. 2022, 391, 133261. [Google Scholar] [CrossRef]
- Anton, D.; Bender, I.; Kaart, T.; Roasto, M.; Heinonen, M.; Luik, A.; Püssa, T. Changes in Polyphenols Contents and Antioxidant Capacities of Organically and Conventionally Cultivated Tomato (Solanum lycopersicum L.) Fruits during Ripening. Int. J. Anal. Chem. 2017, 2017, 2367453. [Google Scholar] [CrossRef]
- Periago, M.J.; Martínez-Valverde, I.; Chesson, A.; Provan, G. Phenolic Compounds, Lycopene and Antioxidant Activity in Commercial Varieties of Tomato (Lycopersicum esculentum). J. Sci. Food Agric. 2002, 82, 323–330. [Google Scholar] [CrossRef]
- Kozukue, N.; Friedman, M. Tomatine, Chlorophyll, β-Carotene and Lycopene Content in Tomatoes during Growth and Maturation. J. Sci. Food Agric. 2003, 83, 195–200. [Google Scholar] [CrossRef]
- Chaudhary, P.; Sharma, A.; Singh, B.; Nagpal, A.K. Bioactivities of Phytochemicals Present in Tomato. J. Food Sci. Technol. 2018, 55, 2833–2849. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Ortega, M.; Ortiz-Moreno, A.; Hernández-Navarro, M.D.; Chamorro-Cevallos, G.; Dorantes-Alvarez, L.; Necoechea-Mondragón, H. Antioxidant, Antinociceptive, and Anti-Inflammatory Effects of Carotenoids Extracted from Dried Pepper (Capsicum annuum L.). J. Biomed Biotechnol. 2012, 2012, 524019. [Google Scholar] [CrossRef] [PubMed]
- Subramoniam, A.; Asha, V.V.; Nair, S.A.; Sasidharan, S.P.; Sureshkumar, P.K.; Rajendran, K.N.; Karunagaran, D.; Ramalingam, K. Chlorophyll Revisited: Anti-Inflammatory Activities of Chlorophyll a and Inhibition of Expression of TNF-α Gene by the Same. Inflammation 2012, 35, 959–966. [Google Scholar] [CrossRef] [PubMed]
- Maisto, M.; Iannuzzo, F.; Schiano, E.; Ciampaglia, R.; Labanca, A.; Montesano, D.; Piccolo, V.; Rossi, P.; Tenore, G.C. Effects of Fortified Laying Hen Diet with Moringa Oleifera Leaves and Goji Berries on Cholesterol and Carotenoid Egg Content. Foods 2022, 11, 3156. [Google Scholar] [CrossRef]
- Dzakovich, M.P.; Hartman, J.L.; Cooperstone, J.L. A High-Throughput Extraction and Analysis Method for Steroidal Glycoalkaloids in Tomato. Front. Plant Sci. 2020, 11, 767. [Google Scholar] [CrossRef]
- Zhang, Q.; Liu, D.; Cui, Y.; Xu, T.; Liu, X.; Liu, K.; Wang, Q.; Li, A.; Zhao, P.; Cheng, Z. Bioactivities and chemical profiling comparison and metabolomic variations of polyphenolics and steroidal glycoalkaloids in different parts of Solanum nigrum L. Phytochemical Analysis. 2023, 1–19. [Google Scholar] [CrossRef]
- Fontaine, T.; Irving, G. Isolation and Partial Characterization of Crystalline Tomatine, an Antibiotic Agent from the Tomato Plant. Arch Biochem. 1948, 18, 467–475. [Google Scholar]
- Friedman, M.; Levin, C.E.; Mcdonald, G.M. α-Tomatine Determination in Tomatoes by HPLC Using Pulsed Amperometric Detection. Food Chem. 1994, 42, 1959–1964. [Google Scholar] [CrossRef]
- Cárdenas, P.D.; Sonawane, P.D.; Heinig, U.; Bocobza, S.E.; Burdman, S.; Aharoni, A. The Bitter Side of the Nightshades: Genomics Drives Discovery in Solanaceae Steroidal Alkaloid Metabolism. Phytochemistry 2015, 113, 24–32. [Google Scholar] [CrossRef] [PubMed]
- González-Lamothe, R.; Mitchell, G.; Gattuso, M.; Diarra, M.S.; Malouin, F.; Bouarab, K. Plant Antimicrobial Agents and Their Effects on Plant and Human Pathogens. Int. J. Mol. Sci. 2009, 10, 3400–3419. [Google Scholar] [CrossRef] [PubMed]
- Iijima, Y.; Watanabe, B.; Sasaki, R.; Takenaka, M.; Ono, H.; Sakurai, N.; Umemoto, N.; Suzuki, H.; Shibata, D.; Aoki, K. Steroidal Glycoalkaloid Profiling and Structures of Glycoalkaloids in Wild Tomato Fruit. Phytochemistry 2013, 95, 145–157. [Google Scholar] [CrossRef] [PubMed]
- Friedman, M. Tomato Glycoalkaloids: Role in the Plant and in the Diet. J. Agric. Food Chem. 2002, 50, 5751–5780. [Google Scholar] [CrossRef] [PubMed]
- Kozukue, N.; Han, J.S.; Lee, K.R.; Friedman, M. Dehydrotomatine and α-Tomatine Content in Tomato Fruits and Vegetative Plant Tissues. J. Agric. Food Chem. 2004, 52, 2079–2083. [Google Scholar] [CrossRef] [PubMed]
- Pardini, A.; Consumi, M.; Leone, G.; Bonechi, C.; Tamasi, G.; Sangiorgio, P.; Verardi, A.; Rossi, C.; Magnani, A. Effect of Different Post-Harvest Storage Conditions and Heat Treatment on Tomatine Content in Commercial Varieties of Green Tomatoes. J. Food Compos. Anal. 2021, 96, 103735. [Google Scholar] [CrossRef]
- Koh, E.; Kaffka, S.; Mitchell, A.E. A Long-Term Comparison of the Influence of Organic and Conventional Crop Management Practices on the Content of the Glycoalkaloid α-Tomatine in Tomatoes. J. Sci. Food Agric. 2013, 93, 1537–1542. [Google Scholar] [CrossRef]
- Tamasi, G.; Pardini, A.; Croce, R.; Consumi, M.; Leone, G.; Bonechi, C.; Rossi, C.; Magnani, A. Combined Experimental and Multivariate Model Approaches for Glycoalkaloid Quantification in Tomatoes. Molecules 2021, 26, 3068. [Google Scholar] [CrossRef]
- Piccolo, V.; Maisto, M.; Schiano, E.; Iannuzzo, F.; Keivani, N.; Manuela Rigano, M.; Santini, A.; Novellino, E.; Carlo Tenore, G.; Summa, V. Phytochemical Investigation and Antioxidant Properties of Unripe Tomato Cultivars (Solanum lycopersicum L.). Food Chem. 2023, 438, 137863. [Google Scholar] [CrossRef]
- Ngo, T.H.; Park, J.; Jo, Y.D.; Jin, C.H.; Jung, C.H.; Nam, B.; Han, A.R.; Nam, J.W. Content of Two Major Steroidal Glycoalkaloids in Tomato (Solanum lycopersicum Cv. Micro-Tom) Mutant Lines at Different Ripening Stages. Plants 2022, 11, 2895. [Google Scholar] [CrossRef]
- Taveira, M.; Ferreres, F.; Gil-Izquierdo, A.; Oliveira, L.; Valentão, P.; Andrade, P.B. Fast Determination of Bioactive Compounds from Lycopersicon Esculentum Mill. Leaves. Food Chem. 2012, 135, 748–755. [Google Scholar] [CrossRef] [PubMed]
- Taveira, M.; Sousa, C.; Valentão, P.; Ferreres, F.; Teixeira, J.P.; Andrade, P.B. Neuroprotective Effect of Steroidal Alkaloids on Glutamate-Induced Toxicity by Preserving Mitochondrial Membrane Potential and Reducing Oxidative Stress. J. Steroid Biochem. Mol. Biol. 2014, 140, 106–115. [Google Scholar] [CrossRef] [PubMed]
- Silva-Beltrán, N.P.; Ruiz-Cruz, S.; Cira-Chávez, L.A.; Estrada-Alvarado, M.I.; Ornelas-Paz, J.D.J.; López-Mata, M.A.; Del-Toro-Sánchez, C.L.; Ayala-Zavala, J.F.; Márquez-Ríos, E. Total Phenolic, Flavonoid, Tomatine, and Tomatidine Contents and Antioxidant and Antimicrobial Activities of Extracts of Tomato Plant. Int. J. Anal. Chem. 2015, 2015, 284071. [Google Scholar] [CrossRef] [PubMed]
- Choi, K.M.; Lee, Y.S.; Shin, D.M.; Lee, S.; Yoo, K.S.; Lee, M.K.; Lee, J.H.; Kim, S.Y.; Lee, Y.M.; Hong, J.T.; et al. Green Tomato Extract Attenuates High-Fat-Diet-Induced Obesity through Activation of the AMPK Pathway in C57BL/6 Mice. J. Nutr. Biochem. 2013, 24, 335–342. [Google Scholar] [CrossRef]
- Friedman, M.; Levin, C.E. Tomatine Content in Tomato and Tomato Products Determined by HPLC with Pulsed Amperometric Detection. J. Agric. Food Chem. 1995, 43, 1507–1511. [Google Scholar] [CrossRef]
- Friedman, M.; Kozukue, N.; Harden, L.A. Preparation and Characterization of Acid Hydrolysis Products of the Tomato Glycoalkaloid R-Tomatine. J. Agric. Food Chem. 1998, 46, 2096–2101. [Google Scholar] [CrossRef]
- Choi, S.H.; Lee, S.H.; Kim, H.J.; Lee, I.S.; Nobuyuki, K.; Levin, C.E.; Mendel, F. Changes in Free Amino Acid, Phenolic, Chlorophyll, Carotenoid, and Glycoalkaloid Contents in Tomatoes during 11 Stages of Growth and Inhibition of Cervical and Lung Human Cancer Cells by Green Tomato Extracts. J. Agric. Food Chem. 2010, 58, 7547–7556. [Google Scholar] [CrossRef]
- Caprioli, G.; Cahill, M.G.; James, K.J. Mass Fragmentation Studies of α-Tomatine and Validation of a Liquid Chromatography LTQ Orbitrap Mass Spectrometry Method for Its Quantification in Tomatoes. Food Anal. Methods 2014, 7, 1565–1571. [Google Scholar] [CrossRef]
- Leonardi, C.; Ambrosino, P.; Esposito, F.; Fogliano, V. Antioxidative Activity and Carotenoid and Tomatine Contents in Different Typologies of Fresh Consumption Tomatoes. J. Agric. Food Chem. 2000, 48, 4723–4727. [Google Scholar] [CrossRef]
- Bushway, R.J.; Perkins, B.; Paradis, L.R.; Vanderpan, S. High-Performance Liquid Chromatographic Determination of the Tomato Glycoalkaloid, Tomatine, in Green and Red Tomatoes. J. Agric. Food Chem. 1994, 42, 2824–2829. [Google Scholar] [CrossRef]
- Rick, C.M.; Uhligt, J.W.; Jones, A.D. High A-Tomatine Content in Ripe Fruit of Andean Lycopersicon Esculentum Var. Cerasiforme: Developmental and Genetic Aspects. Proc. Natl. Acad. Sci. USA 1994, 91, 12877–12881. [Google Scholar] [CrossRef] [PubMed]
- Nepal, B.; Stine, K.J. Glycoalkaloids: Structure, Properties, and Interactions with Model Membrane Systems. Processes 2019, 7, 513. [Google Scholar] [CrossRef]
- Monteiro, T.; Pinheiro, M.A. Lycopersicon Esculentum Miller: From by-Products towards Neuroprotection. Ph.D. Thesis, Universidade do Porto, Porto, Portugal, 2013. [Google Scholar]
- Marcolongo, P.; Gamberucci, A.; Tamasi, G.; Pardini, A.; Bonechi, C.; Rossi, C.; Giunti, R.; Barone, V.; Borghini, A.; Fiorenzani, P.; et al. Chemical Characterisation and Antihypertensive Effects of Locular Gel and Serum of Lycopersicum esculentum L. Var. “Camone” Tomato in Spontaneously Hypertensive Rats. Molecules 2020, 25, 3758. [Google Scholar] [CrossRef] [PubMed]
- Itkin, M.; Rogachev, I.; Alkan, N.; Rosenberg, T.; Malitsky, S.; Masini, L.; Meir, S.; Iijima, Y.; Aoki, K.; de Vos, R.; et al. GLYCOALKALOID METABOLISM1 Is Required for Steroidal Alkaloid Glycosylation and Prevention of Phytotoxicity in Tomato. Plant Cell 2011, 23, 4507–4525. [Google Scholar] [CrossRef] [PubMed]
- Steiner, D.; Krska, R.; Malachová, A.; Taschl, I.; Sulyok, M. Evaluation of Matrix Effects and Extraction Efficiencies of LC-MS/MS Methods as the Essential Part for Proper Validation of Multiclass Contaminants in Complex Feed. J. Agric. Food Chem. 2020, 68, 3868–3880. [Google Scholar] [CrossRef] [PubMed]
- Sonawane, P.D.; Gharat, S.A.; Jozwiak, A.; Barbole, R.; Heinicke, S.; Almekias-Siegl, E.; Meir, S.; Rogachev, I.; Connor, S.E.O.; Giri, A.P.; et al. A BAHD-Type Acyltransferase Concludes the Biosynthetic Pathway of Non-Bitter Glycoalkaloids in Ripe Tomato Fruit. Nat. Commun. 2023, 14, 4540. [Google Scholar] [CrossRef]
- Kozukue, N.; Kozukue, E.; Yamashita, H.; Fujii, S. Alpha-Tomatine Purification and Quantification in Tomatoes by HPLC. J. Food Sci. 1994, 6, 1211–1212. [Google Scholar] [CrossRef]
- Vaananen, T.; Kuronen, P.; Pehu, E. Comparison of Commercial Solid-Phase Extraction Sorbents for the Sample Preparation of Potato Glycoalkaloids. J. Chromatogr. A 2000, 869, 301–305. [Google Scholar] [CrossRef]
- Friedman, M.; Levin, C.E.; Lee, S.U.; Kim, H.J.; Lee, I.S.; Byun, J.O.; Kozukue, N. Tomatine-Containing Green Tomato Extracts Inhibit Growth of Human Breast, Colon, Liver, and Stomach Cancer Cells. J. Agric. Food Chem. 2009, 57, 5727–5733. [Google Scholar] [CrossRef]
- Abbasi-Parizad, P.; Salvino, R.A.; Passera, A.; Follador, A.R.V.; Cosentino, C.; Jucker, C.; Savoldelli, S.; Bacenetti, J.; Casati, P.; Scaglia, B. Development of a Cascade Production System Finalized to the Extraction of All-Tomatine-Rich Fraction Using the Tomato Cannery Waste as Feedstock. J. Clean Prod. 2023, 401, 136743. [Google Scholar] [CrossRef]
- Roddick, J.G.; Melchers, G. Steroidal glycoalkaloid content of potato, tomato and their somatic hybrids. Theor. Appl. Genet. 1985, 70, 655–660. [Google Scholar] [CrossRef] [PubMed]
- Drummer, O.H. Chromatographic screening techniques in systematic toxicological analysis. J. Chromatogr. B Biomed. Sci. Appl. 1999, 1–2, 27–45. [Google Scholar] [CrossRef] [PubMed]
- Klein- Júnior, L.C.; Heyden, Y.V.; Henriques, A.T. Enlarging the bottleneck in the analysis of alkaloids: A review on sample preparation in herbal matrices. Trends Anal. Chem. 2016, 80, 66–82. [Google Scholar] [CrossRef]
- Figueiredo-González, M.; Valentão, P.; Andrade, P.B. Tomato Plant Leaves: From by-Products to the Management of Enzymes in Chronic Diseases. Ind. Crop. Prod. 2016, 94, 621–629. [Google Scholar] [CrossRef]
- Nirmala, F.S.; Lee, H.; Kim, J.S.; Ha, T.; Jung, C.H.; Ahn, J. Green Tomato Extract Prevents Bone Loss in Ovariectomized Rats, a Model of Osteoporosis. Nutrients 2020, 12, 3210. [Google Scholar] [CrossRef]
- Fujimaki, J.; Sayama, N.; Shiotani, S.; Suzuki, T.; Nonaka, M.; Uezono, Y.; Oyabu, M.; Kamei, Y.; Nukaya, H.; Wakabayashi, K.; et al. The Steroidal Alkaloid Tomatidine and Tomatidine-Rich Tomato Leaf Extract Suppress the Human Gastric Cancer-Derived 85As2 Cells In Vitro and In Vivo via Modulation of Interferon-Stimulated Genes. Nutrients 2022, 14, 1023. [Google Scholar] [CrossRef] [PubMed]
- Wolfender, J.L. HPLC in Natural Product Analysis: The Detection Issue. Planta Med. 2009, 75, 719–734. [Google Scholar] [CrossRef]
- Laurila, J.; Laakso, I.; Väänänen, T.; Kuronen, P.; Huopalahti, R.; Pehu, E. Determination of Solanidine- and Tomatidine-Type Glycoalkaloid Aglycons by Gas Chromatography/Mass Spectrometry. J. Agric. Food Chem. 1999, 47, 2738–2742. [Google Scholar] [CrossRef]
- Moco, S.; Bino, R.J.; Vorst, O.; Verhoeven, H.A.; De Groot, J.; Van Beek, T.A.; Vervoort, J.; Ric De Vos, C.H. A Liquid Chromatography-Mass Spectrometry-Based Metabolome Database for Tomato. Plant. Physiol. 2006, 141, 1205–1218. [Google Scholar] [CrossRef]
- Kozukue, N.; Kim, D.S.; Choi, S.H.; Mizuno, M.; Friedman, M. Isomers of the Tomato Glycoalkaloids α-Tomatine and Dehydrotomatine: Relationship to Health Benefits. Molecules 2023, 28, 3621. [Google Scholar] [CrossRef]
- Cichon, M.J.; Riedl, K.M.; Wan, L.; Thomas-Ahner, J.M.; Francis, D.M.; Clinton, S.K.; Schwartz, S.J. Plasma Metabolomics Reveals Steroidal Alkaloids as Novel Biomarkers of Tomato Intake in Mice. Mol. Nutr. Food Res. 2017, 61, 1700241. [Google Scholar] [CrossRef]
- Cooperstone, J.L.; Tober, K.L.; Riedl, K.M.; Teegarden, M.D.; Cichon, M.J.; Francis, D.M.; Schwartz, S.J.; Oberyszyn, T.M. Tomatoes Protect against Development of UV-Induced Keratinocyte Carcinoma via Metabolomic Alterations. Sci. Rep. 2017, 7, 5106. [Google Scholar] [CrossRef] [PubMed]
- Dzakovich, M.P.; Goggans, M.L.; Thomas-Ahner, J.M.; Moran, N.E.; Clinton, S.K.; Francis, D.M.; Cooperstone, J.L.; Cooperstone, J. Transcriptomics and Metabolomics Reveal Tomato Consumption Alters Hepatic Xenobiotic Metabolism and Induces Steroidal Alkaloid Metabolite Accumulation in Mice. Mol. Nutr. Food Res. 2023, 16, 2300239. [Google Scholar] [CrossRef] [PubMed]
- Yamanaka, T.; Vincken, J.P.; De Waard, P.; Sanders, M.; Takada, N.; Gruppen, H. Isolation, Characterization, and Surfactant Properties of the Major Triterpenoid Glycosides from Unripe Tomato Fruits. J. Agric. Food Chem. 2008, 56, 11432–11440. [Google Scholar] [CrossRef] [PubMed]
- Ghisalberti, E.L. Steroidal Glycoalkaloids: Isolation, Structure, Analysis, and Biosynthesis. Nat. Prod. Commun. 2006, 1, 1934578X0600101007. [Google Scholar] [CrossRef]
- Rial, C.; Gómez, E.; Varela, R.M.; Molinillo, J.M.G.; Macías, F.A. Ecological Relevance of the Major Allelochemicals in Lycopersicon Esculentum Roots and Exudates. J. Agric. Food Chem. 2018, 66, 4638–4644. [Google Scholar] [CrossRef] [PubMed]
- Bachmann, H.; Autzen, S.; Frey, U.; Wehr, U.; Rambeck, W.; McCormack, H.; Whitehead, C.C. The Efficacy of a Standardised Product from Dried Leaves of Solanum Glaucophyllum as Source of 1,25-Dihydroxycholecalciferol for Poultry. Br. Poult. Sci. 2013, 54, 642–652. [Google Scholar] [CrossRef]
- Altria, K.D.; Marsh, A.; Sänger-van de Griend, C. Capillary Electrophoresis for the Analysis of Small-Molecule Pharmaceuticals. Electrophoresis 2006, 27, 2263–2282. [Google Scholar] [CrossRef]
- Bianco, G.; Schmitt-Kopplin, P.; De Benedetto, G.; Kettrup, A.; Cataldi, T.R.I. Determination of Glycoalkaloids and Relative Aglycones by Nonaqueous Capillary Electrophoresis Coupled with Electrospray Ionization-Ion Trap Mass Spectrometry. Electrophoresis 2002, 23, 2904–2912. [Google Scholar] [CrossRef]
- Holzapfel, N.P.; Holzapfel, B.M.; Champ, S.; Feldthusen, J.; Clements, J.; Hutmacher, D.W. The Potential Role of Lycopene for the Prevention and Therapy of Prostate Cancer: From Molecular Mechanisms to Clinical Evidence. Int. J. Mol. Sci. 2013, 14, 14620–14646. [Google Scholar] [CrossRef]
- Kumar, M.; Tomar, M.; Bhuyan, D.J.; Punia, S.; Grasso, S.; Sá, A.G.A.; Carciofi, B.A.M.; Arrutia, F.; Changan, S.; Radha; et al. Tomato (Solanum Lycopersicum L.) Seed: A Review on Bioactives and Biomedical Activities. Biomed. Pharmacother. 2021, 142, 112018. [Google Scholar] [CrossRef] [PubMed]
- Khan, U.M.; Sevindik, M.; Zarrabi, A.; Nami, M.; Ozdemir, B.; Kaplan, D.N.; Selamoglu, Z.; Hasan, M.; Kumar, M.; Alshehri, M.M.; et al. Lycopene: Food Sources, Biological Activities, and Human Health Benefits. Oxid. Med. Cell Longev. 2021, 2021, 522. [Google Scholar] [CrossRef] [PubMed]
- Friedman, M. Anticarcinogenic, Cardioprotective, and Other Health Benefits of Tomato Compounds Lycopene, α-Tomatine, and Tomatidine in Pure Form and in Fresh and Processed Tomatoes. J. Agric. Food Chem. 2013, 61, 9534–9550. [Google Scholar] [CrossRef] [PubMed]
- Arena, M.P.; Govers, C.; Lotti, C.; Ricciardi, L.; Wichers, H.J.; Mes, J.J. The Effect of Tomatine on Gene Expression and Cell Monolayer Integrity in Caco-2. Molecules 2018, 23, 644. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Chen, S.; Van Doren, J.; Li, D.; Farichon, C.; He, Y.; Zhang, Q.; Zhang, K.; Conney, A.H.; Goodin, S.; et al. α-Tomatine Inhibits Growth and Induces Apoptosis in HL-60 Human Myeloid Leukemia Cells. Mol. Med. Rep. 2015, 11, 4573–4578. [Google Scholar] [CrossRef] [PubMed]
- Morrow, W.J.W.; Yang, Y.W.; Sheikh, N.A. Immunobiology of the Tomatine Adjuvant. Proc. Vaccine 2004, 22, 2380–2384. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.K.; Zhao, Y.; Chen, S.Y.; Kennelly, E.J. Solanum Steroidal Glycoalkaloids: Structural Diversity, Biological Activities, and Biosynthesis. Nat. Prod Rep. 2021, 38, 1423–1444. [Google Scholar] [CrossRef]
- Tam, C.C.; Nguyen, K.; Nguyen, D.; Hamada, S.; Kwon, O.; Kuang, I.; Gong, S.; Escobar, S.; Liu, M.; Kim, J.; et al. Antimicrobial Properties of Tomato Leaves, Stems, and Fruit and Their Relationship to Chemical Composition. BMC Complement. Med. Ther. 2021, 21, 229. [Google Scholar] [CrossRef]
- Poprawski, T.J.; Greenberg, S.M.; Ciomperlik, M.A. Effect of Host Plant on Beauveria Bassiana-and Paecilomyces Fumosoroseus-Induced Mortality of Trialeurodes Vaporariorum (Homoptera: Aleyrodidae). Environ. Entomol. 2000, 29, 1048–1053. [Google Scholar] [CrossRef]
- McKee, R.K. Affecting the Toxicity of Solanine and Related Alkaloids to Fusarium Caeruleum. Microbiology 1959, 20, 686–696. [Google Scholar] [CrossRef]
- Liu, J.; Kanetake, S.; Wu, Y.H.; Tam, C.; Cheng, L.W.; Land, K.M.; Friedman, M. Antiprotozoal Effects of the Tomato Tetrasaccharide Glycoalkaloid Tomatine and the Aglycone Tomatidine on Mucosal Trichomonads. J. Agric. Food Chem. 2016, 64, 8806–8810. [Google Scholar] [CrossRef]
- Rocha-Hasler, M.; de Oliveira, G.M.; da Gama, A.N.; Fiuza, L.F.d.A.; Fesser, A.F.; Cal, M.; Rocchetti, R.; Peres, R.B.; Guan, X.L.; Kaiser, M.; et al. Combination With Tomatidine Improves the Potency of Posaconazole Against Trypanosoma Cruzi. Front. Cell. Infect. Microbiol. 2021, 11, 617917. [Google Scholar] [CrossRef] [PubMed]
- Elwan, H.A.M.; Elnesr, S.S.; Mohany, M.; Al-Rejaie, S.S. The Effects of Dietary Tomato Powder (Solanum lycopersicum L.) Supplementation on the Haematological, Immunological, Serum Biochemical and Antioxidant Parameters of Growing Rabbits. J. Anim. Physiol. Anim. Nutr. 2019, 103, 534–546. [Google Scholar] [CrossRef] [PubMed]
- Chu, X.; Yu, T.; Huang, X.; Xi, Y.; Ni, B.; Zhang, R.; You, H. Tomatidine Suppresses Inflammation in Primary Articular Chondrocytes and Attenuates Cartilage Degradation in Osteoarthritic Rats. Aging 2020, 12, 12799–12811. [Google Scholar] [CrossRef] [PubMed]
- Chiu, F.L.; Lin, J.K. Tomatidine Inhibits INOS and COX-2 through Suppression of NF-ΚB and JNK Pathways in LPS-Stimulated Mouse Macrophages. FEBS Lett. 2008, 582, 2407–2412. [Google Scholar] [CrossRef] [PubMed]
- Filderman, R.B.; Kovacs, B.A. Anti-Inflammatory Activity of the Steroid Alkaloid Glycoside, Toatine. Br. J. Pharmacol. 1969, 37, 748–755. [Google Scholar] [CrossRef] [PubMed]
- Hu, B.; Sun, X.; Yang, Y.; Ying, Z.; Meng, J.; Zhou, C.; Jiang, G.; Li, S.; Wu, F.; Zhao, X.; et al. Tomatidine Suppresses Osteoclastogenesis and Mitigates Estrogen Deficiency-Induced Bone Mass Loss by Modulating TRAF6-Mediated Signaling. FASEB J. 2019, 33, 2574–2586. [Google Scholar] [CrossRef] [PubMed]
- Dyle, M.C.; Ebert, S.M.; Cook, D.P.; Kunkel, S.D.; Fox, D.K.; Bongers, K.S.; Bullard, S.A.; Dierdorff, J.M.; Adams, C.M. Systems-Based Discovery of Tomatidine as a Natural Small Molecule Inhibitor of Skeletal Muscle Atrophy. J. Biol. Chem. 2014, 289, 14913–14924. [Google Scholar] [CrossRef]
- Lee, K.R.; Kozukue, N.; Han, J.S.; Park, J.H.; Chang, E.Y.; Baek, E.J.; Chang, J.S.; Friedman, M. Glycoalkaloids and Metabolites Inhibit the Growth of Human Colon (HT29) and Liver (HepG2) Cancer Cells. J. Agric. Food Chem. 2004, 52, 2832–2839. [Google Scholar] [CrossRef]
- Echeverría, C.; Martin, A.; Simon, F.; Salas, C.O.; Nazal, M.; Varela, D.; Pérez-Castro, R.A.; Santibanez, J.F.; Valdés-Valdés, R.O.; Forero-Doria, O.; et al. In Vivo and in Vitro Antitumor Activity of Tomatine in Hepatocellular Carcinoma. Front. Pharmacol. 2022, 13, 1003264. [Google Scholar] [CrossRef]
- Shih, Y.W.; Shieh, J.M.; Wu, P.F.; Lee, Y.C.; Chen, Y.Z.; Chiang, T.A. α-Tomatine Inactivates PI3K/Akt and ERK Signaling Pathways in Human Lung Adenocarcinoma A549 Cells: Effect on Metastasis. Food Chem. Toxicol. 2009, 47, 1985–1995. [Google Scholar] [CrossRef] [PubMed]
- Yelken, B.Ö.; Balcı, T.; Süslüer, S.Y.; Kayabaşı, Ç.; Avcı, Ç.B.; Kırmızıbayrak, P.B.; Gündüz, C. The Effect of Tomatine on Metastasis Related Matrix Metalloproteinase (MMP) Activities in Breast Cancer Cell Model. Gene 2017, 627, 408–411. [Google Scholar] [CrossRef] [PubMed]
- Shieh, J.M.; Cheng, T.H.; Shi, M.D.; Wu, P.F.; Chen, Y.; Ko, S.C.; Shih, Y.W. α-Tomatine Suppresses Invasion and Migration of Human Non-Small Cell Lung Cancer NCI-H460 Cells Through Inactivating FAK/PI3K/Akt Signaling Pathway and Reducing Binding Activity of NF-ΚB. Cell Biochem. Biophys. 2011, 60, 297–310. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.T.; Wong, P.F.; He, H.; Da Hooper, J.; Mustafa, M.R. Alpha-Tomatine Attenuation of In Vivo Growth of Subcutaneous and Orthotopic Xenograft Tumors of Human Prostate Carcinoma PC-3 Cells Is Accompanied by Inactivation of Nuclear Factor-Kappa B Signaling. PLoS ONE 2013, 8, e57708. [Google Scholar] [CrossRef] [PubMed]
- Serratì, S.; Porcelli, L.; Guida, S.; Ferretta, A.; Iacobazzi, R.M.; Cocco, T.; Maida, I.; Tamasi, G.; Rossi, C.; Manganelli, M.; et al. Tomatine Displays Antitumor Potential in in Vitro Models of Metastatic Melanoma. Int. J. Mol. Sci. 2020, 21, 5243. [Google Scholar] [CrossRef]
- Huang, W.C.; Wu, S.J.; Chen, Y.L.; Lin, C.F.; Liou, C.J. Tomatidine Improves Pulmonary Inflammation in Mice with Acute Lung Injury. Mediat. Inflamm. 2021, 2021, 4544294. [Google Scholar] [CrossRef]
- Chao, M.W.; Chen, C.H.; Chang, Y.L.; Teng, C.M.; Pan, S.L. α-Tomatine-Mediated Anti-Cancer Activity In Vitro and In Vivo through Cell Cycle- and Caspase-Independent Pathways. PLoS ONE 2012, 7, 44093. [Google Scholar] [CrossRef]
- Friedman, M.; McQuistan, T.; Hendricks, J.D.; Pereira, C.; Bailey, G.S. Protective Effect of Dietary Tomatine against Dibenzo[a,l]Pyrene (DBP)-Induced Liver and Stomach Tumors in Rainbow Trout. Mol. Nutr. Food Res. 2007, 51, 1485–1491. [Google Scholar] [CrossRef]
- Bailly, C. The steroidal alkaloids α-tomatine and tomatidine: Panorama of their mode of action and pharmacological properties. Steroids 2021, 176, 108933. [Google Scholar] [CrossRef]
- Sandrock, R.W.; Vanetten, H.D.; Sandrock, W. Biochemistry and Cell Biology Fungal Sensitivity to and Enzymatic Degradation of the Phytoanticipin α-Tomatine. Phytopathology 1998, 88, 137–143. [Google Scholar] [CrossRef]
- Blankemeyer, J.T.; White, J.B.; Stringer, B.K.; Friedman, M. Effect of c,Tomatine and Tomatidine on Membrane Potential of Frog Embryos and Active Transport of Ions in Frog Skin. Food Chem. Toxicol. 1997, 35, 639–646. [Google Scholar] [CrossRef] [PubMed]
- Ito, S.-I.; Ihara, T.; Tamura, H.; Tanaka, S.; Ikeda, T.; Kajihara, H.; Dissanayake, C.; Abdel-Motaal, F.F.; El-Sayed, M.A. α-Tomatine, the Major Saponin in Tomato, Induces Programmed Cell Death Mediated by Reactive Oxygen Species in the Fungal Pathogen Fusarium Oxysporum. FEBS Lett. 2007, 581, 3217–3222. [Google Scholar] [CrossRef] [PubMed]
- Sueiro, R.A.; Leiro, J.M.; Blanco-Abad, V.; Raaijmakers, J.; de Bruijn, I.; Dirks, R.P.H.; Lamas, J. Plant-and Bacteria-Derived Compounds with Anti-Philasterides Dicentrarchi Activity. Pathogens 2022, 11, 267. [Google Scholar] [CrossRef] [PubMed]
- Zhao, B.; Zhou, B.; Bao, L.; Yang, Y.; Guo, K. Alpha-Tomatine Exhibits Anti-Inflammatory Activity in Lipopolysaccharide-Activated Macrophages. Inflammation 2015, 38, 1769–1776. [Google Scholar] [CrossRef] [PubMed]
- Kovacs, B.A.; Wakkary, J.A.; Goodfriend, L.; Rose, B. Isolation of an Antihistaminic Principle Resembling Tomatine from Crown Gall Tumors. Science 1964, 144, 295–296. [Google Scholar] [CrossRef] [PubMed]
- Yu, T.; Wu, Q.; You, X.; Zhou, H.; Xu, S.; He, W.; Li, Z.; Li, B.; Xia, J.; Zhu, H.; et al. Tomatidine Alleviates Osteoporosis by Downregulation of P53. Med. Sci. Monit. 2020, 26, e923996-1–e923996-9. [Google Scholar] [CrossRef] [PubMed]
- Adams, C.M.; Ebert, S.M.; Dyle, M.C. Role of ATF4 in Skeletal Muscle Atrophy. Curr. Opin Clin. Nutr. Metab. Care 2017, 20, 164–168. [Google Scholar] [CrossRef]
- Ebert, S.M.; Dyle, M.C.; Bullard, S.A.; Dierdorff, J.M.; Murry, D.J.; Fox, D.K.; Bongers, K.S.; Lira, V.A.; Meyerholz, D.K.; Talley, J.J.; et al. Identification and Small Molecule Inhibition of an Activating Transcription Factor 4 (ATF4)-Dependent Pathway to Age-Related Skeletal Muscle Weakness and Atrophy. J. Biol. Chem. 2015, 290, 25497–25511. [Google Scholar] [CrossRef]
- Huang, H.; Chen, X.; Li, D.; He, Y.; Li, Y.; Du, Z.; Zhang, K.; DiPaola, R.; Goodin, S.; Zheng, X. Combination of α-Tomatine and Curcumin Inhibits Growth and Induces Apoptosis in Human Prostate Cancer Cells. PLoS ONE 2015, 10, e0144293. [Google Scholar] [CrossRef]
- Kim, S.P.; Nam, S.H.; Friedman, M. The Tomato Glycoalkaloid α-Tomatine Induces Caspase-Independent Cell Death in Mouse Colon Cancer CT-26 Cells and Transplanted Tumors in Mice. J. Agric. Food Chem. 2015, 63, 1142–1150. [Google Scholar] [CrossRef]
- Zheng, X.; Cheng, M.; Xiang, L.; Liang, J.; Xie, L.; Zhang, R. The AP-1 Transcription Factor Homolog Pf-AP-1 Activates Transcription of Multiple Biomineral Proteins and Potentially Participates in Pinctada Fucata Biomineralization. Sci. Rep. 2015, 5, 14408. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.T.; Wong, P.F.; Cheah, S.C.; Mustafa, M.R. Alpha-Tomatine Induces Apoptosis and Inhibits Nuclear Factor-Kappa B Activation on Human Prostatic Adenocarcinoma PC-3 Cells. PLoS ONE 2011, 6, e18915. [Google Scholar] [CrossRef] [PubMed]
- Green, D.R.; Llambi, F. Cell Death Signaling. Cold Spring Harb. Perspect Biol. 2015, 7, a006080. [Google Scholar] [CrossRef] [PubMed]
- Hemmings, B.A.; Restuccia, D.F. PI3K-PKB/Akt Pathway. Cold Spring Harb. Perspect. Biol. 2012, 4, a011189. [Google Scholar] [CrossRef] [PubMed]
- Schrenk, D.; Bignami, M.; Bodin, L.; Chipman, J.K.; del Mazo, J.; Hogstrand, C.; Hoogenboom, L.; Leblanc, J.C.; Nebbia, C.S.; Nielsen, E.; et al. Risk Assessment of Glycoalkaloids in Feed and Food, in Particular in Potatoes and Potato-Derived Products. EFSA J. 2020, 18, e06222. [Google Scholar] [CrossRef] [PubMed]
- Kasimir, M.; Wolbeck, A.; Behrens, M.; Humpf, H.U. Intestinal Metabolism of Selected Steroidal Glycoalkaloids in the Pig Cecum Model. ACS Omega 2023, 8, 18266–18274. [Google Scholar] [CrossRef] [PubMed]
- Rose, E.C.; Blikslager, A.T.; Ziegler, A.L. Porcine Models of the Intestinal Microbiota: The Translational Key to Understanding How Gut Commensals Contribute to Gastrointestinal Disease. Front Vet. Sci. 2022, 9, 834598. [Google Scholar] [CrossRef]
- Hövelmann, Y.; Jagels, A.; Schmid, R.; Hübner, F.; Humpf, H.U. Identification of Potential Human Urinary Biomarkers for Tomato Juice Intake by Mass Spectrometry-Based Metabolomics. Eur. J. Nutr. 2020, 59, 685–697. [Google Scholar] [CrossRef]
- Yamashoji, S.; Onoda, E. Detoxification and Function of Immature Tomato. Food Chem. 2016, 209, 171–176. [Google Scholar] [CrossRef]
- Wilson, R.H.; Poley, G.W.; Deeds, F.; Fontaine, T.D. Some Pharmacologic and Toxicologic Properties of Tomatine and Its Derivatives. Toxicol. Appl. Pharmacol. 1961, 3, 39–48. [Google Scholar] [CrossRef]
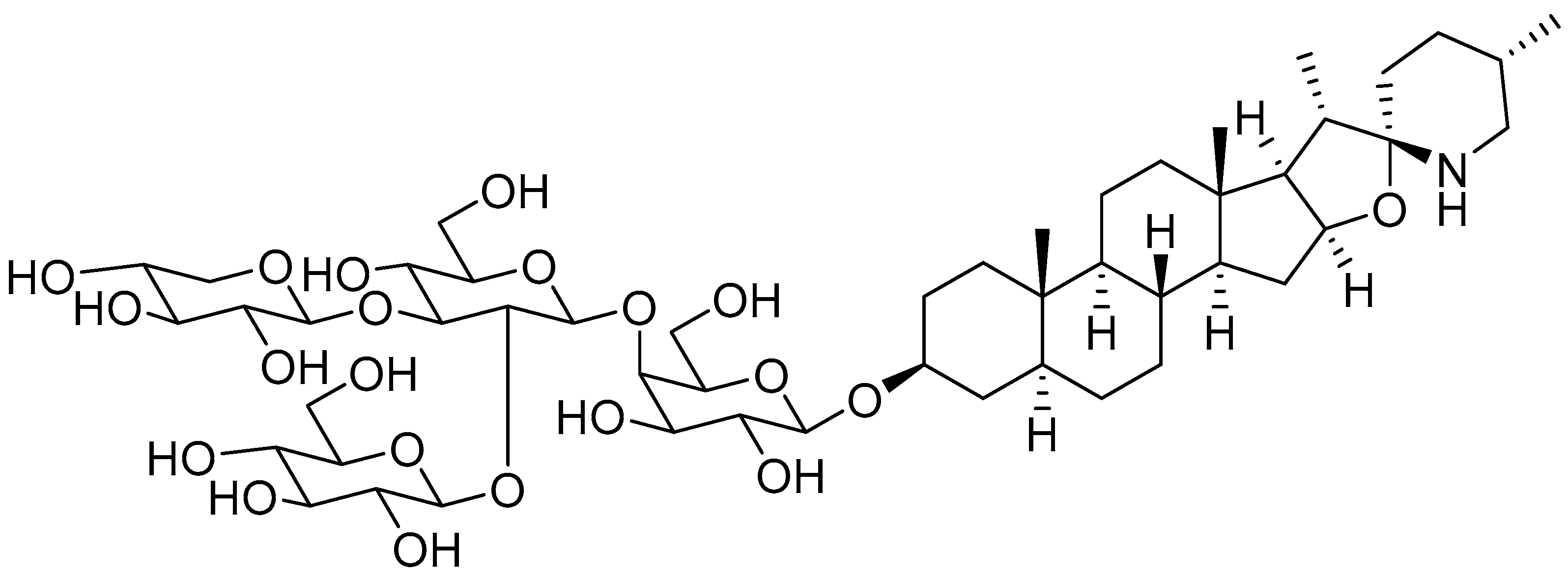
| Sample | α-Tomatine Content (mg/g) | Weight | Reference |
|---|---|---|---|
| Lyophilized green tomatoes | 0.734 ± 0.028–0.175 ± 0.011 | DW | [28] |
| Air-dried red tomatoes | 0.112–0.005 | DW | [29] |
| Lyophilized green tomatoes | 1.993 ± 0.033–0.181 ± 0.012 | DW | [30] |
| Lyophilized green tomatoes | 34.354 ± 1.094–0.282 ± 0.019 | DW | [31] |
| Fresh green tomatoes | 1.894 ± 0.008–0.278 ± 0.004 | FW | [32] |
| Lyophilized green tomatoes | 2.240 ± 0.010–1.130 ± 0.035 | DW | [22] |
| Lyophilized leaves | 9.620 ± 0.201–6.030 ± 0.303 | DW | [33] |
| Lyophilized leaves | 0.640 ± 0.002–0.402 ± 0.001 | DW | [34] |
| Hot-air-dried sample | 2.430 ± 0.467–0.170 ± 0.002 | DW | [35] |
| Fresh sample | 0.087 ± 0.027–0.036 ± 0.014 | FW | [36] |
| Fresh sample | 0.089 ± 0.004–0.010 ± 0.001 | FW | [14] |
| Lyophilized samples | 7.435 ± 0.015–0.003 ± 0.001 | DW | [37] |
| Fresh samples | 4.985 ± 0.115–0.144 ± 0.005 | FW | [38] |
| Fresh samples | 3.610 ± 0.255–0.138 ± 0.010 | FW | [39] |
| Fresh samples | 0.308–0.003 | FW | [40] |
| Fresh samples | 0.041–0.008 | FW | [41] |
| Fresh samples | 0.065–0.006 | FW | [42] |
| Hot-air-dried sample | 31.000–0.001 | DW | [43] |
| Sample (Amount in g) | Extraction Solvent | Extraction Condition | Clean-Up | Reference |
|---|---|---|---|---|
| Lyophilized sample (0.1) | 10 mL of acetic acid 1% in aqueous EtOH 80% (v/v) | UAE (10 min), centrifugation (5 min, 4000 rpm) for two cycles | [28,30] | |
| Lyophilized sample (0.1) | 10 mL of acetic acid 1% in aqueous EtOH 70% (v/v) | UAE (15 min), centrifugation (5 min, 4000 rpm) for two cycles | [46] | |
| Fresh samples (10) | 10 mL of methanol | UAE (1 h), centrifugation (10 min, 12,000 rpm) | [32] | |
| Hot-air-dried sample (35) | Acetic acid 5% in EtOH | UAE (20 min) | (NH4)OH 5% (v/v) solution | [35] |
| Fresh sample |
| UAE, centrifugation (10 min, 9800 g) | (NH4)OH 2% (v/v) solution | [36] |
| Fresh sample (1–20) | Chloroform/MeOH, 2:1 (v/v) | (NH4)OH 2% (v/v) solution | [14,50] | |
| Lyophilized sample (0.5) | 100 mL of aqueous acetic acid 5% | UAE (10 min) | Different SPE sorbents (C18, CN, SCX, Oasis HLB) | [51] |
| Fresh sample (2) | 10 mL of formic acid 1% in aqueous MeOH 70% (v/v) | Centrifugation (10 min, 2600 g) | [48] | |
| Lyophilized sample (0.16) | 2 mL of aqueous acetic acid 5% | UAE (60 min), centrifugation (5 min, 12,000 rpm) | SPE (SCX) | [33,34,53,58] |
| Lyophilized sample (0.2) | 4 mL of aqueous acetic acid 5% | UAE (30 min), centrifugation (5 min, 12,000 rpm) | SPE (SCX) | [57] |
| Air-dried sample (0.25) | 10 mL of aqueous acetic acid 1% | Centrifugation (15 min) | SPE (C18) | [29] |
| Lyophilized sample (1) | 30 mL of aqueous acetic acid 1% | Centrifugation (10 min, 13,300 rpm) for two cycles | SPE (C18), LLE with 1-butanol | [22,37] |
| Lyophilized sample (1) | 30 mL of aqueous acetic acid 1% | Centrifugation (10 min, 13,300 rpm) for two cycles | SPE (C18) | [38] |
| Fresh sample (1–22) | 100 mL of acetic acid 2% in MeOH | Centrifugation (10 min, 18,000 g) | (NH4)OH 2% (v/v) solution | [39] |
| Fresh sample (3–41) | 100 mL of acetic acid 2% in MeOH | Centrifugation (5 min, 18,000 g) | (NH4)OH 2% (v/v) solution | [52] |
| Fresh sample | 45 mL of MeOH | Centrifugation (5 min, 3000 rpm) for three cycles | SPE (C18) | [40] |
| Lyophilized sample (1) | 20 mL of aqueous acetic acid 1% | SPE (C18) | [41] | |
| Lyophilized sample (0.5) | 5 mL of acetic acid 2% in MeOH | Centrifugation (15 min, 8000 rpm) for two cycles | (NH4)OH 25% (v/v) solution | [53] |
| Lyophilized sample (1.5) | 15 mL of formic acid 1% in aqueous MeOH 80% (v/v) | UAE (10 min), centrifugation (10 min, 9000 rpm) for two cycles | SPE (C18) | [31] |
| Fresh sample (55) | 50 mL of THF/H2O/Acn/Acetic acid (50/30/20/1 v/v) | SPE (C18) | [42] | |
| Lyophilized sample (0.05) | 15 mL of MeOH | Centrifugation (5 min, 3000 g) | [19] | |
| Fresh sample (0.2) | 20 mL of formic acid 0.1% in aqueous MeOH 80% (v/v) | Centrifugation (10 min, 20,000 g) | [47] |
| Pharmacological Effects | Experimental Model | Extracts/Pure Molecule | α-Tomatine/Tomatidine Content | IC50 | Reference |
|---|---|---|---|---|---|
| Antimicrobial | Escherichia coli K12 | Dried green tomato peel extract | 12 mg of α-tomatine/kg of DW; 2 mg of dehydrotomatine/kg of DW | 8 mm of zone of inhibition (treatment at 10% w/v) | [81] |
| Salmonella enterica | Dried green tomato peel extract | 12 mg of α-tomatine/kg of DW; 2 mg of dehydrotomatine/kg of DW | 7 mm of zone of inhibition (treatment at 10% w/v) | [81] | |
| Candida albicans | Green tomato extract Dried leaf extract Dried stem extract | n.r. 11 mg of α-tomatine/kg of DW; 7 mg of dehydrotomatine/kg of DW | 11.5 mm of zone of inhibition (treatment at 10% w/v) | [81] | |
| Salmonella enterica | Dried leaf extract | 11 mg of α-tomatine/kg of DW; 7 mg of dehydrotomatine/kg of DW | 8 mm of zone of inhibition (treatment at 10% w/v) | [81] | |
| Bacillus cereus | Dried leaf extract | 11 mg of α-tomatine/kg of DW; 7 mg of dehydrotomatine/kg of DW | 13 mm of zone of inhibition (treatment at 10% w/v) | [81] | |
| Beauveria brassiana | α-tomatine | 1 mM | [82] | ||
| Fusarium caereleum | Tomatine | 7–460 µM | [83] | ||
| Paecilomyces fumosoreus | α-tomatine | 500 µM | [82] | ||
| Trichomonas vaginalis | α-tomatine | 8 µM range | [84] | ||
| Trichomonas foetus | α-tomatine | 2 µM range | [84] | ||
| Trypanosoma cruzi | α-tomatine | 10.14 µM | [85] | ||
| Anti-inflammatory | In vivo rat model | Locular gel and serum extracts of Solanum lycopersicum L. var “Camone” | 61.7 ± 0.9 mg of α-tomatine/kg of FW locular gel; 12.5 ± 0.5 mg of α-tomatine/kg of FW serum | 12.40 g DW/kg rat p.o. | [46] |
| In vivo rabbit model | Dried tomatoes powder | Diet of 1% and 2% tomato powder | [86] | ||
| In vivo rat model | Tomatidine | 2.5–10 μM | [87] | ||
| In vitro murine macrophage cultures | Tomatidine | 10–40 µM | [88] | ||
| In vivo rat model | α-tomatine | 1–10 mg/kg i.m. 15–30 mg/kg p.o. 5–10 mg/kg s.c. | [89] | ||
| Anti-aging | In vivo rat model | Dried green tomato extract | 1.06 ± 0.11 mg of tomatidine/100 g of DW matrix | [58] | |
| In vitro primary cultures from rat model | Tomatidine | 8 µM | [90] | ||
| In vitro cultures of neuronal cells | Leaf extract of Solanum lycopersicum L. var “Cherry” | 640.0 ± 2.0 µg of tomatine/mg of DW; 9.34 ± 0.10 µg of tomatidine/mg of DW | 197.50 µg/mL | [34] | |
| In vitro cultures of neuronal cells | Leaf extract of Solanum lycopersicum L. var “Bull’s heart” | 402.0 ± 1.2 µg of tomatine/mg of DW; 5.39 ± 0.10 µg of tomatidine/mg of DW | 197.50 µg/mL | [34] | |
| In vivo mouse model | Tomatidine | 25 mg/kg (i.p.) | [91] | ||
| Anti-tumoral | In vitro breast cancer cells (MCF-7) | Dried green tomato extract of Solanum lycopersicon L. var Sancheri premium (S.p), Chobok Power (C.p), Yoyo (Y), Rokusanmaru (R) | S.p: 10.8 ± 0.69 µg of α-tomatine mg/100 g of FW; S.p: 5.75 ± 0.29 µg of α-tomatine mg/100 g of FW; Y: 8.30 ± 0.07 µg of α-tomatine mg/100 g of FW; R: 11.53 ± 1.11 µg of α-tomatine mg/100 g of FW; R: 9.36 ± 0.32 µg of α-tomatine mg/100 g of FW | 0.33 ppm; 2.41 ppm; ≤0.1 ppm; 18 ppm; 9 ppm | [52] |
| In vitro colon cancer cells (HT-29) | Dried green tomato extract of Solanum lycopersicon L. var Sancheri premium (S.p), Chobok Power (C.p), Yoyo (Y), Rokusanmaru (R) | S.p: 10.8 ± 0.69 µg of α-tomatine mg/100 g of FW; S.p: 5.75 ± 0.29 µg of α-tomatine mg/100 g of FW; Y: 8.30 ± 0.07 µg of α-tomatine mg/100 g of FW; R: 11.53 ± 1.11 µg of α-tomatine mg/100 g of FW; R: 9.36 ± 0.32 µg of α-tomatine mg/100 g of FW | ≤0.1 ppm; ≤0.1 ppm; 5.4 ppm; 1.3 ppm | [52] | |
| In vitro hepatocarcinoma cells (HepG2) | Dried green tomato extract of Solanum lycopersicon L. var Sancheri premium (S.p), Chobok Power (C.p), Yoyo (Y), Rokusanmaru (R) | S.p: 31.4 ± 1.97 µg of α-tomatine mg/100 g of FW; S.p: 10.8 ± 0.69 µg of α-tomatine mg/100 g of FW; S.p: 5.75 ± 0.29 µg of α-tomatine mg/100 g of FW; Y: 8.30 ± 0.07 µg of α-tomatine mg/100 g of FW; R: 11.53 ± 1.11 µg of α-tomatine mg/100 g of FW; R: 9.36 ± 0.32 µg of α-tomatine mg/100 g of FW | 12.3 ppm; 3.2 ppm; 1 ppm; ≤0.8 ppm; 0.2 ppm; 0.9 ppm | [52] | |
| In vitro stomach cancer cells (AGS) | Dried green tomato extract of Solanum lycopersicon L. var Sancheri premium (S.p), Chobok Power (C.p), Yoyo (Y), Rokusanmaru (R) | S.p: 31.4 ± 1.97 µg of α-tomatine mg/100 g of FW; S.p: 10.8 ± 0.69 µg of α-tomatine mg/100 g of FW; S.p: 5.75 ± 0.29 µg of α-tomatine mg/100 g of FW; Y: 8.30 ± 0.07 µg of α-tomatine mg/100 g of FW; R: 11.53 ± 1.11 µg of α-tomatine mg/100 g of FW; R: 9.36 ± 0.32 µg of α-tomatine mg/100 g of FW | 11.4 ppm; 2 ppm; 1.4 ppm; 1.7 ppm; 0.3 ppm; 1.2 ppm | [52] | |
| In vitro hepatocarcinoma cells (HepG2) | α-tomatine | 1 μM | [92] | ||
| In vitro hepatocarcinoma cells (HepG2) | α-tomatine | 30 µM | [93] | ||
| In vitro lung cancer cells (A549) | α-tomatine | 1 µM | [94] | ||
| In vitro breast cancer cells (MCF-7) | α-tomatine | 7.07 µM | [95] | ||
| In vitro non-small lung cancer cells (NCI-H460) | α-tomatine | 2 µM | [96] | ||
| In vitro prostate cancer cells (PC3) | α-tomatine | 2 µM | [97] | ||
| In vitro melanoma cells (BRAF, V600BRAF) | Tomatine (α-tomatine 87.1 ± 1.6%; dehydrotomatine 13.0 ± 0.8%) | 1 µM | [98] | ||
| In vitro leukemia cells (HL60, K562) | α-tomatine | 5 µM | [99,100] | ||
| In vivo Hasta strain rainbow trout | α-tomatine | 100–2000 ppm | [101] | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Piccolo, V.; Pastore, A.; Maisto, M.; Keivani, N.; Tenore, G.C.; Stornaiuolo, M.; Summa, V. Agri-Food Waste Recycling for Healthy Remedies: Biomedical Potential of Nutraceuticals from Unripe Tomatoes (Solanum lycopersicum L.). Foods 2024, 13, 331. https://doi.org/10.3390/foods13020331
Piccolo V, Pastore A, Maisto M, Keivani N, Tenore GC, Stornaiuolo M, Summa V. Agri-Food Waste Recycling for Healthy Remedies: Biomedical Potential of Nutraceuticals from Unripe Tomatoes (Solanum lycopersicum L.). Foods. 2024; 13(2):331. https://doi.org/10.3390/foods13020331
Chicago/Turabian StylePiccolo, Vincenzo, Arianna Pastore, Maria Maisto, Niloufar Keivani, Gian Carlo Tenore, Mariano Stornaiuolo, and Vincenzo Summa. 2024. "Agri-Food Waste Recycling for Healthy Remedies: Biomedical Potential of Nutraceuticals from Unripe Tomatoes (Solanum lycopersicum L.)" Foods 13, no. 2: 331. https://doi.org/10.3390/foods13020331











