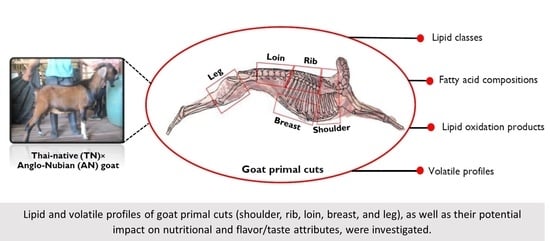
Lipid and Volatile Profiles of Various Goat Primal Cuts: Aspects of Nutritional Value and Flavor/Taste Attributes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Collection and Preparation
2.2. Measurement of Lipid Classes
2.3. Measurement of Fatty Acid Compositions
2.4. Measurement of Lipid Oxidation Products (CD, PV, TBARS)
2.5. Measurement of Volatile Profiles (GC-MS)
2.6. Statistical Analysis
3. Results and Discussion
3.1. Proximate Composition
3.2. Lipid Classes
3.3. Fatty Acid Profiles
3.4. Lipid Oxidation Products
3.5. Volatile Compounds
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mazhangara, I.R.; Chivandi, E.; Mupangwa, J.F.; Muchenje, V. The potential of goat meat in the red meat industry. Sustainability 2009, 11, 3671. [Google Scholar] [CrossRef]
- Madruga, M.; Resosemito, F.; Narain, N.; Souza, W.; Cunha, M.; Ramos, J. Effect of raising conditions of goats on physicochemical and chemical quality of its meat. Cienc. Tecnol. Aliment. 2006, 5, 100–104. [Google Scholar] [CrossRef]
- Hunt, M.R.; Legako, J.F.; Dinh, T.T.N.; Garmyn, A.J.; O’Quinn, T.G.; Corbin, C.H.; Rathmann, R.J.; Brooks, J.C.; Miller, M.F. Assessment of volatile compounds, neutral and polar lipid fatty acids of four beef muscles from USDA choice and select graded carcasses and their relationships with consumer palatability scores and intramuscular fat content. Meat Sci. 2016, 116, 91–101. [Google Scholar] [CrossRef]
- Indriani, S.; Srisakultiew, N.; Sangsawad, P.; Paengkoum, P.; Pongsetkul, J. Characterization of the non-volatiles and volatiles in correlation with flavor development of cooked goat meat as affected by different cooking methods. Food Sci. Anim. Resour. 2024. [Google Scholar] [CrossRef]
- Okeudo, N.J.; Moss, B.W. Interrelationships amongst carcass and meat quality characteristics of sheep. Meat Sci. 2005, 69, 1–8. [Google Scholar] [CrossRef]
- Tshabalala, P.A.; Strydom, P.E.; Webb, E.C.; de Kock, H.L. Meat quality of designated South African indigenous goat and sheep breeds. Meat Sci. 2003, 65, 563–570. [Google Scholar] [CrossRef]
- Mottram, D.S. Flavour formation in meat and meat products: A review. Food Chem. 1998, 62, 415–424. [Google Scholar] [CrossRef]
- Gawat, M.; Kaur, L.; Singh, J.; Boland, M. Physicochemical and quality characteristics of New Zealand goat meat and its ultrastructural features. Food Res. Int. 2022, 161, 111736. [Google Scholar] [CrossRef]
- Ali, M.; Choi, Y.S.; Nam, K.C. Physicochemical attributes, free amino acids, and fatty acids of the five major cuts from Korean native black goat. Anim. Technol. 2021, 8, 23–33. [Google Scholar] [CrossRef]
- Machado, B.; Gomes, I.; Nunes, C.; Andrade, G.; Nery, T.; Reis, J.; Viana, J.; Druzian, J. Lipid content and fatty acids compositions in commercial cuts of young goat meat. Ciência Rural. 2019, 49, 1–8. [Google Scholar] [CrossRef]
- Anneke, A.; Wattanachant, C.; Wattanachant, S.; Ann, A. Effects of supplementing crude glycerin in concentrated diet and castration on carcass characteristics and meat quality of Thai native × Anglo Nubian goats. Walailak J. Sci. Technol. (WJST) 2019, 16, 477–486. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis, 16th ed.; Association of Official Analytical Chemists: Washington, DC, USA, 2000. [Google Scholar]
- Pongsetkul, J.; Benjakul, S.; Vongkamjan, K.; Sumpavapol, P.; Osako, K. Changes in lipids of shrimp (Acetes vulgaris) during salting and fermentation. Eur. J. Lipid Sci. Technol. 2017, 1119, 1700253. [Google Scholar] [CrossRef]
- Frankel, E.N. Lipid oxidation: Mechanisms, products and biological significance. J. Am. Oil Chem. Soc. 1984, 61, 1908–1917. [Google Scholar] [CrossRef]
- Pongsetkul, J.; Benjakul, S.; Boonchuen, P. Changes in volatile compounds and quality characteristics of salted shrimp paste stored in different packaging containers. Fermentation 2022, 8, 69. [Google Scholar] [CrossRef]
- Food Advisory Committee. Report on Review of Food Labelling and Advertising; Food Advisory Committee: London, UK, 1990.
- Casey, N.; Niekerk, W.; Webb, E. Goat Meat. In Encyclopaedia of Food Sciences and Nutrition; Academic Press: London, UK, 2003; pp. 2937–2944. [Google Scholar]
- Werdi Pratiwi, N.M.; Murray, P.J.; Taylor, D.G. Total cholesterol concentrations of the muscles in castrated Boer goats. Small Rum. Res. 2006, 64, 77–81. [Google Scholar] [CrossRef]
- Verdiglione, R.; Cassandro, M. Characterization of muscle fiber type in the pectoralis major muscle of slow-growing local and commercial chicken strains. Poult. Sci. 2013, 92, 2433–2437. [Google Scholar] [CrossRef]
- American Heart Association. Dietary Guidelines for Healthy American Adults; Cholesterol Fat Heart and Stroke Encyclopedia: Dallas, TX, USA, 2008. [Google Scholar]
- Soliman, G.A. Dietary cholesterol and the lack of evidence in cardiovascular disease. Nutrients 2018, 10, 780. [Google Scholar] [CrossRef]
- Laguerre, M.; Lecomte, J.; Villeneuve, P. Evaluation of the ability of antioxidants to counteract lipid oxidation: Existing methods, new trends and challenges. Prog. Lipid Res. 2007, 46, 244–282. [Google Scholar] [CrossRef]
- Pereira, A.L.; Abreu, V. Lipid peroxidation in meat and meat products. In Lipid Peroxidation Research; IntechOpen: Rijeka, Croatia, 2018; p. Ch.3. [Google Scholar]
- Domínguez, R.; Pateiro, M.; Gagaoua, M.; Barba, F.J.; Zhang, W.; Lorenzo, J.M. A comprehensive review on lipid oxidation in meat and meat products. Antioxidants 2019, 8, 429. [Google Scholar] [CrossRef]
- Chew, S.C.; Nyam, K.L. Refining of edible oils. In Lipids and Edible Oils; Academic Press: Cambridge, MA, USA, 2020; pp. 213–241. [Google Scholar]
- Rhee, K.S. Fatty acids in meats and meat products. In Fatty Acids in Foods and Their Health Implications; Marcel Dekker Inc.: New York, NY, USA, 1992; pp. 65–93. [Google Scholar]
- Erickson, M.C. Lipid oxidation of muscle foods. In Food Lipids: Chemistry, Nutrition, and Biotechnology; Marcel Dekker, Inc.: New York, NY, USA, 2002; pp. 384–430. [Google Scholar]
- Webb, E.C. Goat meat production, composition, and quality. Anim. Front. 2014, 4, 33–37. [Google Scholar] [CrossRef]
- Williams, C.D.; Whitley, B.M.; Hoyo, C.; Grant, D.J.; Iraggi, J.D.; Newman, K.A.; Gerber, L.; Taylor, L.A.; McKeever, M.G.; Freedland, S.J. A high ratio of dietary n-6/n-3 polyunsaturated fatty acids is associated with increased risk of prostate cancer. Nutr. Res. 2011, 31, 1–8. [Google Scholar] [CrossRef]
- Min, B.; Ahn, D.U. Mechanism of lipid peroxidation in meat and meat products—A review. Food Sci. Biotechnol. 2005, 14, 152–163. [Google Scholar]
- Barriuso, B.; Astiasarán, I.; Ansorena, D. A review of analytical methods measuring lipid oxidation status in foods: A challenging task. Eur. Food Res. Technol. 2013, 236, 1–15. [Google Scholar] [CrossRef]
- Han, D.; McMillin, K.; Godber, J. Hemoglobin, myoglobin, and total pigments in beef and chicken muscles: Chromatographic determination. J. Food Sci. 2006, 59, 1279–1282. [Google Scholar] [CrossRef]
- Dinh, T.; To, K.; Schilling, W. Fatty acid composition of meat animals as flavor precursors. Meat Muscle Biol. 2021, 5, 1–16. [Google Scholar] [CrossRef]
- deMan, J.H.; John, M. Lipids. In Principle of Food Chemistry; Springer: New York, NY, USA, 1999; pp. 33–110. [Google Scholar]
- Caporaso, F.S.; Dimick, P.S.; Mussinan, C.J.; Sanderson, A. Volatile flavor constituents of ovine adipose tissue. J. Agric. Food Chem. 1977, 25, 1230–1234. [Google Scholar] [CrossRef]
- Lamikanra, V.T.; Dupuy, H.P. Analysis of volatiles related to warmed over flavor of cooked chevon. J. Food Sci. 1990, 55, 861–862. [Google Scholar] [CrossRef]
- Zhang, K.; Li, D.; Zang, M.; Zhang, Z.; Li, X.; Wang, S.; Zhang, S.; Zhao, B. Comparative characterization of fatty acids, reheating volatile compounds, and warmed-over flavor (WOF) of Chinese indigenous pork and hybrid pork. LWT 2022, 155, 112981. [Google Scholar] [CrossRef]
- Shi, Y.; Li, X.; Huang, A. A metabolomics-based approach investigates volatile flavor formation and characteristic compounds of the Dahe black pig dry-cured ham. Meat Sci. 2019, 158, 107904. [Google Scholar] [CrossRef]
- Varlet, V.; Fernandez, X. Review. Sulfur-containing volatile compounds in seafood: Occurrence, odorant properties and mechanisms of formation. Food Sci. Technol. Int. 2010, 16, 463–503. [Google Scholar] [CrossRef]
- Pongsetkul, J.; Yongsawadigul, J.; Boonanuntanasarn, S.; Benjakul, S. Development of flavor and taste components of sous-vide-cooked nile tilapia (Oreochromis niloticus) fillet as affected by various conditions. Foods 2022, 11, 3681. [Google Scholar] [CrossRef]
- Estevez, M.; Ventanas, S.; Ramirez, R.; Cava, R. Influence of addition of rosemary essential oil on the volatiles pattern of porcine frankfurter. J. Agric. Food Chem. 2004, 53, 8317–8324. [Google Scholar] [CrossRef]
- Paleari, M.A.; Moretti, V.M.; Beretta, G.; Caprino, F. Chemical parameters, fatty acids and volatile compounds of salted and ripened goat thigh. Small Rum. Res. 2008, 74, 140–148. [Google Scholar] [CrossRef]
- Cramer, D.A. Chemical compounds implicated in lamb flavor. Food Tech. 1983, 37, 429–457. [Google Scholar]
- Lee, J.; Kannan, G.; Kouakou, B. Tenderness and flavor of leg cuts from meat goats influenced by calcium chloride injection. Int. J. Food Prop. 2018, 21, 357–363. [Google Scholar] [CrossRef]



| Parameters | Shoulder | Rib | Loin | Breast | Leg |
|---|---|---|---|---|---|
| Moisture (%) | 77.45 ± 0.29 b | 78.38 ± 0.30 a | 77.76 ± 0.33 ab | 76.46 ± 0.34 c | 78.39 ± 0.45 a |
| Protein (%) | 19.05 ± 0.52 a | 18.30 ± 0.54 a | 18.79 ± 0.31 a | 16.26 ± 0.40 b | 18.26 ± 0.51 a |
| Fat (%) | 2.29 ± 0.31 b | 1.90 ± 0.30 bc | 1.74 ± 0.21 bc | 4.35 ± 0.26 a | 1.44 ± 0.25 c |
| Ash (%) | 1.07 ± 0.13 | 1.08 ± 0.09 | 1.13 ± 0.05 | 1.23 ± 0.14 | 1.01 ± 0.12 |
| Cholesterol (mg/100 g meat) | 64.23 ± 3.21 ab | 63.65 ± 5.35 ab | 62.42 ± 3.19 b | 66.88 ± 2.24 ab | 69.02 ± 3.30 a |
| Lipid classes (% of total lipids) | |||||
| Triacylglycerol | 87.75 ± 2.23 a | 86.84 ± 3.04 ab | 88.01 ± 2.55 a | 82.22 ± 2.92 b | 86.22 ± 3.01 ab |
| Diacylglycerol | 1.74 ± 0.15 c | 2.03 ± 0.29 c | 1.90 ± 0.25 c | 4.54 ± 0.22 a | 2.95 ± 0.48 b |
| Monoacylglycerol | ND | ND | ND | 0.23 ± 0.09 | ND |
| Phospholipid | 9.55 ± 1.03 | 9.81 ± 0.77 | 10.01 ± 0.70 | 9.98 ± 0.69 | 9.76 ± 1.01 |
| Free fatty acid | 0.31 ± 0.06 c | 0.50 ± 0.09 bc | 0.39 ± 0.08 c | 1.92 ± 0.11 a | 0.66 ± 0.12 b |
| FA Compositions | Shoulder | Rib | Loin | Breast | Leg |
|---|---|---|---|---|---|
| SFA * | |||||
| C10:0 | ND | ND | ND | 0.24 ± 0.03 | ND |
| C12:0 | ND | ND | ND | 0.66 ± 0.24 | ND |
| C14:0 | 3.71 ± 0.51 a | 1.64 ± 0.44 c | 3.17 ± 1.23 ab | 1.51 ± 0.50 c | 2.30 ± 0.45 bc |
| C15:0 | 2.35 ± 0.77 | 3.41 ± 1.25 | 2.26 ± 0.30 | 2.18 ± 0.76 | 2.55 ± 0.43 |
| C16:0 | 21.22 ± 1.06 a | 24.35 ± 1.54 a | 23.55 ± 2.12 a | 17.88 ± 1.15 b | 18.60 ± 1.23 b |
| C17:0 | 8.88 ± 0.96 a | 7.00 ± 0.91 ab | 6.45 ± 0.84 b | 7.66 ± 0.32 a | 8.21 ± 0.49 a |
| C18:0 | 16.08 ± 1.11 a | 14.95 ± 1.25 a | 15.62 ± 1.06 a | 7.11 ± 1.24 b | 5.65 ± 0.78 b |
| C22:0 | 0.23 ± 0.04 a | 0.09 ± 0.01 c | 0.14 ± 0.06 c | 1.55 ± 0.10 b | ND |
| Total SFA | 52.47 ± 2.45 a | 51.44 ± 4.01 a | 51.19 ± 4.53 a | 38.79 ± 3.69 b | 37.31 ± 2.41 b |
| MUFA | |||||
| C15:1 | ND | ND | ND | 0.14 ± 0.03 | ND |
| C16:1 | ND | ND | ND | 0.26 ± 0.10 | ND |
| C16:1n7 | 0.11 ± 0.08 b | 0.29 ± 0.11 b | 0.24 ± 0.08 b | 2.05 ± 0.08 a | 2.22 ± 0.14 a |
| C17:1 | 3.66 ± 0.23 a | 3.05 ± 0.40 a | 3.10 ± 0.33 a | 2.03 ± 0.56 b | 1.18 ± 0.28 c |
| C18:1n9 | 35.55 ± 2.15 b | 33.31 ± 2.06 b | 31.39 ± 3.22 b | 45.45 ± 2.14 a | 49.02 ± 4.09 a |
| C20:1 | 2.07 ± 0.21 b | 2.15 ± 0.33 b | 3.06 ± 0.29 a | 2.77 ± 0.50 a | 3.40 ± 0.43 a |
| C24:1 | 1.09 ± 0.08 c | 2.53 ± 0.16 b | 4.45 ± 0.26 a | 1.29 ± 0.23 c | 1.03 ± 0.12 c |
| Total MUFA | 42.48 ± 3.05 b | 41.33 ± 2.94 b | 42.24 ± 4.07 b | 53.99 ± 4.14 a | 56.85 ± 5.13 a |
| PUFA | |||||
| C18:2n6 | 2.01 ± 0.22 b | 3.50 ± 0.44 a | 2.05 ± 0.65 ab | 3.02 ± 0.43 a | 3.05 ± 0.51 a |
| C18:3n3 | 1.46 ± 0.26 a | 0.51 ± 0.11 b | 1.02 ± 0.29 a | 0.24 ± 0.13 b | 0.53 ± 0.10 b |
| C18:3n6 | 0.20 ± 0.04 b | 0.36 ± 0.09 a | 0.34 ± 0.09 a | 0.09 ± 0.01 c | 0.26 ± 0.09 ab |
| C20:2n6 | 0.09 ± 0.02 | 0.10 ± 0.03 | 0.15 ± 0.06 | ND | ND |
| C20:3n3 | 0.41 ± 0.05 | ND | ND | ND | ND |
| C20:4n6 (AA) ** | ND | ND | 0.61 ± 0.04 c | 2.04 ± 0.31 a | 1.05 ± 0.49 b |
| C20:5n3 (EPA) | 0.24 ± 0.03 c | 0.53 ± 0.14 b | 1.49 ± 0.25 a | 0.67 ± 0.21 b | 0.43 ± 0.06 b |
| C22:6n3 (DHA) | 0.11 ± 0.03 | 0.15 ± 0.05 | 0.14 ± 0.03 | ND | ND |
| Total PUFA | 4.52 ± 0.52 | 5.15 ± 0.43 | 5.80 ± 1.04 | 6.06 ± 0.97 | 5.32 ± 1.01 |
| Total UFA | 47.00 ± 0.04 b | 46.48 ± 0.02 b | 48.04 ± 0.07 b | 60.05 ± 0.04 a | 62.17 ± 0.02 a |
| MUFA/SFA | 0.81 ± 0.02 b | 0.80 ± 0.02 b | 0.82 ± 0.04 b | 1.39 ± 0.02 a | 1.52 ± 0.03 a |
| PUFA/SFA | 0.08 ± 0.01 | 0.10 ± 0.03 | 0.11 ± 0.01 | 0.15 ± 0.02 | 0.14 ± 0.01 |
| n-6 | 2.30 ± 0.02 c | 3.96 ± 0.04 b | 3.15 ± 0.05 b | 5.15 ± 0.07 a | 4.36 ± 0.03 ab |
| n-3 | 2.22 ± 0.03 a | 1.19 ± 0.07 b | 2.65 ± 0.06 a | 0.91 ± 0.03 c | 0.96 ± 0.05 c |
| n-6/n-3 | 1.03 ± 0.03 d | 3.32 ± 0.04 c | 1.19 ± 0.05 d | 5.65 ± 0.02 a | 4.54 ± 0.03 b |
| Volatile Compounds | Shoulder | Rib | Loin | Breast | Leg |
|---|---|---|---|---|---|
| Aldehydes (12) | |||||
| 3-methyl-butanal | 1.52 | 1.05 | 2.01 | 6.02 | 3.04 |
| 2-methyl-2-betenal | ND | 1.13 | 1.55 | ND | ND |
| Acetaldehyde | 0.23 | 0.50 | 0.93 | 0.84 | 0.78 |
| Pentanal | 4.02 | 4.49 | 4.15 | 5.06 | 3.98 |
| Hexanal | 10.15 | 11.09 | 10.10 | 12.77 | 13.08 |
| 2-heptanal | 1.53 | 1.06 | 2.02 | 1.44 | 1.78 |
| Octanal | 1.40 | 2.06 | 1.02 | 1.15 | 1.03 |
| 2-octenal | ND | ND | ND | 0.26 | ND |
| Nonanal | 2.18 | 3.45 | 2.01 | 3.44 | 3.06 |
| 2-undecenal | 0.22 | 0.40 | 0.78 | 1.56 | 0.54 |
| 4-pentylbenzaldehyde | ND | ND | ND | 0.32 | 0.41 |
| Decanal | 1.10 | 0.78 | 0.65 | 1.14 | 0.96 |
| Total aldehydes | 22.35 | 26.01 | 25.22 | 34.00 | 28.66 |
| Ketones (12) | |||||
| Acetone | 0.10 | 0.15 | 0.09 | 0.26 | 0.50 |
| 2-propanone | 0.65 | 0.40 | 0.53 | 1.51 | 1.22 |
| 2-butanone | 4.03 | 3.45 | 2.02 | 2.40 | 1.88 |
| 2,3-butanedione | ND | ND | 0.32 | 2.13 | 2.16 |
| 2-pentanone | 1.01 | 1.41 | 1.50 | 0.26 | 0.50 |
| 2-heptanone | 1.02 | 1.16 | 0.65 | 0.54 | 1.22 |
| 2,3-actanedione | ND | ND | ND | ND | 0.99 |
| 6-methyl-5-hepten-2-one | ND | ND | ND | 1.01 | 0.23 |
| 2,3-octanedione | 2.05 | 3.14 | 2.12 | 5.58 | 6.04 |
| 2-octanone | 0.12 | 0.26 | 0.10 | 0.23 | 0.20 |
| 2-nonanone | 0.08 | 0.14 | 0.50 | 0.22 | 0.09 |
| 2-decanone | 0.06 | 0.22 | 0.25 | 0.09 | 0.08 |
| Total ketones | 9.12 | 10.33 | 8.08 | 14.23 | 15.11 |
| Alcohols (13) | |||||
| Ethanol | 0.53 | 0.26 | 0.23 | 0.20 | 0.35 |
| 2-methyl-1-proponol | 0.06 | 0.10 | 0.14 | ND | 0.02 |
| 1-butanol | 3.04 | 1.55 | 2.02 | 3.16 | 4.07 |
| 2-butanol | 1.20 | 2.23 | 1.06 | 1.44 | 1.03 |
| 3-methyl-butanol | 0.34 | 0.50 | 0.45 | 0.88 | 0.43 |
| 1-pentanol | 1.75 | 1.24 | 2.03 | 3.23 | 2.40 |
| 1-penten-3-ol | ND | ND | ND | 0.05 | 0.12 |
| 1-hexanol | 0.25 | 0.10 | 0.42 | 0.17 | 0.16 |
| 1-heptanol | 0.20 | 0.13 | 0.14 | 0.04 | 0.07 |
| 1-octen-3-ol | 7.12 | 11.44 | 9.28 | 12.05 | 12.11 |
| 2-ethyl-1-hexanol | 1.78 | 1.23 | 0.24 | 1.01 | 1.44 |
| 2-octen-1-ol | 0.13 | ND | ND | ND | ND |
| 1-octanol | 3.72 | 2.77 | 2.05 | 3.06 | 2.02 |
| Total alcohols | 20.12 | 21.55 | 18.06 | 25.29 | 24.22 |
| Hydrocarbons (10) | |||||
| 1,1-diethoxy-ethane | 0.21 | 0.05 | 0.08 | ND | ND |
| Pentane | 1.44 | 1.42 | 1.30 | 2.21 | 2.64 |
| 3-methyl-pentane | 0.55 | 0.32 | 0.27 | 0.29 | 0.18 |
| Octane | 0.08 | 0.26 | 0.19 | 0.15 | 0.31 |
| Nonane | 0.52 | 0.89 | 0.20 | ND | 0.24 |
| 1-nitro-hexane | ND | 0.20 | 0.09 | ND | ND |
| Dodecane | 0.34 | ND | ND | ND | ND |
| Tetradecane | 1.01 | 0.08 | 0.10 | 0.14 | 0.13 |
| Toluene | 0.55 | 0.32 | 0.24 | 0.17 | 0.22 |
| Benzene | 2.75 | 1.07 | 1.22 | 0.45 | 0.23 |
| Total hydrocarbons | 7.45 | 4.61 | 3.69 | 3.41 | 3.95 |
| Acids (4) | |||||
| Hexanoic acid | 1.01 | 0.53 | 1.22 | 0.41 | 0.98 |
| Octanoic acid | 0.17 | 0.34 | 0.67 | 0.07 | 0.26 |
| Nonanoic acid | 0.10 | 0.09 | 0.32 | 0.04 | 0.26 |
| Tetradecanoic acid | 0.25 | 0.09 | 0.30 | ND | ND |
| Total acids | 1.53 | 1.05 | 2.51 | 0.52 | 1.50 |
| Esters (4) | |||||
| Ethyl acetate | 0.15 | ND | ND | ND | ND |
| Hexanoic acid, ethyl ester | 0.53 | 0.16 | 0.46 | 1.01 | 1.43 |
| Decanoic acid, ethyl ester | 0.31 | 0.09 | 0.21 | 0.90 | 0.42 |
| Octadecenoic acid, methyl ester | 0.67 | 0.20 | 0.34 | 0.51 | 0.27 |
| Total esters | 1.66 | 0.45 | 1.01 | 2.42 | 2.12 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saengsuk, N.; Sangsawad, P.; Paengkoum, P.; Pongsetkul, J. Lipid and Volatile Profiles of Various Goat Primal Cuts: Aspects of Nutritional Value and Flavor/Taste Attributes. Foods 2024, 13, 492. https://doi.org/10.3390/foods13030492
Saengsuk N, Sangsawad P, Paengkoum P, Pongsetkul J. Lipid and Volatile Profiles of Various Goat Primal Cuts: Aspects of Nutritional Value and Flavor/Taste Attributes. Foods. 2024; 13(3):492. https://doi.org/10.3390/foods13030492
Chicago/Turabian StyleSaengsuk, Nachomkamon, Papungkorn Sangsawad, Pramote Paengkoum, and Jaksuma Pongsetkul. 2024. "Lipid and Volatile Profiles of Various Goat Primal Cuts: Aspects of Nutritional Value and Flavor/Taste Attributes" Foods 13, no. 3: 492. https://doi.org/10.3390/foods13030492