Preparation and Investigation of Sustained-Release Nanocapsules Containing Cumin Essential Oil for Their Bacteriostatic Properties
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of CENPs
2.3. Characterization of CENPs
2.3.1. Particle Size and Zeta Potential
2.3.2. Embedding Efficiency (EE)
2.3.3. Scanning Electron Microscopy (SEM)
2.3.4. Microscopy
2.3.5. Confocal Laser Scanning Microscopy (CLSM)
2.3.6. Determination of CEO Release from CENPs
2.3.7. Differential Scanning Calorimetry (DSC)
2.3.8. Antibacterial Activity
2.4. Application of Nanocapsules on Mutton Preservation
Microbiological Analyses
2.5. Statistical Analysis
3. Results and Discussion
3.1. Encapsulation Efficiency (EE) of CENPs
3.2. Characterization of CENPs
3.2.1. Particle Size and Zeta Potential
3.2.2. Morphology of CENPs
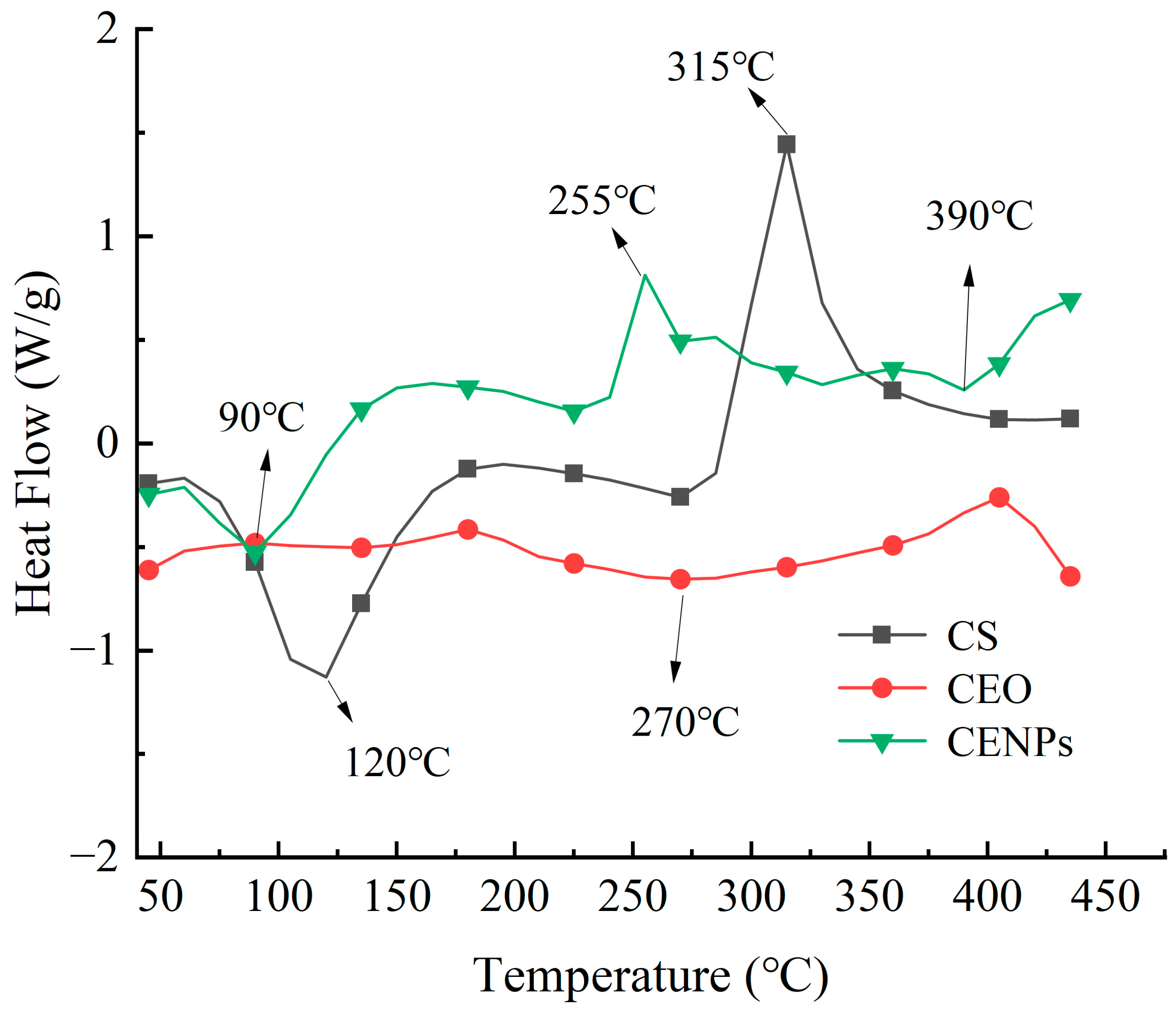
3.2.3. Thermal Stability
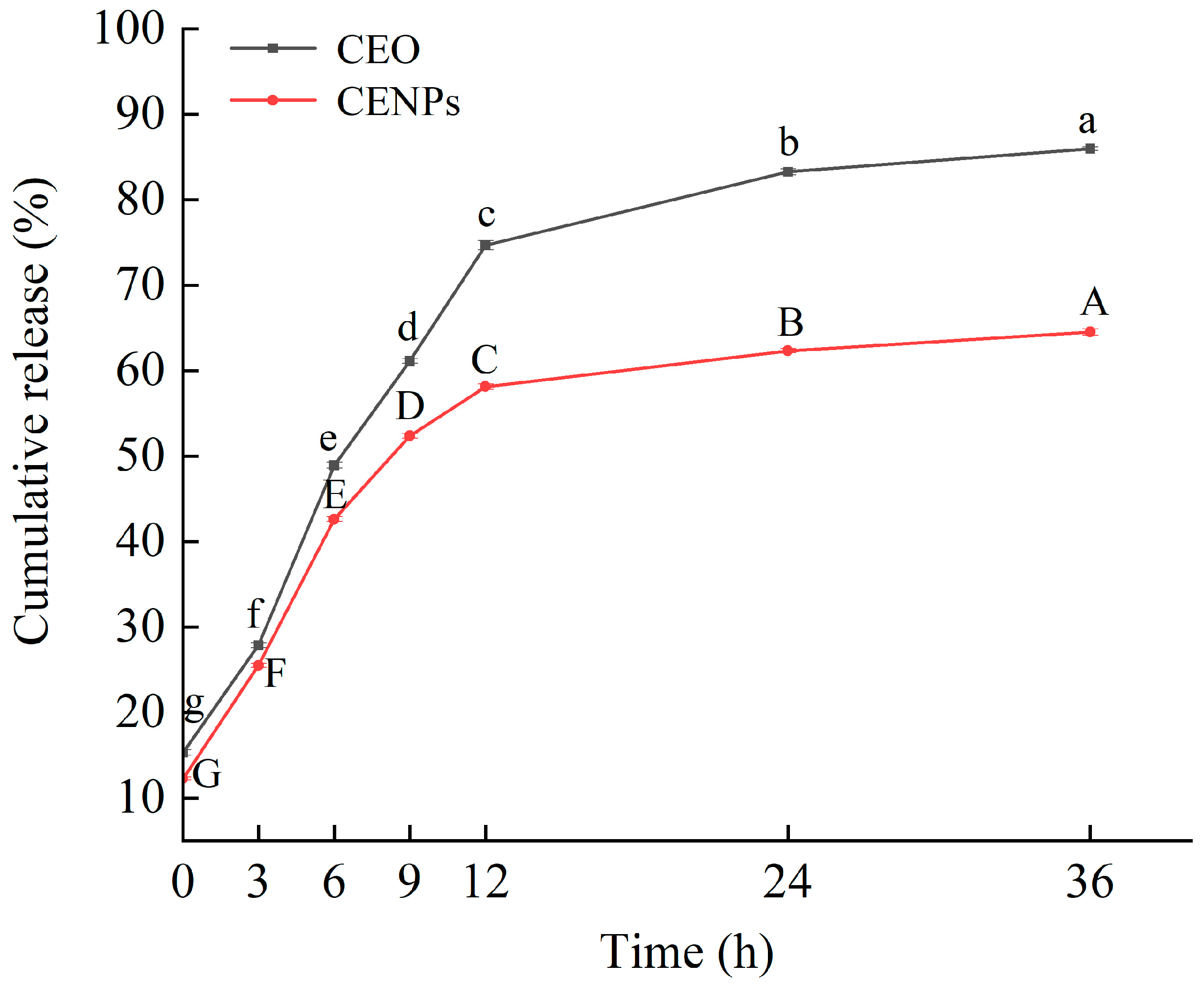
3.3. In Vitro Release of CENPs
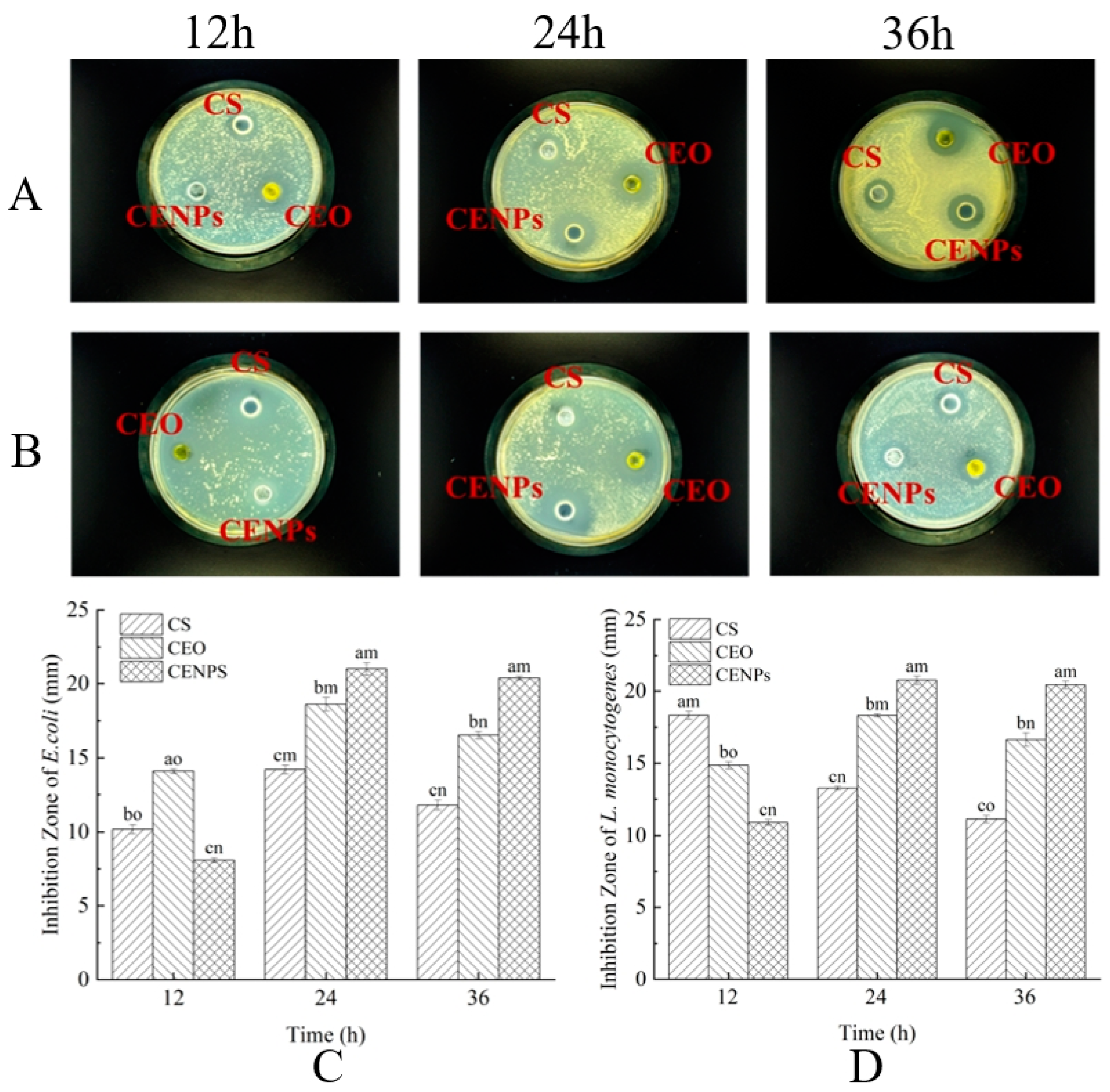
3.4. Comparison of Antibacterial Activity between CEO and CENPs
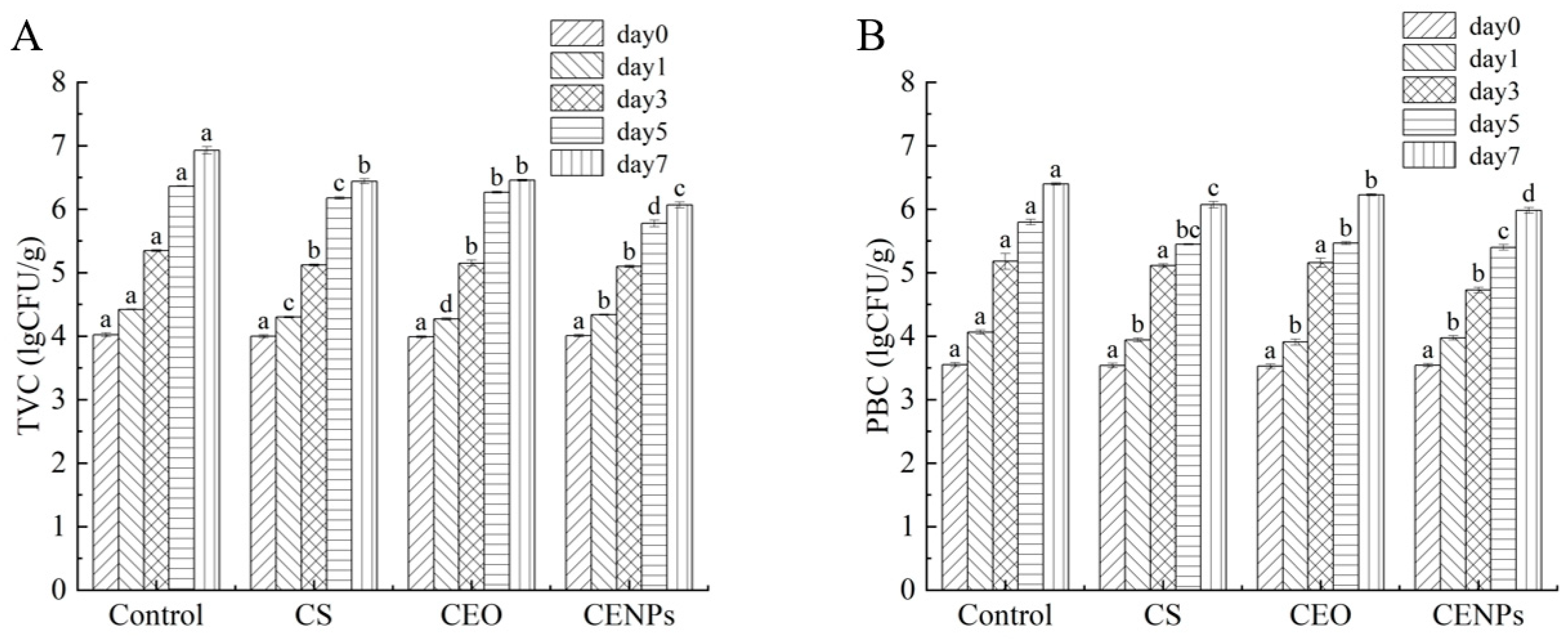
3.5. Microbiological Analyses of Mutton
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sharma, S.; Barkauskaite, S.; Jaiswal, A.K.; Jaiswal, S. Essential Oils as Additives in Active Food Packaging. Food Chem. 2021, 343, 128403. [Google Scholar] [CrossRef] [PubMed]
- Vianna, T.C.; Marinho, C.O.; Marangoni Júnior, L.; Ibrahim, S.A.; Vieira, R.P. Essential Oils as Additives in Active Starch-Based Food Packaging Films: A Review. Int. J. Biol. Macromol. 2021, 182, 1803–1819. [Google Scholar] [CrossRef]
- Aziz, M.; Karboune, S. Natural Antimicrobial/Antioxidant Agents in Meat and Poultry Products as Well as Fruits and Vegetables: A Review. Crit. Rev. Food Sci. Nutr. 2018, 58, 486–511. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Wang, Y.; Pan, D.-D.; Cao, J.-X.; Shao, X.-F.; Chen, Y.-J.; Sun, Y.-Y.; Ou, C.-R. Effect of Black Pepper Essential Oil on the Quality of Fresh Pork During Storage. Meat Sci. 2016, 117, 130–136. [Google Scholar] [CrossRef]
- Noori, S.; Zeynali, F.; Almasi, H. Antimicrobial and Antioxidant Efficiency of Nanoemulsion-Based Edible Coating Containing Ginger (Zingiber officinale) Essential Oil and Its Effect on Safety and Quality Attributes of Chicken Breast Fillets. Food Control 2018, 84, 312–320. [Google Scholar] [CrossRef]
- Dini, H.; Fallah, A.A.; Bonyadian, M.; Abbasvali, M.; Soleimani, M. Effect of Edible Composite Film Based on Chitosan and Cumin Essential Oil-Loaded Nanoemulsion Combined with Low-Dose Gamma Irradiation on Microbiological Safety and Quality of Beef Loins During Refrigerated Storage. Int. J. Biol. Macromol. 2020, 164, 1501–1509. [Google Scholar] [CrossRef]
- Kedia, A.; Prakash, B.; Mishra, P.K.; Dubey, N.K. Antifungal and Antiaflatoxigenic Properties of Cuminum cyminum (L.) Seed Essential Oil and Its Efficacy as a Preservative in Stored Commodities. Int. J. Food Microbiol. 2014, 168–169, 1–7. [Google Scholar] [CrossRef]
- Alizadeh Behbahani, B.; Noshad, M.; Falah, F. Cumin Essential Oil: Phytochemical Analysis, Antimicrobial Activity and Investigation of Its Mechanism of Action through Scanning Electron Microscopy. Microb. Pathog. 2019, 136, 103716. [Google Scholar] [CrossRef]
- Shanbehzadeh, F.; Saei-Dehkordi, S.S.; Semnani, D. Fabrication and Characterization of Electrospun Nanofibrous Mats of Polycaprolactone/Gelatin Containing Zno Nanoparticles and Cumin Essential Oil and Their Anti-Staphylococcal Potency in White Cheese. Food Biosci. 2022, 49, 101904. [Google Scholar] [CrossRef]
- Hasani-Javanmardi, M.; Fallah, A.A.; Abbasvali, M. Effect of Safflower Oil Nanoemulsion and Cumin Essential Oil Combined with Oxygen Absorber Packaging on the Quality and Shelf-Life of Refrigerated Lamb Loins. LWT 2021, 147, 111557. [Google Scholar] [CrossRef]
- Zhang, W.; Jiang, H.; Rhim, J.-W.; Cao, J.; Jiang, W. Effective Strategies of Sustained Release and Retention Enhancement of Essential Oils in Active Food Packaging Films/Coatings. Food Chem. 2022, 367, 130671. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Ezati, P.; Khan, A.; Assadpour, E.; Rhim, J.-W.; Jafari, S.M. Encapsulation and Delivery Systems of Cinnamon Essential Oil for Food Preservation Applications. Adv. Colloid Interface Sci. 2023, 318, 102965. [Google Scholar] [CrossRef] [PubMed]
- Rao, J.P.; Geckeler, K.E. Polymer Nanoparticles: Preparation Techniques and Size-Control Parameters. Prog. Polym. Sci. 2011, 36, 887–913. [Google Scholar] [CrossRef]
- dos Santos, P.P.; Flôres, S.H.; de Oliveira Rios, A.; Chisté, R.C. Biodegradable Polymers as Wall Materials to the Synthesis of Bioactive Compound Nanocapsules. Trends Food Sci. Technol. 2016, 53, 23–33. [Google Scholar] [CrossRef]
- Benavides, S.; Cortés, P.; Parada, J.; Franco, W. Development of Alginate Microspheres Containing Thyme Essential Oil Using Ionic Gelation. Food Chem. 2016, 204, 77–83. [Google Scholar] [CrossRef] [PubMed]
- Shetta, A.; Kegere, J.; Mamdouh, W. Comparative Study of Encapsulated Peppermint and Green Tea Essential Oils in Chitosan Nanoparticles: Encapsulation, Thermal Stability, in-Vitro Release, Antioxidant and Antibacterial Activities. Int. J. Biol. Macromol. 2019, 126, 731–742. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Chen, H.; Zhang, L.; Xu, Y. Clove Essential Oil Loaded Chitosan Nanocapsules on Quality and Shelf-Life of Blueberries. Int. J. Biol. Macromol. 2023, 249, 126091. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Liang, Y.; Li, X.; Kang, H. Effect of Chitosan-Gelatin Coating Containing Nano-Encapsulated Tarragon Essential Oil on the Preservation of Pork Slices. Meat Sci. 2020, 166, 108137. [Google Scholar] [CrossRef]
- Negi, H.; Verma, P.; Singh, R.K. A Comprehensive Review on the Applications of Functionalized Chitosan in Petroleum Industry. Carbohydr. Polym. 2021, 266, 118125. [Google Scholar] [CrossRef]
- Riseh, R.S.; Hassanisaadi, M.; Vatankhah, M.; Babaki, S.A.; Barka, E.A. Chitosan as a Potential Natural Compound to Manage Plant Diseases. Int. J. Biol. Macromol. 2022, 220, 998–1009. [Google Scholar] [CrossRef]
- Hosseini, S.F.; Zandi, M.; Rezaei, M.; Farahmandghavi, F. Two-Step Method for Encapsulation of Oregano Essential Oil in Chitosan Nanoparticles: Preparation, Characterization and in Vitro Release Study. Carbohydr. Polym. 2013, 95, 50–56. [Google Scholar] [CrossRef] [PubMed]
- Keawchaoon, L.; Yoksan, R. Preparation, Characterization and in Vitro Release Study of Carvacrol-Loaded Chitosan Nanoparticles. Colloids Surf. B 2011, 84, 163–171. [Google Scholar] [CrossRef] [PubMed]
- Huang, P.; Huang, C.; Ma, X.; Gao, C.; Sun, F.; Yang, N.; Nishinari, K. Effect of Ph on the Mechanical, Interfacial, and Emulsification Properties of Chitosan Microgels. Food Hydrocoll. 2021, 121, 106972. [Google Scholar] [CrossRef]
- Feyzioglu, G.C.; Tornuk, F. Development of Chitosan Nanoparticles Loaded with Summer Savory (Satureja hortensis L.) Essential Oil for Antimicrobial and Antioxidant Delivery Applications. LWT 2016, 70, 104–110. [Google Scholar] [CrossRef]
- GB 4789.2-2022; Food Microbiological Examination. China National Standard. Standardization Administration of China: Beijing, China, 2022.
- Hu, J.; Zhang, Y.; Xiao, Z.; Wang, X. Preparation and Properties of Cinnamon-Thyme-Ginger Composite Essential Oil Nanocapsules. Ind. Crops Prod. 2018, 122, 85–92. [Google Scholar] [CrossRef]
- Ma, Q.; Zhang, Y.; Critzer, F.; Davidson, P.M.; Zivanovic, S.; Zhong, Q. Physical, Mechanical, and Antimicrobial Properties of Chitosan Films with Microemulsions of Cinnamon Bark Oil and Soybean Oil. Food Hydrocoll. 2016, 52, 533–542. [Google Scholar] [CrossRef]
- Liu, C.-G.; Desai, K.G.H.; Chen, X.-G.; Park, H.-J. Linolenic Acid-Modified Chitosan for Formation of Self-Assembled Nanoparticles. J. Agric. Food Chem. 2005, 53, 437–441. [Google Scholar] [CrossRef] [PubMed]
- Dickinson, E. Hydrocolloids as Emulsifiers and Emulsion Stabilizers. Food Hydrocoll. 2009, 23, 1473–1482. [Google Scholar] [CrossRef]
- Shao, Y.; Wu, C.; Wu, T.; Li, Y.; Chen, S.; Yuan, C.; Hu, Y. Eugenol-Chitosan Nanoemulsions by Ultrasound-Mediated Emulsification: Formulation, Characterization and Antimicrobial Activity. Carbohydr. Polym. 2018, 193, 144–152. [Google Scholar] [CrossRef]
- Du, W.-L.; Xu, Y.-L.; Xu, Z.-R.; Fan, C.-L. Preparation, Characterization and Antibacterial Properties against E. Coli k88 of Chitosan Nanoparticle Loaded Copper Ions. Nanotechnology 2008, 19, 085707. [Google Scholar] [CrossRef]
- Hasani, M.; Elhami-Rad, A.; Mohammadhosseini, M.; Shahidi Noghabi, M. Physicochemical Characteristic of Microencapsulated Fish Oil by Freeze-Drying Using Different Combinations of Wall Materials. Biosci. Biotechnol. Res. Asia 2015, 12, 45–51. [Google Scholar] [CrossRef]
- Zhu, H.; Zhang, Y.; Tian, J.; Chu, Z. Effect of a New Shell Material—Jackfruit Seed Starch on Novel Flavor Microcapsules Containing Vanilla Oil. Ind. Crops Prod. 2018, 112, 47–52. [Google Scholar] [CrossRef]
- Hasani, S.; Ojagh, S.M.; Ghorbani, M. Nanoencapsulation of Lemon Essential Oil in Chitosan-Hicap System. Part 1: Study on Its Physical and Structural Characteristics. Int. J. Biol. Macromol. 2018, 115, 143–151. [Google Scholar] [CrossRef]
- Wang, Y.; Li, L.; Hu, J. Development of Biobased Multifunctional Films Incorporated with Essential Oils@Polydopamine Nanocapsules for Food Preservation Applications. Int. J. Biol. Macromol. 2023, 253, 127161. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.; Liu, A.; Hu, A.; Li, J.; Zen, Z.; Liu, Y.; Tang, S.; Li, C. Preparation and Characterization of Cinnamon Essential Oil Nanocapsules and Comparison of Volatile Components and Antibacterial Ability of Cinnamon Essential Oil before and after Encapsulation. Food Control 2021, 123, 107783. [Google Scholar] [CrossRef]
- Cai, C.; Ma, R.; Duan, M.; Lu, D. Preparation and Antimicrobial Activity of Thyme Essential Oil Microcapsules Prepared with Gum Arabic. RSC Adv. 2019, 9, 19740–19747. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Tang, Q.; Lei, H.; Zhen, X.; Zheng, N.; Qiu, P.; Liu, L.; Zhao, J. Preparation, Physicochemical and Biological Evaluation of Chitosan Pleurotus Ostreatus Polysaccharides Active Films for Food Packaging. Int. J. Biol. Macromol. 2024, 254, 127470. [Google Scholar] [CrossRef] [PubMed]
- Timilsena, Y.P.; Adhikari, R.; Kasapis, S.; Adhikari, B. Molecular and Functional Characteristics of Purified Gum from Australian Chia Seeds. Carbohydr. Polym. 2016, 136, 128–136. [Google Scholar] [CrossRef] [PubMed]
- Zohuriaan, M.J.; Shokrolahi, F. Thermal Studies on Natural and Modified Gums. Polym. Test. 2004, 23, 575–579. [Google Scholar] [CrossRef]
- Hariharan, S.; Bhardwaj, V.; Bala, I.; Sitterberg, J.; Bakowsky, U.; Ravi Kumar, M.N.V. Design of Estradiol Loaded Plga Nanoparticulate Formulations: A Potential Oral Delivery System for Hormone Therapy. Pharm. Res. 2006, 23, 184–195. [Google Scholar] [CrossRef] [PubMed]
- Anitha, A.; Deepagan, V.G.; Divya Rani, V.V.; Menon, D.; Nair, S.V.; Jayakumar, R. Preparation, Characterization, in Vitro Drug Release and Biological Studies of Curcumin Loaded Dextran Sulphate–Chitosan Nanoparticles. Carbohydr. Polym. 2011, 84, 1158–1164. [Google Scholar] [CrossRef]
- Frazier, A.N.; Yang, H. Utilizing a Crispr-Cas9 Targeted Foodborne Pathogen Antimicrobial within Livestock Feed: A Market Research Perspective. J. Agric. Food Chem. 2023, 14, 100758. [Google Scholar] [CrossRef]
- El-Zehery, H.R.A.; Zaghloul, R.A.; Abdel-Rahman, H.M.; Salem, A.A.; El-Dougdoug, K.A. Novel Strategies of Essential Oils, Chitosan, and Nano- Chitosan for Inhibition of Multi-Drug Resistant: E. coli O157:H7 and Listeria Monocytogenes. Saudi J. Biol. Sci. 2022, 29, 2582–2590. [Google Scholar] [CrossRef]
- Sun, L.; Sun, J.; Chen, L.; Niu, P.; Yang, X.; Guo, Y. Preparation and Characterization of Chitosan Film Incorporated with Thinned Young Apple Polyphenols as an Active Packaging Material. Carbohydr. Polym. 2017, 163, 81–91. [Google Scholar] [CrossRef]
- Souza, V.G.L.; Pires, J.R.; Rodrigues, P.F.; Lopes, A.A.; Fernandes, F.M.; Duarte, M.P.; Coelhoso, I.M.; Fernando, A.L. Bionanocomposites of Chitosan/Montmorillonite Incorporated with Rosmarinus officinalis Essential Oil: Development and Physical Characterization. Food Packaging Shelf. 2018, 16, 148–156. [Google Scholar] [CrossRef]
- Wei, Q.; Wang, X.; Sun, D.-W.; Pu, H. Rapid Detection and Control of Psychrotrophic Microorganisms in Cold Storage Foods: A Review. Trends Food Sci. Technol. 2019, 86, 453–464. [Google Scholar] [CrossRef]
- Özogul, Y.; Durmus, M.; Ucar, Y.; Özogul, F.; Regenstein, J.M. Comparative Study of Nanoemulsions Based on Commercial Oils (Sunflower, Canola, Corn, Olive, Soybean, and Hazelnut Oils): Effect on Microbial, Sensory, and Chemical Qualities of Refrigerated Farmed Sea Bass. Innov. Food Sci. Emerg. Technol. 2016, 33, 422–430. [Google Scholar] [CrossRef]
- Yazgan, H.; Ozogul, Y.; Durmuş, M.; Balikçi, E.; Gökdoğan, S.; Uçar, Y.; Aksun, E.T. Effects of Oil-in-Water Nanoemulsion Based on Sunflower Oil on the Quality of Farmed Sea Bass and Gilthead Sea Bream Stored at Chilled Temperature (2 ± 2 °C). J. Aquat. Food Prod. Technol. 2017, 26, 979–992. [Google Scholar] [CrossRef]
- Durmuş, M.; Ozogul, Y.; Köşker, A.R.; Ucar, Y.; Boğa, E.K.; Ceylan, Z.; Ozogul, F. The Function of Nanoemulsion on Preservation of Rainbow Trout Fillet. J. Food Sci. Technol. 2020, 57, 895–904. [Google Scholar] [CrossRef]
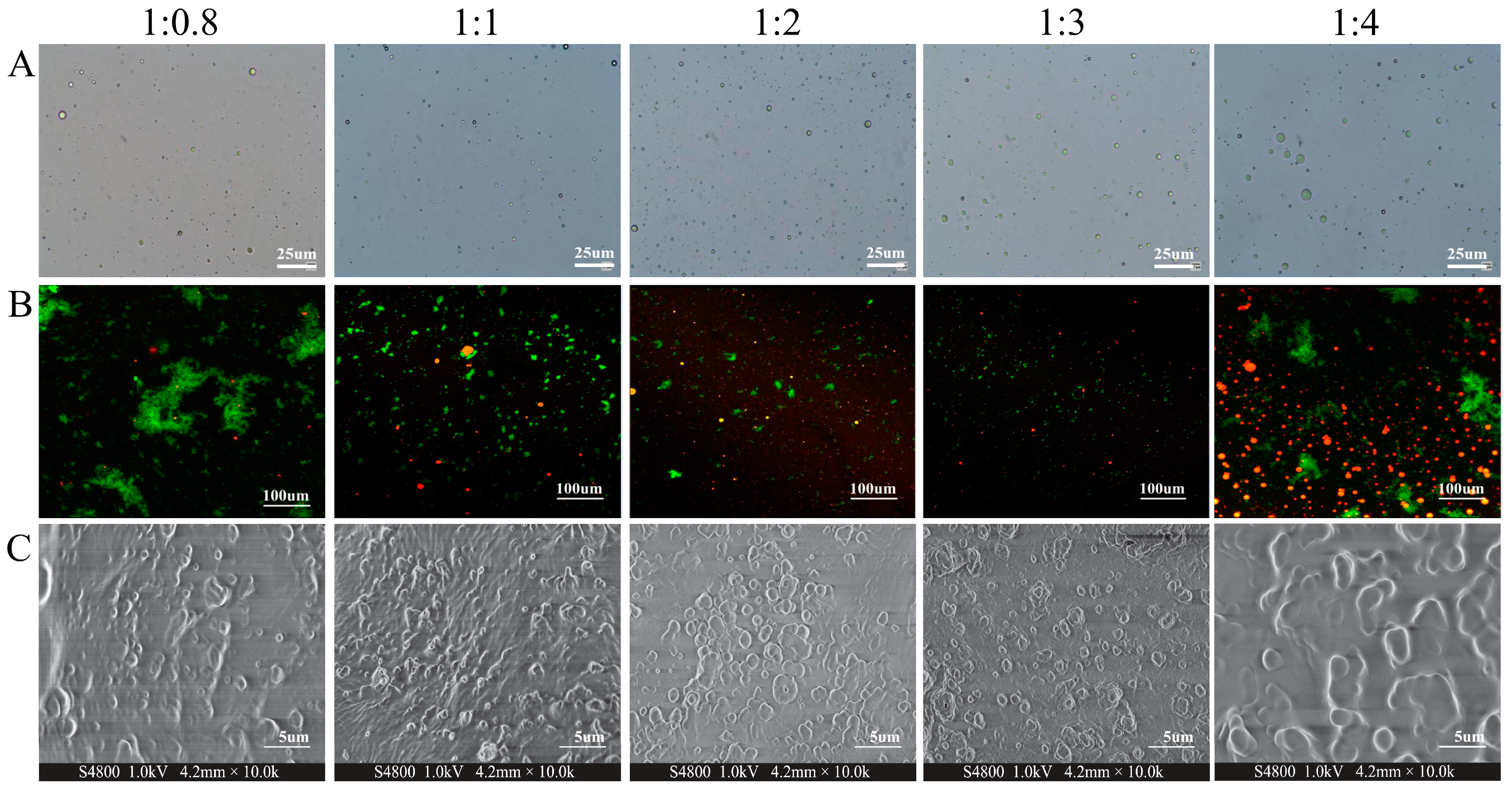
| CS/CEO Ratio | Particle Size (nm) | Zeta Potential (mV) | PDI | EE (%) |
|---|---|---|---|---|
| 1:0.8 | 810.00 ± 2.70 a | 29.03 ± 0.31 b | 0.276 ± 0.02 b | 38.13 ± 0.35 c |
| 1:1 | 605.00 ± 1.73 c | 25.80 ± 0.32 e | 0.292 ± 0.01 b | 42.10 ± 0.25 b |
| 1:2 | 609.33 ± 2.51 c | 27.93 ± 0.28 c | 0.306 ± 0.02 b | 42.04 ± 0.18 b |
| 1:3 | 584.67 ± 2.62 d | 33.77 ± 0.15 a | 0.354 ± 0.02 a | 52.00 ± 0.27 a |
| 1:4 | 618.67 ± 2.52 b | 27.13 ± 0.21 d | 0.143 ± 0.01 c | 37.68 ± 0.20 c |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, M.; Li, M.; Zhang, D.; Yu, Y.; Zhu, K.; Zang, X.; Liu, D. Preparation and Investigation of Sustained-Release Nanocapsules Containing Cumin Essential Oil for Their Bacteriostatic Properties. Foods 2024, 13, 947. https://doi.org/10.3390/foods13060947
Zhang M, Li M, Zhang D, Yu Y, Zhu K, Zang X, Liu D. Preparation and Investigation of Sustained-Release Nanocapsules Containing Cumin Essential Oil for Their Bacteriostatic Properties. Foods. 2024; 13(6):947. https://doi.org/10.3390/foods13060947
Chicago/Turabian StyleZhang, Mingcheng, Mingyang Li, Danyang Zhang, Ying Yu, Kaixian Zhu, Xiaodan Zang, and Dengyong Liu. 2024. "Preparation and Investigation of Sustained-Release Nanocapsules Containing Cumin Essential Oil for Their Bacteriostatic Properties" Foods 13, no. 6: 947. https://doi.org/10.3390/foods13060947





