Sea Cucumber Polysaccharide from Stichopus japonicu and Its Photocatalytic Degradation Product Alleviate Acute Alcoholic Liver Injury in Mice
Abstract
:1. Introduction
2. Material and Methods
2.1. Preparation and Degradation
2.2. High-Performance Gel Permeation Chromatography (HPGPC) Analysis
2.3. Thin Layer Chromatography (TLC) Analysis
2.4. Chemical Composition Analysis
2.5. Monosaccharide Composition Analysis of SCSP and Its Degradation Products
2.6. HPLC-PDA-MSn Analysis
2.7. Fourier Transform-Infrared (FT-IR) Spectroscopy
2.8. NMR Spectroscopic Analysis
2.9. Animal Experiments
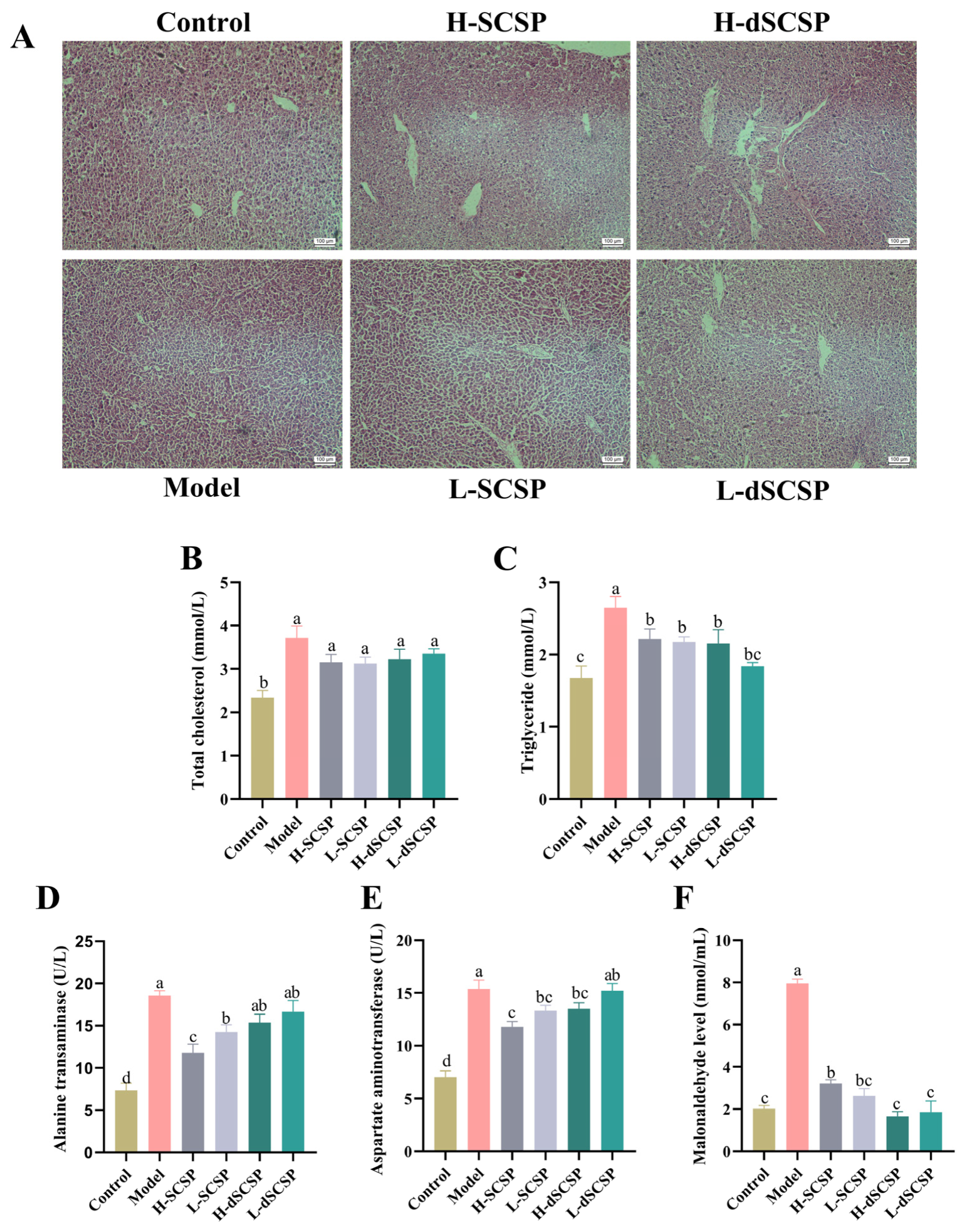
2.10. Hepatic Histological Analysis
2.11. Biochemical Index Detection
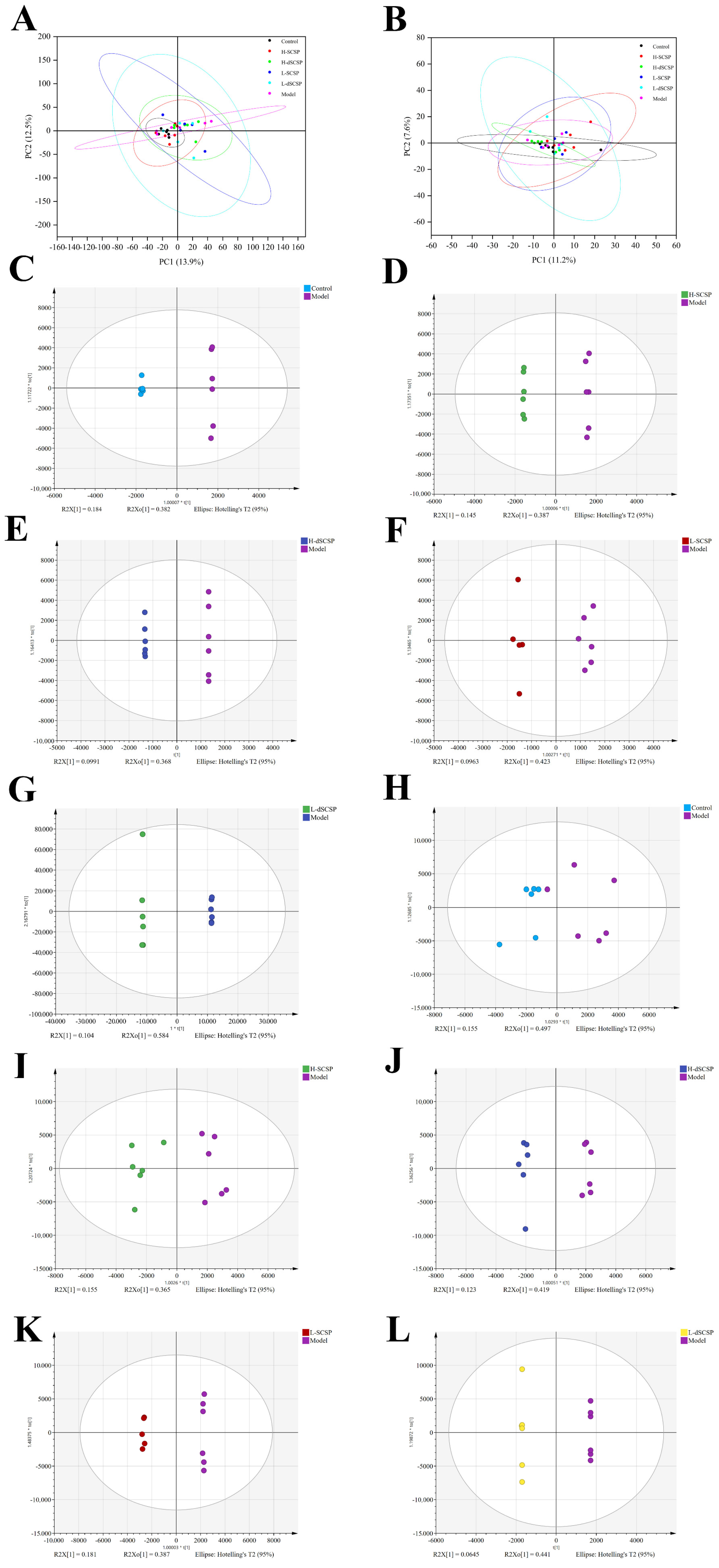
2.12. Lipidomics Analysis
2.13. Statistical Analysis
3. Results
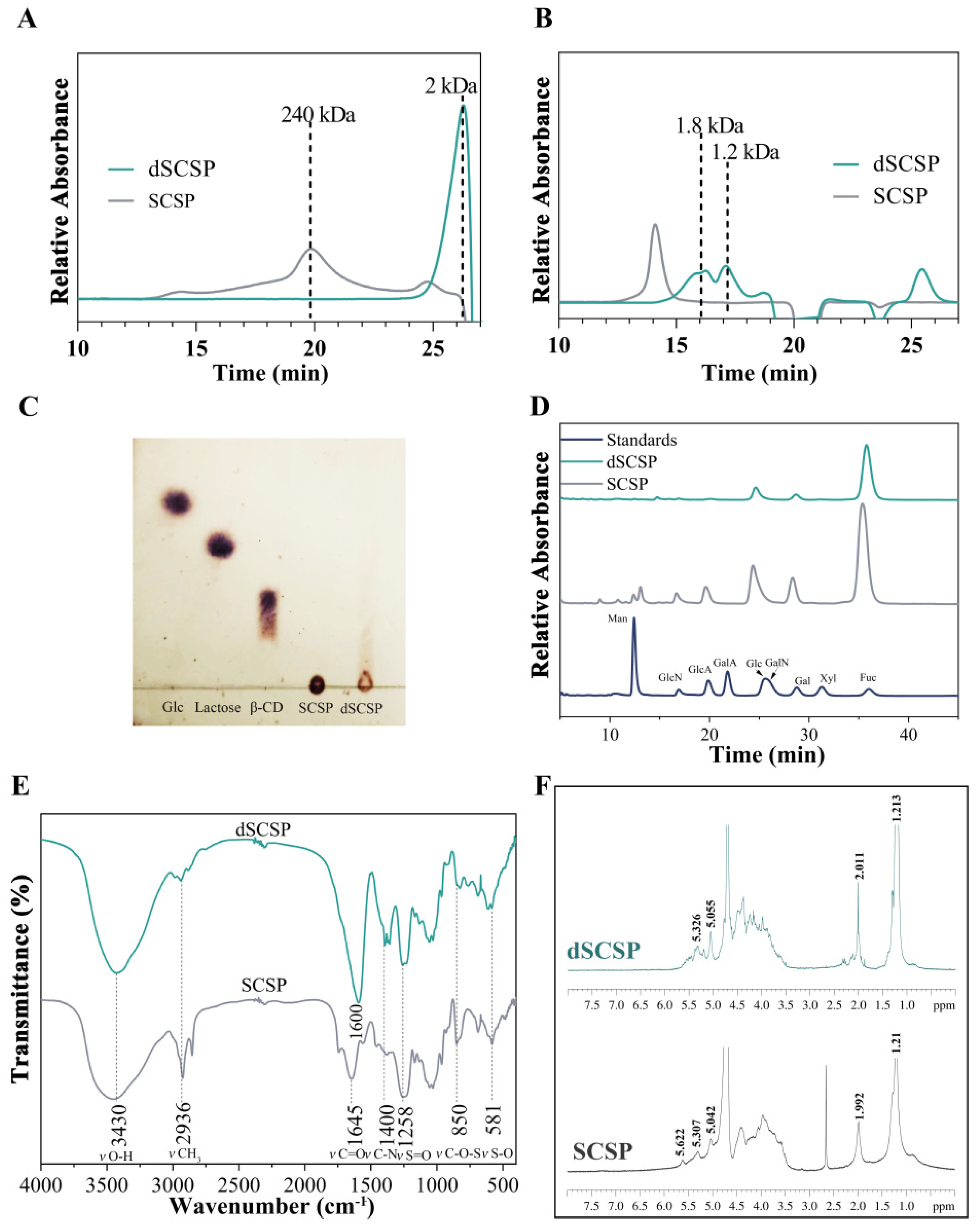
3.1. Characterization of SCSP and Its Degradation Products
3.2. Identification of Oligosaccharides in dSCSP
3.3. Addition of SCSP and dSCSP in Baijiu Reduces Alcoholic Liver Injury
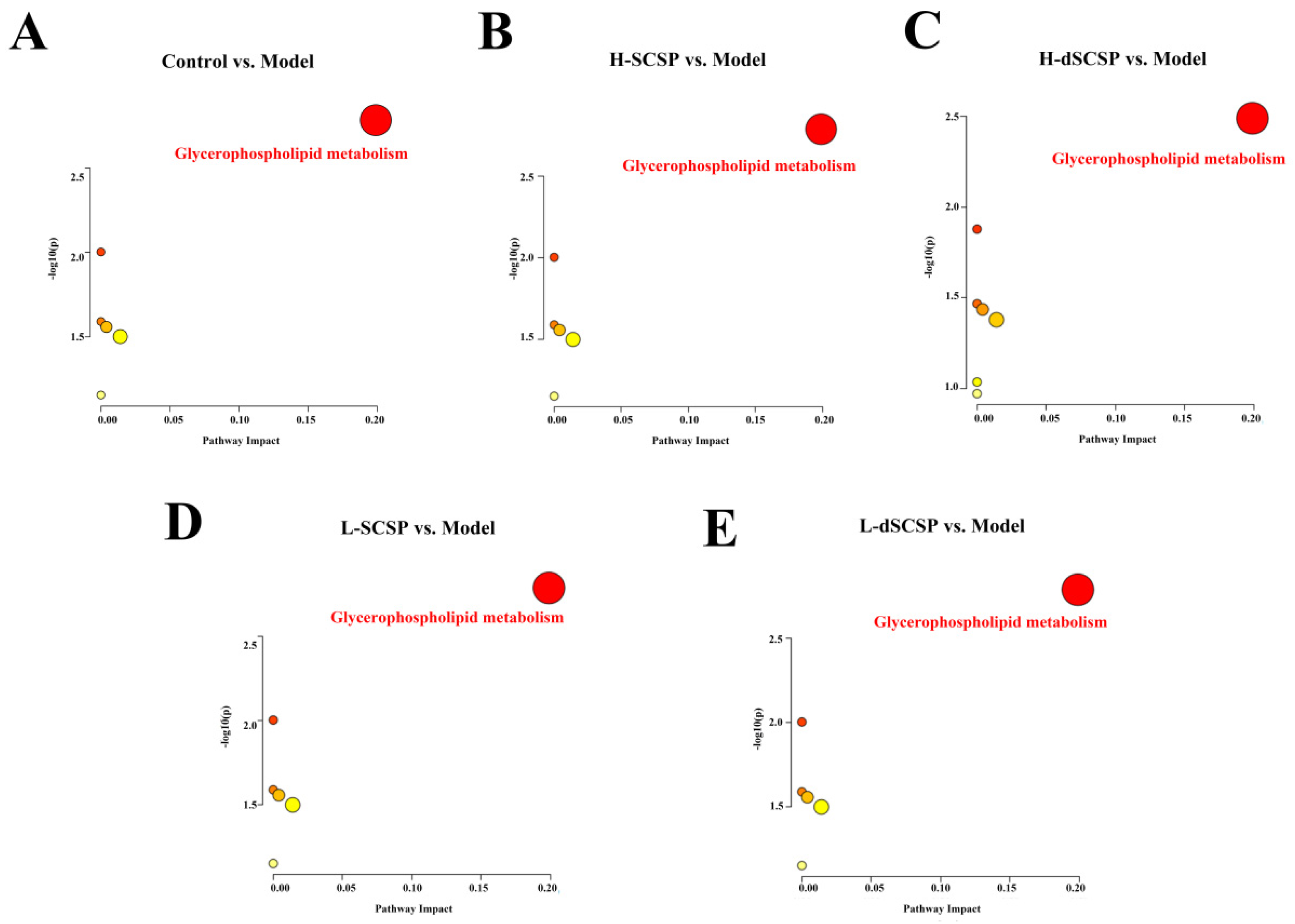
3.4. Regulation of SCSP/dSCSP on Hepatic Lipid Metabolism
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mourão, P.; Bastos, I. Highly acidic glycans from sea cucumbers. Isolation and fractionation of fucose-rich sulfated polysaccharides from the body wall of Ludwigothurea grisea. Eur. J. Biochem. 1987, 166, 639–645. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.; Zhang, L.; Tang, X.; Hu, W.; Zhou, P. Major yolk protein from sea cucumber (Stichopus japonicus) attenuates acute colitis via regulation of microbial dysbiosis and inflammatory responses. Food Res. Int. 2022, 151, 110841. [Google Scholar] [CrossRef] [PubMed]
- Mishra, V.; Tomar, S.; Yadav, P.; Singh, M.P. Promising anticancer activity of polysaccharides and other macromolecules derived from oyster mushroom (Pleurotus sp.): An updated review. Int. J. Biol. Macromol. 2021, 182, 1628–1637. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Q.; Lin, L.; Zhao, M. Sulfated fucan/fucosylated chondroitin sulfate-dominated polysaccharide fraction from low-edible-value sea cucumber ameliorates type 2 diabetes in rats: New prospects for sea cucumber polysaccharide based-hypoglycemic functional food. Int. J. Biol. Macromol. 2020, 159, 34–45. [Google Scholar] [CrossRef] [PubMed]
- Gong, P.; Wu, Y.; Liu, Y.; Lv, S.; You, Y.; Zhou, Z.; Chen, X.; Li, H. Structure and hypoglycemic effect of a neutral polysaccharide isolated from sea cucumber Stichopus japonicus. Int. J. Biol. Macromol. 2022, 216, 14–23. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Yuan, Q.; Lv, K.; Ma, H.; Gao, C.; Liu, Y.; Zhang, S.; Zhao, L. Low-molecular-weight fucosylated glycosaminoglycan and its oligosaccharides from sea cucumber as novel anticoagulants: A review. Carbohydr. Polym. 2021, 251, 117034. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Gao, N.; Zuo, Z.; Li, S.; Zheng, W.; Shi, X.; Liu, Q.; Ma, T.; Yin, R.; Li, X.; et al. Five distinct fucan sulfates from sea cucumber Pattalus mollis: Purification, structural characterization and anticoagulant activities. Int. J. Biol. Macromol. 2021, 186, 535–543. [Google Scholar] [CrossRef]
- Li, Q.; Jiang, S.; Shi, W.; Qi, X.; Song, W.; Mou, J.; Yang, J. Structure characterization, antioxidant and immunoregulatory properties of a novel fucoidan from the sea cucumber Stichopus chloronotus. Carbohydr. Polym. 2020, 231, 115767. [Google Scholar] [CrossRef]
- Mou, J.; Li, Q.; Qi, X.; Yang, J. Structural comparison, antioxidant and anti-inflammatory properties of fucosylated chondroitin sulfate of three edible sea cucumbers. Carbohydr. Polym. 2018, 185, 41–47. [Google Scholar] [CrossRef]
- Lee, H.-G.; Kim, H.-S.; Oh, J.-Y.; Lee, D.-S.; Yang, H.-W.; Kang, M.-C.; Kim, E.-A.; Kang, N.; Kim, J.; Heo, S.-J.; et al. Potential antioxidant properties of enzymatic hydrolysates from Stichopus japonicus against hydrogen peroxide-induced oxidative stress. Antioxidants 2021, 10, 110. [Google Scholar] [CrossRef]
- Liu, X.; Sun, Z.; Zhang, M.; Meng, X.; Xia, X.; Yuan, W.; Xue, F.; Liu, C. Antioxidant and antihyperlipidemic activities of polysaccharides from sea cucumber Apostichopus japonicus. Carbohydr. Polym. 2012, 90, 1664–1670. [Google Scholar] [CrossRef]
- Zhu, Z.; Zhu, B.; Sun, Y.; Ai, C.; Wu, S.; Wang, L.; Song, S.; Liu, X. Sulfated polysaccharide from sea cucumber modulates the gut microbiota and its metabolites in normal mice. Int. J. Biol. Macromol. 2018, 120, 502–512. [Google Scholar] [CrossRef] [PubMed]
- Zhaz, F.; Liu, Q.; Cao, J.; Xu, Y.; Li, C. A sea cucumber (Holothuria leucospilota) polysaccharide improves the gut microbiome to alleviate the symptoms of type 2 diabetes mellitus in Goto-Kakizaki rats. Food Chem. Toxicol. 2019, 135, 110886. [Google Scholar]
- Zhu, Z.; Dong, X.; Yan, C.; Ai, C.; Zhou, D.; Yang, J.; Zhang, H.; Liu, X.; Song, S.; Xiao, H.; et al. Stichopus japonicus structural features and digestive behavior of fucosylated chondroitin sulfate from sea cucumbers. J. Agric. Food Chem. 2019, 67, 10534–10542. [Google Scholar] [CrossRef] [PubMed]
- Hao, J.; Ye, L.; Meng, G.; Song, Y.; Fu, J.; Wu, X. The protective effect and crucial biological pathways analysis of Trametes lactinea mycelium polysaccharides on acute alcoholic liver injury in mice based on transcriptomics and metabonomics. Food Sci. Hum. Wellness 2021, 10, 480–489. [Google Scholar] [CrossRef]
- Gao, B.; Bataller, R. Alcoholic liver disease: Pathogenesis and new therapeutic targets. Gastroenterology 2011, 141, 1572–1585. [Google Scholar] [CrossRef] [PubMed]
- Ishii, H.; Kurose, I.; Kato, S. Pathogenesis of alcoholic liver disease with particular emphasis on oxidative stress. J. Gastroenterol. Hepatol. 1997, 12, S272–S282. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Su, W.; Zhang, C.; Xue, C.; Chang, Y.; Wu, X.; Tang, Q.; Wang, J. Protective effect of sea cucumber (Acaudina molpadioides) fucoidan against ethanol-induced gastric damage. Food Chem. 2012, 133, 1414–1419. [Google Scholar] [CrossRef]
- Qi, Y.; Wang, L.; You, Y.; Sun, X.; Wen, C.; Fu, Y.; Song, S. Preparation of low-molecular-weight fucoidan with anticoagulant activity by photocatalytic degradation method. Foods 2022, 11, 822. [Google Scholar] [CrossRef]
- Saravana, P.; Cho, Y.; Park, Y.; Woo, H.; Chun, B. Structural, antioxidant, and emulsifying activities of fucoidan from Saccharina japonica using pressurized liquid extraction. Carbohydr. Polym. 2016, 153, 518–525. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, F.; Xu, Z.; Ding, Z. Bioactive Mushroom Polysaccharides: A Review on Monosaccharide Composition, Biosynthesis and Regulation. Molecules 2017, 22, 955. [Google Scholar] [CrossRef]
- Song, S.; Zhang, B.; Wu, S.; Huang, L.; Ai, C.; Pan, J.; Su, Y.; Wang, Z.; Wen, C. Structural characterization and osteogenic bioactivity of a sulfated polysaccharide from pacific abalone (Haliotis discus hannai Ino). Carbohydr. Polym. 2018, 182, 207–214. [Google Scholar] [CrossRef]
- Ming, Y.N.; Zhang, J.Y.; Wang, X.L.; Li, C.M.; Ma, S.C.; Wang, Z.Y.; Liu, X.L.; Li, X.B.; Mao, Y.M. Liquid chromatography mass spectrometry-based profiling of phosphatidylcholine and phosphatidylethanolamine in the plasma and liver of acetaminophen-induced liver injured mice. Lipids Health Dis. 2017, 16, 153. [Google Scholar] [CrossRef]
- Zhang, L.; Na, X.; Lai, B.; Song, Y.; Wang, H.; Tan, M. Effects of fluorescent carbon dots from the baked lamb on energy and lipid metabolism. Food Chem. 2021, 338, 127832. [Google Scholar] [CrossRef]
- Xuan, Q.; Hu, C.; Yu, D.; Wang, L.; Zhou, Y.; Zhao, X.; Li, Q.; Hou, X.; Xu, G. Development of a high coverage pseudotargeted lipidomics method based on ultra-high performance liquid chromatography–mass spectrometry. Anal. Chem. 2018, 90, 7608–7616. [Google Scholar] [CrossRef]
- Zheng, W.; Zhou, L.; Lin, L.; Cai, Y.; Sun, H.; Zhao, L.; Gao, N.; Yin, R.; Zhao, J. Physicochemical characteristics and anticoagulant activities of the polysaccharides from sea cucumber Pattalus mollis. Mar. Drugs 2019, 17, 198. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Owoseni, E.; Salamat, J.; Cederbaum, A.I.; Lu, Y. Nicotine enhances alcoholic fatty liver in mice: Role of CYP2A5. Arch. Biochem. Biophys. 2018, 657, 65–73. [Google Scholar] [CrossRef] [PubMed]
- Yuan, R.; Tao, X.; Liang, S.; Pan, Y.; He, L.; Sun, J.; Wenbo, J.; Li, X.; Chen, J.; Wang, C. Protective effect of acidic polysaccharide from Schisandra chinensis on acute ethanol-induced liver injury through reducing CYP2E1-dependent oxidative stress. Biomed. Pharmacother. 2018, 99, 537–542. [Google Scholar] [CrossRef] [PubMed]
- Yimam, M.; Jiao, P.; Hong, M.; Jia, Q. A Standardized composition from extracts of Myristica Fragrans, Astragalus Membranaceus, and Poria Cocos protects liver from acute ethanol insult. J. Med. Food 2016, 19, 780–788. [Google Scholar] [CrossRef] [PubMed]
- Boukhris, M.; Tomasello, S.; Marzà, F.; Bregante, S.; Pluchinotta, F.; Galassi, A. Coronary Heart Disease in Postmenopausal Women with Type II Diabetes Mellitus and the Impact of Estrogen Replacement Therapy: A Narrative Review. Int. J. Endocrinol. 2014, 2014, 413920. [Google Scholar] [CrossRef] [PubMed]
- Ciccone, M.; Cortese, F.; Gesualdo, M.; Carbonara, S.; Zito, A.; Ricci, G.; De Pascalis, F.; Scicchitano, P.; Riccioni, G. Dietary intake of carotenoids and their antioxidant and anti-inflammatory effects in cardiovascular care. Mediat. Inflamm. 2013, 2013, 782137. [Google Scholar] [CrossRef] [PubMed]
- Zimmet, P.; Alberti, K.G.; Shaw, J. Global and societal implications of the diabetes epidemic. Nature 2001, 414, 782–787. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, Y.; Liu, R.; Li, X.; Cui, Y.; Qu, L. Geniposide protects against acute alcohol-induced liver injury in mice via up-regulating the expression of the main antioxidant enzymes. Can. J. Physiol. Pharmacol. 2015, 93, 261–267. [Google Scholar] [CrossRef]
- Ceni, E.; Mello, T.; Galli, A. Pathogenesis of alcoholic liver disease: Role of oxidative metabolism. World J. Gastroenterol. 2014, 20, 17756–17772. [Google Scholar] [CrossRef] [PubMed]
- Volynets, V.; Küper, M.; Strahl, S.; Maier, I.; Spruss, A.; Wagnerberger, S.; Königsrainer, A.; Bischoff, S.; Bergheim, I. Nutrition, intestinal permeability, and blood ethanol levels are altered in patients with nonalcoholic fatty liver disease (NAFLD). Dig. Dis. Sci. 2012, 57, 1932–1941. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.-H.; Wang, L.; Chen, W.-D.; Duan, Y.-T.; Sun, M.-J.; Huang, J.-J.; Peng, D.-Y.; Yu, N.-J.; Wang, Y.-Y.; Zhang, Y. Poria cocos polysaccharide prevents alcohol-induced hepatic injury and inflammation by repressing oxidative stress and gut leakiness. Front. Nutr. 2022, 9, 963598. [Google Scholar] [CrossRef]
- Wang, K.L.; Lu, Z.M.; Mao, X.; Chen, L.; Gong, J.S.; Ren, Y.; Geng, Y.; Li, H.; Xu, H.Y.; Xu, G.H.; et al. Structural characterization and anti-alcoholic liver injury activity of a polysaccharide from Coriolus versicolor mycelia. Int. J. Biol. Macromol. 2019, 137, 1102–1111. [Google Scholar] [CrossRef]
- Peng, Q.; Li, C.; Zhao, Y.; Sun, X.; Liu, H.; Ouyang, J. Protective Effect of Degraded Porphyra yezoensis Polysaccharides on the Oxidative Damage of Renal Epithelial Cells and on the Adhesion and Endocytosis of Nanocalcium Oxalate Crystals. Oxidative Med. Cell. Longev. 2021, 2021, 6463281. [Google Scholar] [CrossRef]
- Fang, T.; Zhang, X.; Hu, S.; Yu, Y.; Sun, X.; Xu, N. Enzymatic Degradation of Gracilariopsis lemaneiformis Polysaccharide and the Antioxidant Activity of Its Degradation Products. Marine Drugs 2021, 19, 270. [Google Scholar] [CrossRef]
- Song, X.; Shen, Q.; Liu, M.; Zhang, C.; Zhang, L.; Ren, Z.; Wang, W.; Dong, Y.; Wang, X.; Zhang, J.; et al. Antioxidant and hepatoprotective effects of intracellular mycelium polysaccharides from Pleurotus geesteranus against alcoholic liver diseases. Int. J. Biol. Macromol. 2018, 114, 979–988. [Google Scholar] [CrossRef]
- van der Veen, J.N.; Kennelly, J.P.; Wan, S.; Vance, J.E.; Vance, D.E.; Jacobs, R.L. The critical role of phosphatidylcholine and phosphatidylethanolamine metabolism in health and disease. Biochim. Biophys. Acta (BBA) Biomembr. 2017, 1859, 1558–1572. [Google Scholar] [CrossRef] [PubMed]
- Bian, L.; Chen, H.; Zhou, X. Untargeted lipidomics analysis of Mori Fructus polysaccharide on acute alcoholic liver injury in mice using ultra performance liquid chromatography-quadrupole-orbitrap-high resolution mass spectrometry. Int. Immunopharmacol. 2021, 97, 107521. [Google Scholar] [CrossRef] [PubMed]
- Lei, P.; Lü, J.; Yao, T.; Zhang, P.; Chai, X.; Wang, Y.; Jiang, M. Verbascoside exerts an anti-atherosclerotic effect by regulating liver glycerophospholipid metabolism. Food Sci. Hum. Wellness 2023, 12, 2314–2323. [Google Scholar] [CrossRef]
- Wang, X.-Y.; Luo, J.-P.; Chen, R.; Zha, X.-Q.; Pan, L.-H. Dendrobium huoshanense polysaccharide prevents ethanol-induced liver injury in mice by metabolomic analysis. Int. J. Biol. Macromol. 2015, 78, 354–362. [Google Scholar] [CrossRef]

 ) represents dGal, the yellow circle with green border (
) represents dGal, the yellow circle with green border ( ) stands for GalNAc, the red triangle (
) stands for GalNAc, the red triangle ( ) stands for Fuc, the pink triangle (
) stands for Fuc, the pink triangle ( ) stands for dFuc, and the blue small circle (
) stands for dFuc, and the blue small circle ( ) stands for sulfate groups. The product ions at m/z 641 (A), m/z 611 (B), m/z 698 (C), m/z 788 (D), m/z 721 (E), m/z 717 (F), m/z 771 (G), m/z 481 (H).
) stands for sulfate groups. The product ions at m/z 641 (A), m/z 611 (B), m/z 698 (C), m/z 788 (D), m/z 721 (E), m/z 717 (F), m/z 771 (G), m/z 481 (H).
 ) represents dGal, the yellow circle with green border (
) represents dGal, the yellow circle with green border ( ) stands for GalNAc, the red triangle (
) stands for GalNAc, the red triangle ( ) stands for Fuc, the pink triangle (
) stands for Fuc, the pink triangle ( ) stands for dFuc, and the blue small circle (
) stands for dFuc, and the blue small circle ( ) stands for sulfate groups. The product ions at m/z 641 (A), m/z 611 (B), m/z 698 (C), m/z 788 (D), m/z 721 (E), m/z 717 (F), m/z 771 (G), m/z 481 (H).
) stands for sulfate groups. The product ions at m/z 641 (A), m/z 611 (B), m/z 698 (C), m/z 788 (D), m/z 721 (E), m/z 717 (F), m/z 771 (G), m/z 481 (H).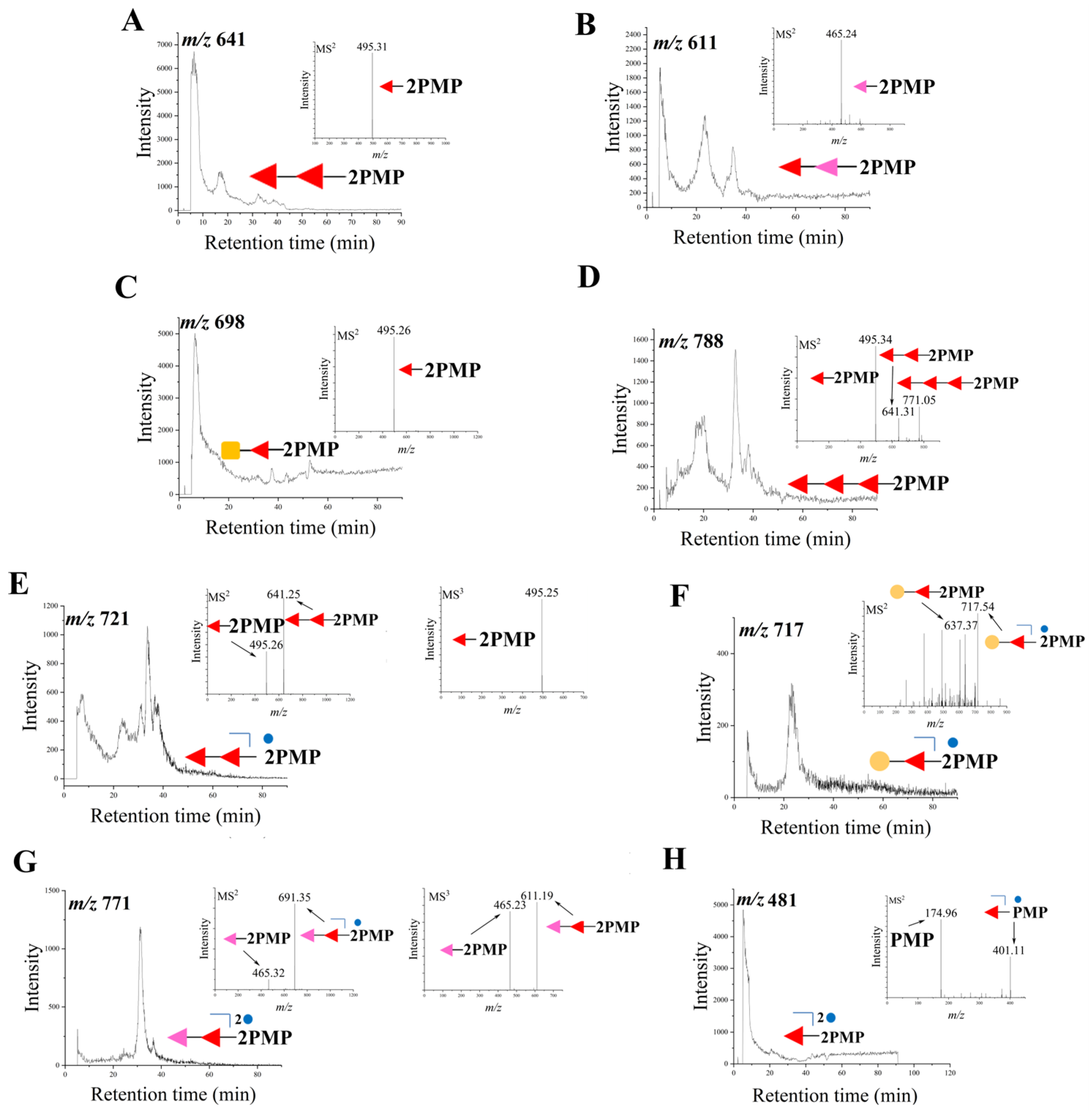

| Group | Experimental Treatments | |||
|---|---|---|---|---|
| Distilled Water (mL) | SCSP in 40% Mimic Baijiu (mg/kg Body Weight) | dSCSP in 40% Mimic Baijiu (mg/kg Body Weight) | 40% Mimic Baijiu (mL) | |
| Control | 0.3 | — | — | — |
| Model | — | — | — | 0.3 |
| H-SCSP | — | 360 | — | 0.3 |
| L-SCSP | — | 60 | — | 0.3 |
| H-dSCSP | — | — | 360 | 0.3 |
| L-dSCSP | — | — | 60 | 0.3 |
| Uronic Acid Content (%) | Sulfate Group Content (%) | Monosaccharide Composition (GlcN:GalN:Gal:Fuc) | |
|---|---|---|---|
| SCSP | 15.09 ± 1.28 | 13.10 ± 0.69 | 2.79:1.00:2.43:17.82 |
| dSCSP | 13.08 ± 0.40 | 11.57 ± 1.09 | 1.00:1.48:1.93:32.35 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, H.; Song, C.; Yan, C.; Yang, J.; Song, S. Sea Cucumber Polysaccharide from Stichopus japonicu and Its Photocatalytic Degradation Product Alleviate Acute Alcoholic Liver Injury in Mice. Foods 2024, 13, 963. https://doi.org/10.3390/foods13060963
Song H, Song C, Yan C, Yang J, Song S. Sea Cucumber Polysaccharide from Stichopus japonicu and Its Photocatalytic Degradation Product Alleviate Acute Alcoholic Liver Injury in Mice. Foods. 2024; 13(6):963. https://doi.org/10.3390/foods13060963
Chicago/Turabian StyleSong, Haoran, Chen Song, Chunhong Yan, Jingfeng Yang, and Shuang Song. 2024. "Sea Cucumber Polysaccharide from Stichopus japonicu and Its Photocatalytic Degradation Product Alleviate Acute Alcoholic Liver Injury in Mice" Foods 13, no. 6: 963. https://doi.org/10.3390/foods13060963





