Inhibition and Mechanism of Protein Nonenzymatic Glycation by Lactobacillus fermentum
Abstract
:1. Introduction
2. Material and Methods
2.1. Materials and Chemicals
2.2. Preparation of Complete Cell Suspension of LAB
2.3. The Antiglycation Capacity of L. fermentum in a BSA + Glu Model
2.3.1. Fluorescent AGEs
2.4. Measurement of pH and Glucose Concentration
2.5. Amadori Product and α-Dicarbonyl Compounds
2.6. Determination of Functional Group Changes
2.6.1. Protein-Bound Carbonyl
2.6.2. Sulfhydryl (SH) Groups
2.6.3. Free Amino Groups
2.7. Evaluation of Structural Changes
2.7.1. Intrinsic Tryptophan Fluorescence
2.7.2. ANS-Fluorescence
2.7.3. SDS-PAGE
2.7.4. Fourier Transform Infrared Spectroscopy (FTIR) Spectra
2.8. Determination and Verification of Trapping Methylglyoxal/Glyoxal
2.8.1. Methylglyoxal/Glyoxal Trapping Ability Determination
2.8.2. The Antiglycative Capacity of L. fermentum in Methylglyoxal + BSA/Methylglyoxal + Arg Models
2.9. Antioxidant Activity Assays
2.9.1. DPPH (2,2,-Diphenyl-1-picrylhydrazyl) Radical Scavenging Assay
2.9.2. Hydroxyl Radical-Scavenging Rate
2.9.3. ABTS Radical Cation-Scavenging Activity
2.9.4. Total Reducing Power Assay
2.10. Total Phenolic Content
2.11. Determination of the Total Viable Count (TVC) and Morphological Changes of L. fermentum
2.12. The Anti-AGE Activity of Intracellular Substances
2.13. Preparation of Active Components of L. fermentum
2.14. Statistical Analysis
3. Results and Discussion
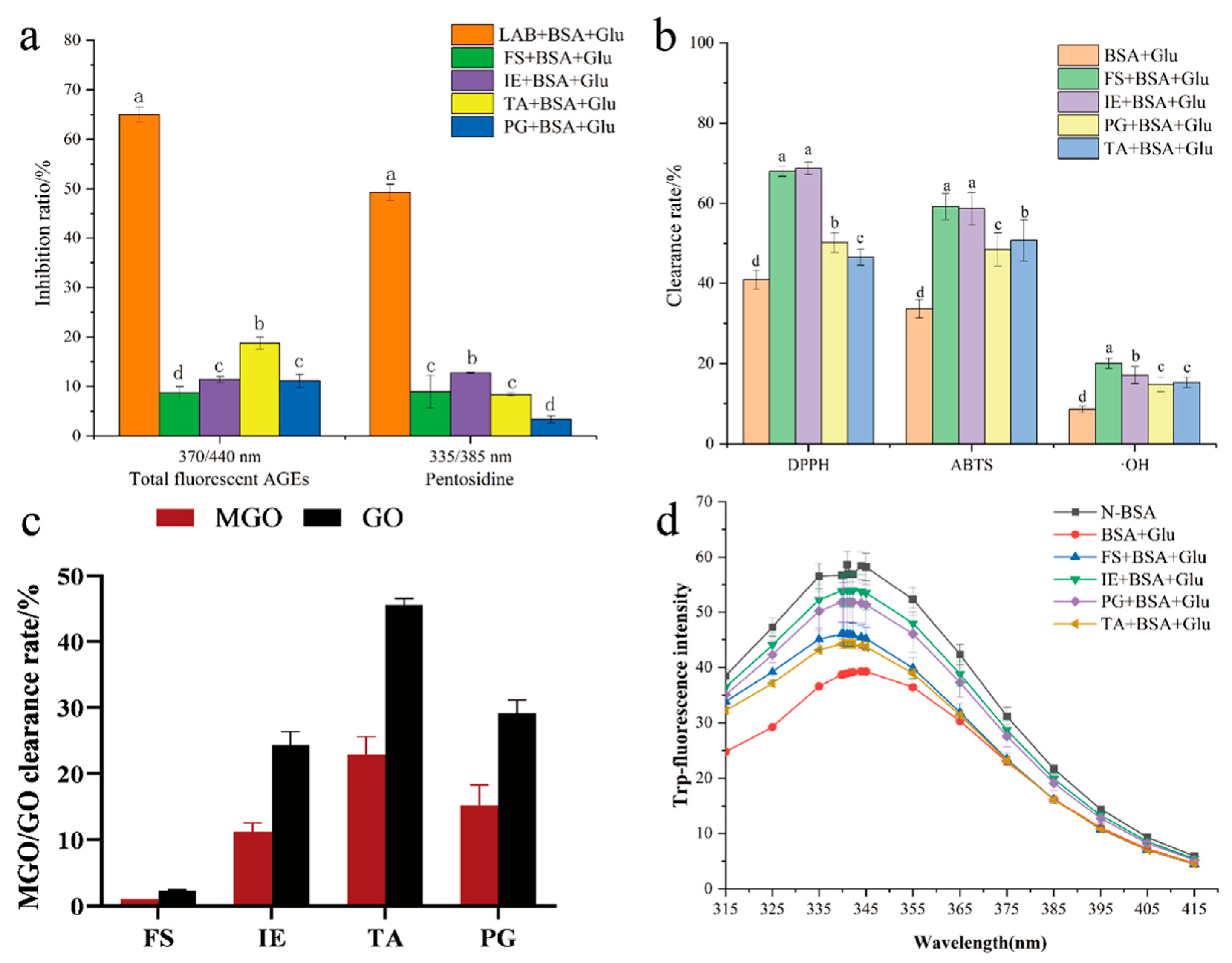
3.1. Inhibition of Fluorescent AGE Formation
3.2. Effect on the Physical and Chemical Properties of the System
3.3. Inhibition of Early and Mid-Stage Glycation Products
3.4. Analysis of Functional Group Changes
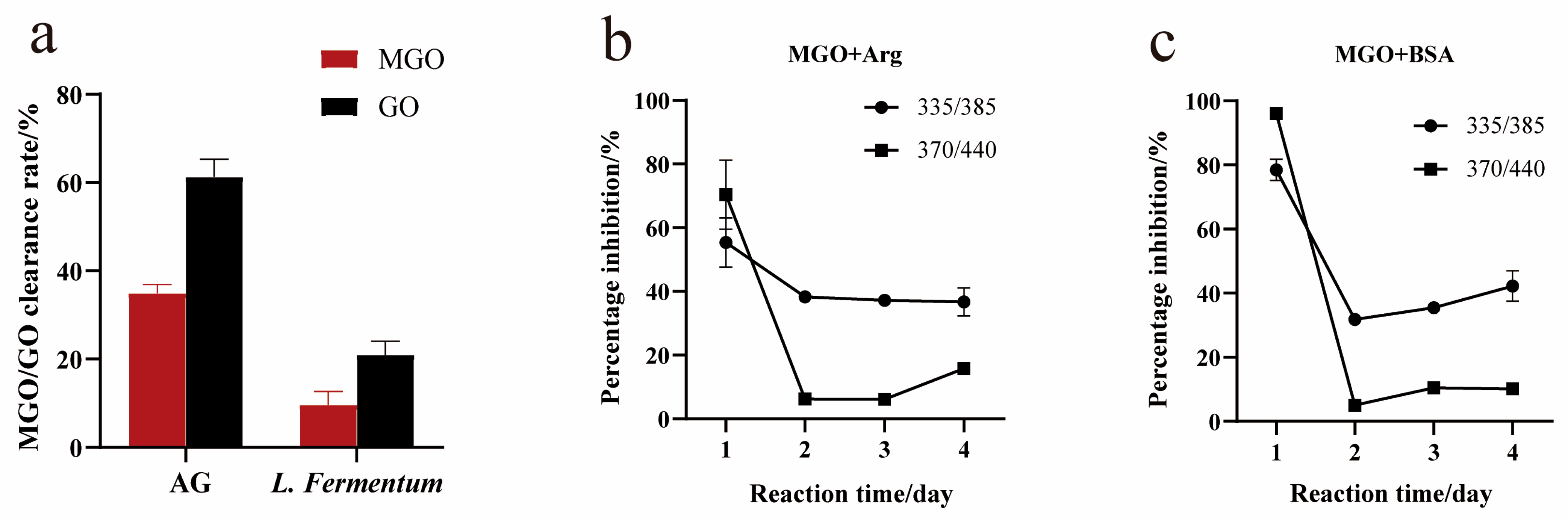
3.5. Capture Ability for Methylglyoxal/Glyoxal
3.6. Antioxidant Activity Assay
3.7. The Survival of LAB in the Glycation Model
3.8. The BSA Structure Analysis
3.9. Effect of Active Components of L. fermentum on Glycation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Monnier, V.M.; Cerami, A. Nonenzymatic browning in vivo: Possible process for aging of long-lived proteins. Science 1981, 211, 491–493. [Google Scholar] [CrossRef] [PubMed]
- Cerami, A. Hypothesis: Glucose as a mediator of aging. J. Am. Geriatr. Soc. 1985, 33, 626–634. [Google Scholar] [CrossRef] [PubMed]
- Emel’yanov, V.V. Glycation, antiglycation, and deglycation: Their role in aging mechanisms and geroprotective effects (literature review). Adv. Gerontol. 2017, 7, 1–9. [Google Scholar] [CrossRef]
- Singh, R.; Barden, A.; Mori, T.; Beilin, L. Advanced glycation end-products: A review. Diabetologia 2001, 44, 129–146. [Google Scholar] [CrossRef] [PubMed]
- Byun, K.; Yoo, Y.; Son, M.; Lee, J.; Jeong, G.B.; Park, Y.M.; Salekdeh, G.H.; Lee, B. Advanced glycation end-products produced systemically and by macrophages: A common contributor to inflammation and degenerative diseases. Pharmacol. Ther. 2017, 177, 44–55. [Google Scholar] [CrossRef] [PubMed]
- Gill, V.; Kumar, V.; Singh, K.; Kumar, A.; Kim, J.J. Advanced glycation end products (AGEs) may be a striking link between modern diet and health. Biomolecules 2019, 9, 888. [Google Scholar] [CrossRef] [PubMed]
- Corzo-Martínez, M.; Ávila, M.; Moreno, F.J.; Requena, T.; Villamiel, M. Effect of milk protein glycation and gastrointestinal digestion on the growth of bifidobacteria and lactic acid bacteria. Int. J. Food Microbiol. 2012, 153, 420–427. [Google Scholar] [CrossRef] [PubMed]
- Huang, G.L.; Lv, M.J.; Hu, J.C.; Huang, K.L.; Xu, H. Glycosylation and Activities of Natural Products. Mini Rev. Med. Chem. 2016, 16, 1013–1016. [Google Scholar] [CrossRef] [PubMed]
- Jafarnejad, A.; Bathaie, S.Z.; Nakhjavani, M.; Hassan, M.Z. Effect of spermine on lipid profile and HDL functionality in the streptozotocin-induced diabetic rat model. Life Sci. 2008, 82, 301–307. [Google Scholar] [CrossRef]
- Zhang, Q.Z.; Huang, Z.J.; Wang, Y.; Wang, Y.B.; Fu, L.L.; Su, L.J. Chinese bayberry (Myrica rubra) phenolics mitigated protein glycoxidation and formation of advanced glycation end-products: A mechanistic investigation. Food Chem. 2021, 361, 130102. [Google Scholar] [CrossRef]
- Lan, M.Y.; Li, H.M.; Tao, G.; Lin, J.; Lu, M.W.; Yan, R.A.; Huang, J.Q. Effects of four bamboo derived flavonoids on advanced glycation end products formation in vitro. J. Funct. Foods 2020, 71, 103976. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, C.J.; Tu, Z.C.; Yang, W.H.; Zhao, Y.; Xin, Z.Q.; Wang, H.; Sha, X.M.; Chen, J. Nelumbo nucifera leaf extracts inhibit the formation of advanced glycation end-products and mechanism revealed by Nano LC-Orbitrap-MS/MS. J. Funct. Foods 2018, 42, 254–261. [Google Scholar] [CrossRef]
- Yang, L.; Li, X.; Wu, Z.; Feng, C.; Zhang, T.; Dai, S.; Dong, Q. Inhibition of methylglyoxal-induced histone H1 N(epsilon)-carboxymethyllysine formation by (+)-catechin. J. Agric. Food Chem. 2018, 66, 5812–5820. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Liu, G.J.; Zhang, W.; Zhou, Y.L.; Ling, T.J.; Wan, X.C.; Bao, G.H. Novel flavoalkaloids from White Tea with inhibitory activity against the formation of advanced glycation end products. J. Agric. Food Chem. 2018, 66, 4621–4629. [Google Scholar] [CrossRef] [PubMed]
- Song, Q.H.; Liu, J.J.; Dong, L.Y.; Wang, X.L.; Zhang, X.D. Novel advances in inhibiting advanced glycation end product formation using natural compounds. Biomed. Pharmacother. 2021, 140, 111750. [Google Scholar] [CrossRef] [PubMed]
- Governa, P.; Manetti, F.; Miraldi, E.; Biagi, M. Effects of in vitro simulated digestion on the antioxidant activity of different Camellia sinensis (L.) Kuntze leaves extracts. Eur. Food Res. Technol. 2021, 248, 119–128. [Google Scholar] [CrossRef]
- Sánchez, B.; Delgado, S.; Blanco-Míguez, A.; Lourenço, A.; Gueimonde, M.; Margolles, A. Probiotics, gut microbiota, and their influence on host health and disease. Mol. Nutr. Food Res. 2017, 61, 1600240. [Google Scholar] [CrossRef]
- Farnworth, E.R. The evidence to support health claims for probiotics. J. Nutr. 2008, 138, 1250S–1254S. [Google Scholar] [CrossRef]
- Zhang, L.H.; Ma, H.C.; Kulyar, M.F.; Pan, H.C.; Li, K.W.; Li, A.Y.; Mo, Q.; Wang, Y.P.; Dong, H.L.; Bao, Y.H.; et al. Complete genome analysis of Lactobacillus fermentum YLF016 and its probiotic characteristics. Microb. Pathog. 2021, 162, 105212. [Google Scholar] [CrossRef]
- Zhang, M.M.; Otake, K.; Miyauchi, Y.; Yagi, M.; Yonei, Y.; Miyakawa, T.; Tanokura, M. Comprehensive NMR analysis of two kinds of post-fermented tea and their anti-glycation activities in vitro. Food Chem. 2019, 277, 735–743. [Google Scholar] [CrossRef]
- Zieliński, H.; Szawara-Nowak, D.; Wronkowska, M. Bioaccessibility of anti-AGEs activity, antioxidant capacity and phenolics from water biscuits prepared from fermented buckwheat flours. LWT 2020, 123, 109051. [Google Scholar] [CrossRef]
- Kaga, Y.; Kuda, T.; Taniguchi, M.; Yamaguchi, Y.; Takenaka, H.; Takahashi, H.; Kimura, B. The effects of fermentation with lactic acid bacteria on the antioxidant and anti-glycation properties of edible cyanobacteria and microalgae. LWT 2021, 135, 110029. [Google Scholar] [CrossRef]
- Peng, X.F.; Zheng, Z.P.; Cheng, K.W.; Shan, F.; Ren, G.X.; Chen, F.; Wang, M.F. Inhibitory effect of mung bean extract and its constituents vitexin and isovitexin on the formation of advanced glycation endproducts. Food Chem. 2008, 106, 475–481. [Google Scholar] [CrossRef]
- Lee, H.H.L.; Jun, L.J.; Yoon, C.S.; Yoonsook, K.; Jinyoung, H. Inhibitory effect of sea buckthorn extracts on advanced glycation endproduct formation. Food Chem. 2022, 373, 131364. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.C.; Hu, S.T.; Chen, F.; Wang, M.F. Treatment of proteins with dietary polyphenols lowers the formation of AGEs and AGE-induced toxicity. Food Funct. 2014, 5, 2656–2661. [Google Scholar] [CrossRef] [PubMed]
- Sang, S.M.; Shao, X.; Bai, N.S.; Lo, C.Y.; Yang, C.S.; Ho, C.T. Tea polyphenol (-)-epigallocatechin-3-gallate: A new trapping agent of reactive dicarbonyl species. Chem. Res. Toxicol. 2007, 20, 1862–1870. [Google Scholar] [CrossRef] [PubMed]
- Eze, F.N.; Tola, A.J. Protein glycation and oxidation inhibitory activity of Centella asiatica phenolics (CAP) in glucose-mediated bovine serum albumin glycoxidation. Food Chem. 2020, 332, 127302. [Google Scholar] [CrossRef] [PubMed]
- Sirichai, A.; Thavaree, T.; Charoonsri, C. Mesona Chinensis Benth extract prevents AGE formation and protein oxidation against fructose-induced protein glycation in vitro. BMC Complement. Complement. Altern. Med. 2014, 14, 130. [Google Scholar]
- Chen, K.Y.; Gao, C.C.; Han, X.M.; Li, D.; Wang, H.K.; Lu, F.P. Co-fermentation of lentils using lactic acid bacteria and Bacillus subtilis natto increases functional and antioxidant components. J. Food Sci. 2020, 86, 475–483. [Google Scholar] [CrossRef]
- Khan, M.W.A.; Otaibi, A.A.; Al-Zahrani, S.A.; Alshammari, E.M.; Haque, A.; Alouffi, S.; Khan, W.A.; Khan, S.N. Experimental and theoretical insight into resistance to glycation of bovine serum albumin. J. Mol. Struct. 2020, 1230, 129645. [Google Scholar] [CrossRef]
- Sobhy, R.; Khalifa, I.; Liang, H.S.; Li, B. Phytosterols disaggregate bovine serum albumin under the glycation conditions through interacting with its glycation sites and altering its secondary structure elements. Bioorganic Chem. 2020, 101, 104047. [Google Scholar] [CrossRef] [PubMed]
- Kulwant, S.; Islam, H.; Vibhor, M.; Akhtar, S. New insight on 8-anilino-1-naphthalene sulfonic acid interaction with TgFNR for hydrophobic exposure analysis. Int. J. Biol. Macromol. 2019, 122, 636–643. [Google Scholar]
- Zhang, L.; Lu, Y.; Ye, Y.H.; Yang, S.H.; Tu, Z.C.; Chen, J.; Wang, H.; Wang, H.H.; Yuan, T. Insights into the Mechanism of Quercetin against BSA-Fructose Glycation by Spectroscopy and High Resolution Mass Spectrometry: Effect on Physicochemical Properties. J. Agric. Food Chem. 2018, 67, 236–246. [Google Scholar] [CrossRef] [PubMed]
- Jian, W.J.; Wang, L.Y.; Wu, L.L.; Sun, Y.M. Physicochemical Properties of Bovine Serum Albumin-Glucose and Bovine Serum Albumin-Mannose Conjugates Prepared by Pulsed Electric Fields Treatment. Molecules 2018, 23, 570. [Google Scholar] [CrossRef]
- Cheng, Z.J.; Zhang, Y.T. Spectroscopic investigation on the interaction of salidroside with bovine serum albumin. J. Mol. Struct. 2008, 889, 20–27. [Google Scholar] [CrossRef]
- Wu, X.Q.; Zhang, G.W.; Hu, X.; Pan, J.H.; Liao, Y.J.; Ding, H.F. Inhibitory effect of epicatechin gallate on protein glycation. Food Res. Int. 2019, 122, 230–240. [Google Scholar] [CrossRef]
- Li, X.Y.; Gao, J.; Simal-Gandara, J.; Wang, X.H.; Caprioli, G.; Mi, S.; Sang, Y.S. Effect of fermentation by Lactobacillus acidophilus CH-2 on the enzymatic browning of pear juice. LWT 2021, 147, 111489. [Google Scholar] [CrossRef]
- Zhang, Q.; Tong, X.; Li, Y.; Wang, H.; Wang, Z.; Qi, B.; Jiang, L. Purification and characterization of antioxidant peptides from alcalase-hydrolyzed soybean (Glycine max L.) hydrolysate and their cytoprotective effects in human intestinal Caco-2 cells. J. Agric. Food Chem. 2019, 67, 5772–5781. [Google Scholar] [CrossRef] [PubMed]
- Du, L.; Shen, Y.; Zhang, X.; Prinyawiwatkul, W.; Xu, Z. Antioxidant-rich phytochemicals in miracle berry (Synsepalum dul-cificum) and antioxidant activity of its extracts. Food Chem. 2014, 153, 279–284. [Google Scholar] [CrossRef]
- Suktham, T.; Jones, A.; Soliven, A.; Dennis, G.R.; Andrew, R.; Shalliker, R.A. A comparison of the performance of the cupric reducing antioxidant potential assay and the ferric reducing antioxidant power assay for the analysis of antioxidants using reaction flow chromatography. Microchem. J. 2019, 149, 104046. [Google Scholar] [CrossRef]
- Zhou, M.Z.; Zheng, X.; Zhu, H.J.; Li, L.B.; Zhang, L.; Liu, M.L.; Liu, Z.P.; Peng, M.Y.; Wang, C.; Li, Q.; et al. Effect of Lactobacillus plantarum enriched with organic/inorganic selenium on the quality and microbial communities of fermented pickles. Food Chem. 2021, 365, 130495. [Google Scholar] [CrossRef]
- Li, Q.; Li, L.B.; Zhu, H.J.; Yang, F.; Xiao, K.; Zhang, L.; Zhang, M.L.; Peng, Y.S.; Wang, C.; Li, D.S.; et al. Lactobacillus fermentum as a new inhibitor to control advanced glycation end-product formation during vinegar fermentation. Food Sci. Hum. Wellness 2022, 11, 1409–1418. [Google Scholar] [CrossRef]
- Uribarri, J.; Woodruff, S.; Goodman, S.; Cai, W.J.; Chen, X.; Pyzik, R.; Yong, A.; Striker, G.E.; Vlassara, H. Advanced Glycation End Products in Foods and a Practical Guide to Their Reduction in the Die. J. Am. Diet. Assoc. 2010, 110, 911–916. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.B.; Cai, P.J.; Zhang, N.; Wu, T.T.; Sun, A.D.; Jia, G.L. Inhibitory effects of polyphenols from black chokeberry on advanced glycation end-products (AGEs) formation. Food Chem. 2022, 392, 133295. [Google Scholar] [CrossRef]
- Raâfet, B.K.M.; Jawhar, H.; Mohamed, H.; Mohamed, H. Inhibition of Protein Glycation by Combined Antioxidant and Antiglycation Constituents from a Phenolic Fraction of Sage (Salvia officinalis L.). Plant Foods Hum. Nutr. 2020, 75, 505–511. [Google Scholar]
- Suantawee, T.; Cheng, H.; Adisakwattana, S. Protective effect of cyanidin against glucose- and methylglyoxal-induced protein glycation and oxidative DNA damage. Int. J. Biol. Macromol. 2016, 93, 814–821. [Google Scholar] [CrossRef]
- Cao, Y.; Xiong, Y.L. Chlorogenic acid-mediated gel formation of oxidatively stressed myofibrillar protein. Food Chem. 2015, 180, 235–243. [Google Scholar] [CrossRef] [PubMed]
- Dominika, Ś.; Arjan, N.; Karyn, R.P.; Henryk, K. The study on the impact of glycated pea proteins on human intestinal bacteria. Int. J. Food Microbiol. 2011, 145, 267–272. [Google Scholar] [CrossRef]
- Wu, Q.; Zhao, K.Q.; Chen, Y.Y.; Ouyang, Y.; Feng, Y.N.; Li, S.Y.; Zhang, L.; Feng, N.J. Effect of lotus seedpod oligomeric procyanidins on AGEs formation in simulated gastrointestinal tract and cytotoxicity in Caco-2 cells. Food Funct. 2021, 12, 3527–3538. [Google Scholar] [CrossRef]







| Reaction Time/Day | BSA + Glu | LAB + BSA + Glu | AG + BSA + Glu |
|---|---|---|---|
| 2 | 0.181 ± 0.0015 a | 0.165 ± 0.0025 b | 0.187 ± 0.0015 a |
| 4 | 0.179 ± 0.0010 a | 0.186 ± 0.0010 a | 0.177 ± 0.0035 a |
| 6 | 0.202 ± 0.0015 b | 0.204 ± 0.0017 b | 0.219 ± 0.0035 a |
| 8 | 0.181 ± 0.0015 b | 0.2 ± 0.0015 a | 0.199 ± 0.0036 a |
| 10 | 0.18 ± 0.0014 b | 0.198 ± 0.0100 a | 0.202 ± 0.0015 a |
| Conformation | N-BSA | BSA + Glu | LAB + BSA + Glu | AG + BSA + Glu |
|---|---|---|---|---|
| β-sheet | 37.23 ± 0.03 a | 33.40 ± 0.04 c (−3.83%) | 36.52 ± 0.01 a (−0.71%) | 35.12 ± 0.002 b (−2.11%) |
| Random coil | 20.11 ± 0.02 a | 19.71 ± 0.04 b (−0.4%) | 19.60 ± 0.01 b (−0.51%) | 20.35 ± 0.0001 a (+0.24%) |
| α-helix | 19.55 ± 0.05 b | 20.69 ± 0.02 a (+1.14%) | 19.75 ± 0.004 b (+0.2%) | 19.95 ± 0.005 b (+0.4%) |
| β-turn | 23.10 ± 0.04 c | 26.20 ± 0.01 a (+3.1%) | 24.54 ± 0.03 b (+1.44%) | 23.58 ± 0.01 c (+0.48%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Q.; Xiao, K.; Yi, C.; Yu, F.; Wang, W.; Rao, J.; Liu, M.; Zhang, L.; Mu, Y.; Wang, C.; et al. Inhibition and Mechanism of Protein Nonenzymatic Glycation by Lactobacillus fermentum. Foods 2024, 13, 1183. https://doi.org/10.3390/foods13081183
Li Q, Xiao K, Yi C, Yu F, Wang W, Rao J, Liu M, Zhang L, Mu Y, Wang C, et al. Inhibition and Mechanism of Protein Nonenzymatic Glycation by Lactobacillus fermentum. Foods. 2024; 13(8):1183. https://doi.org/10.3390/foods13081183
Chicago/Turabian StyleLi, Qin, Ke Xiao, Chi Yi, Fan Yu, Wenyue Wang, Junhui Rao, Menglin Liu, Lin Zhang, Yang Mu, Chao Wang, and et al. 2024. "Inhibition and Mechanism of Protein Nonenzymatic Glycation by Lactobacillus fermentum" Foods 13, no. 8: 1183. https://doi.org/10.3390/foods13081183






