Chemical Compositions and Characteristics of Biocalcium from Pre-Cooked Tuna Bone as Influenced by Sodium Chloride Pretreatment and Defatting by Asian Seabass Lipase
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
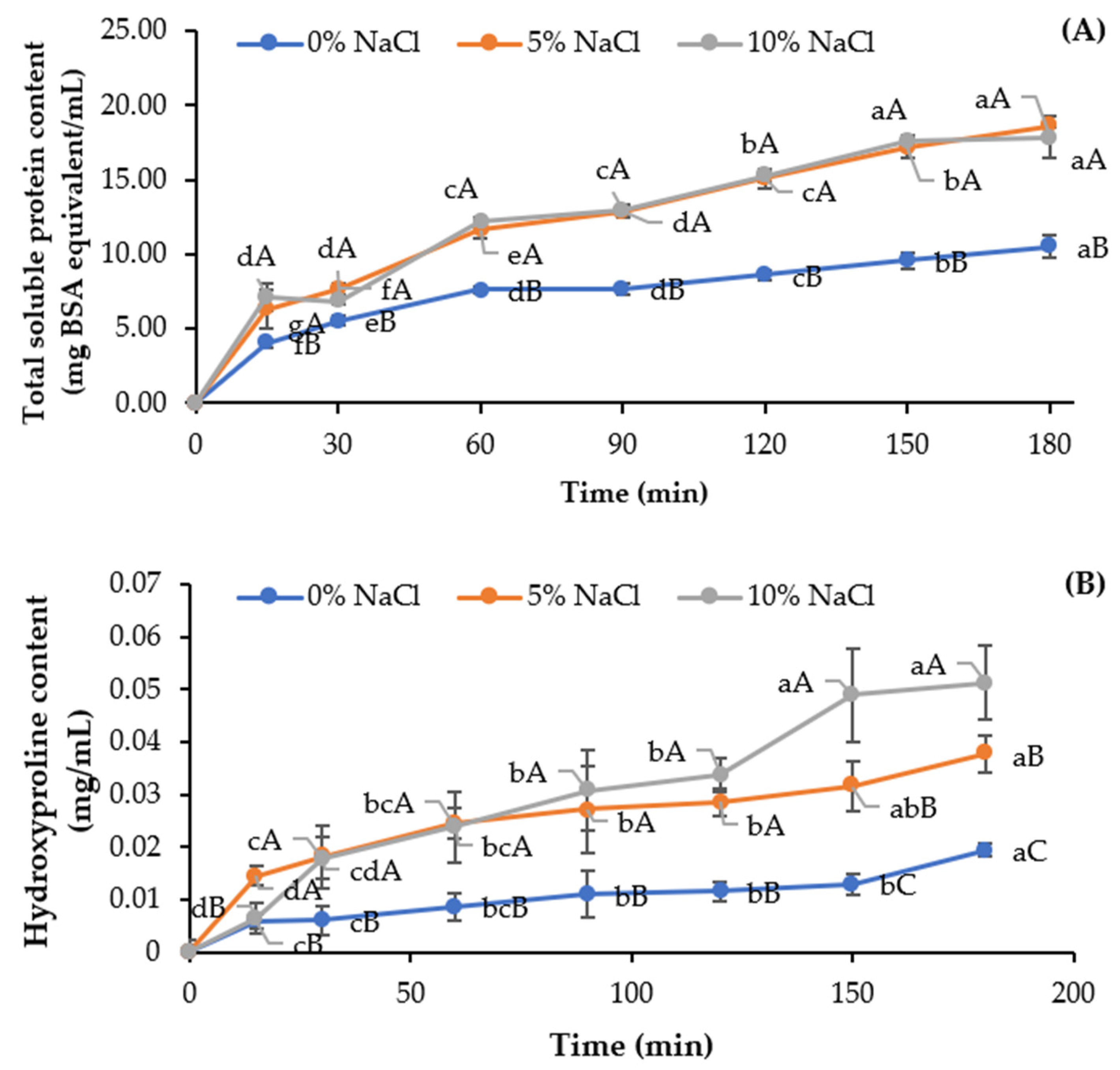
2.2. Effect of Sodium Chloride in Combination with Heat on Non-Collagenous Protein Removal of Pre-Cooked Tuna Bone
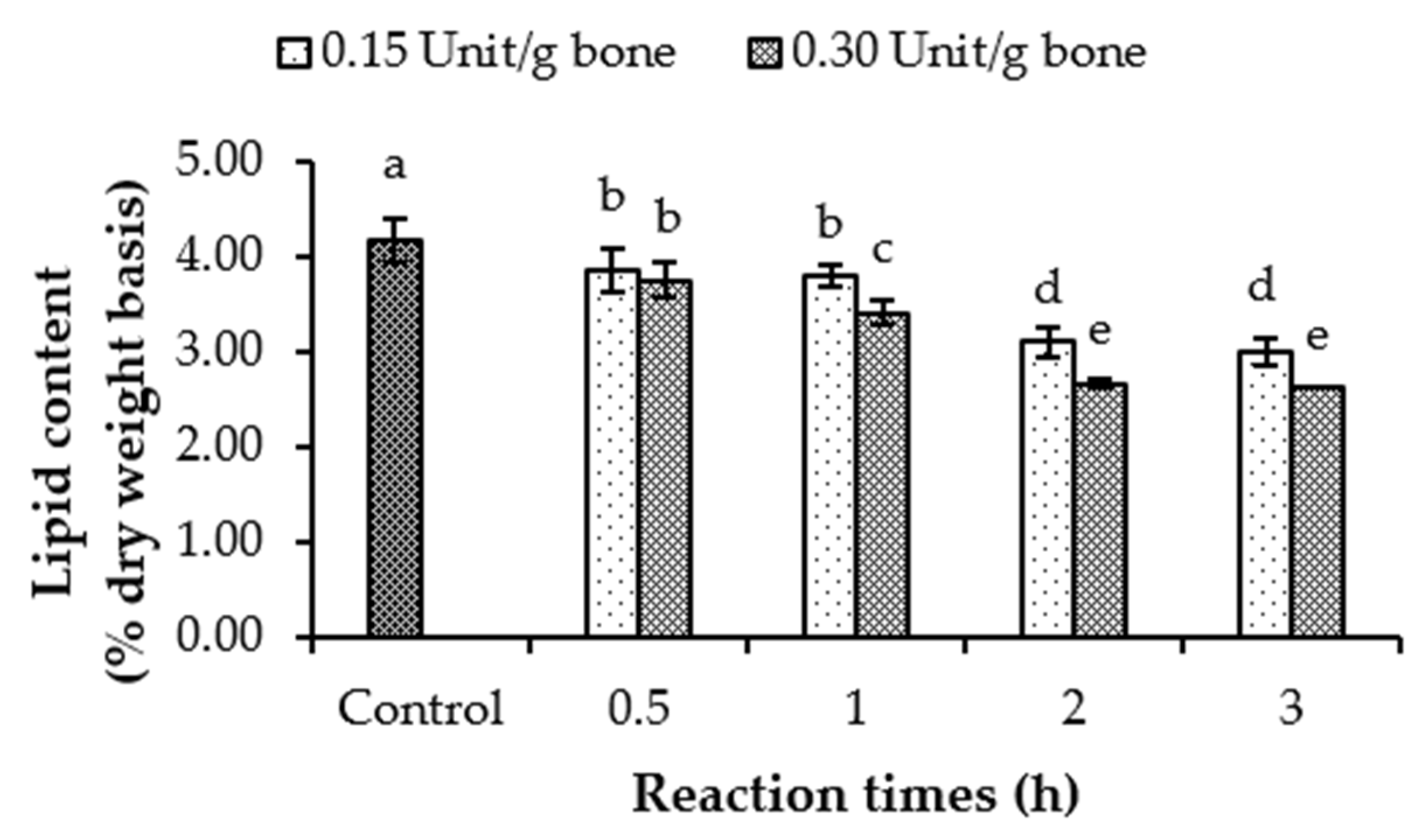
2.3. Impact of Lipase from Asian Seabass Liver on Defatting of Tuna Bone
2.3.1. Preparation and Extraction of Crude Lipase
2.3.2. Defatting Process of Tuna Bone with CLE
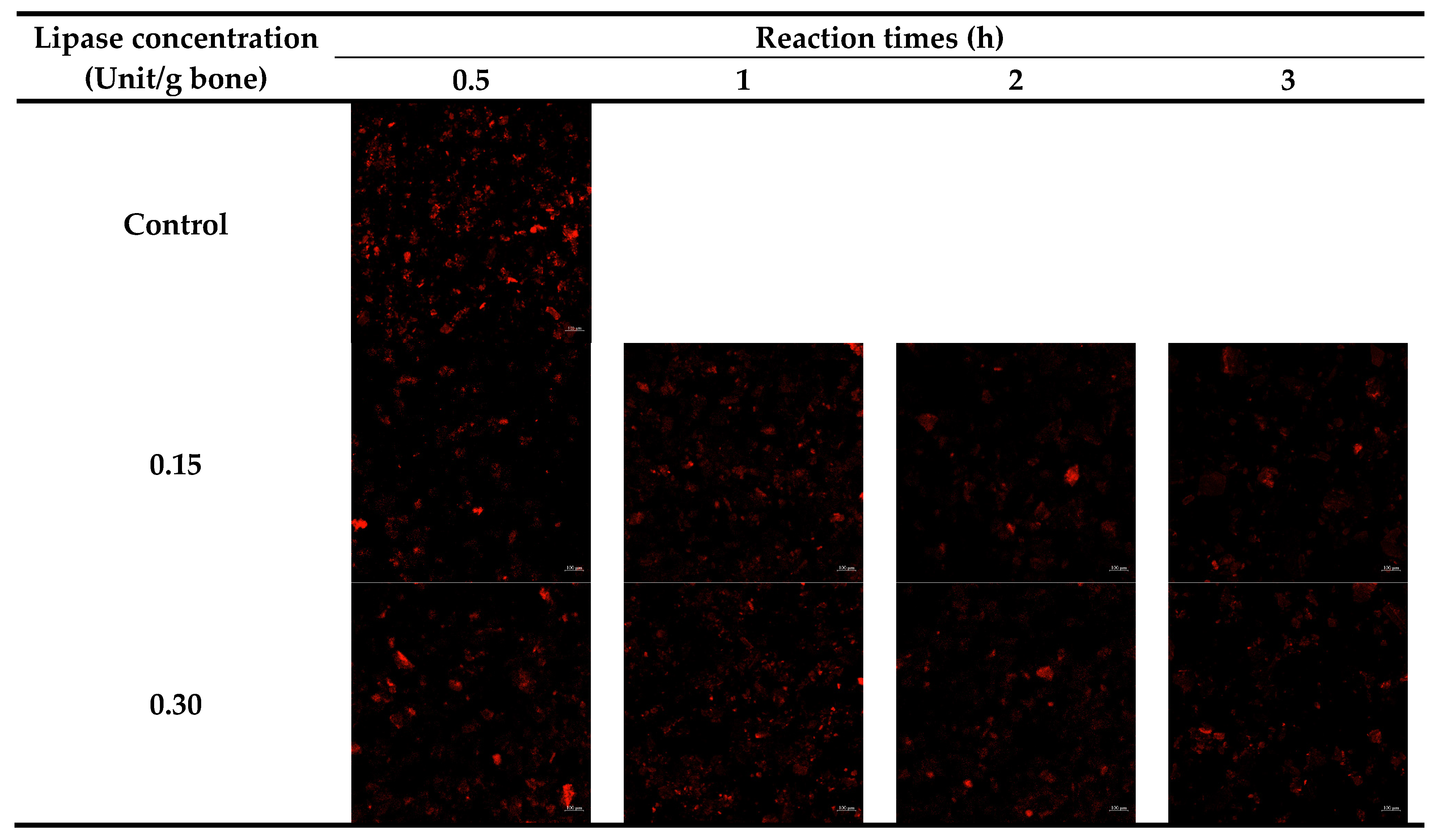
2.3.3. Lipid Content and Distribution
2.4. Production and Characterization of Biocalcium
2.4.1. Yield
2.4.2. Hydroxyproline Content
2.4.3. Chemical Composition
2.4.4. Calcium and Phosphorus Contents
2.4.5. Color
2.4.6. Mean Particle Size (MPS)
2.4.7. X-ray Diffraction (XRD)
2.4.8. Scanning Electron Microscopy with Energy Dispersive X-ray Spectroscopy (SEM–EDX)
2.5. Statistical Analysis
3. Results and Discussion
3.1. Impact of Sodium Chloride in Combination with Heat on Non-Collagenous Protein Removal of Pre-Cooked Tuna Bone
3.2. Effect of Lipase from Asian Seabass Liver on Defatting of Tuna Bone
3.2.1. Lipid Content
3.2.2. Lipid Distribution
3.3. Yield, Composition, and Characteristics of Biocalcium
3.3.1. Yield
3.3.2. Hydroxyproline Content
3.3.3. Chemical Compositions
3.3.4. Calcium and Phosphorus Contents
3.3.5. Color
3.3.6. Mean Particle Size (MPS)
3.3.7. X-ray Diffraction (XRD) Diffractograms
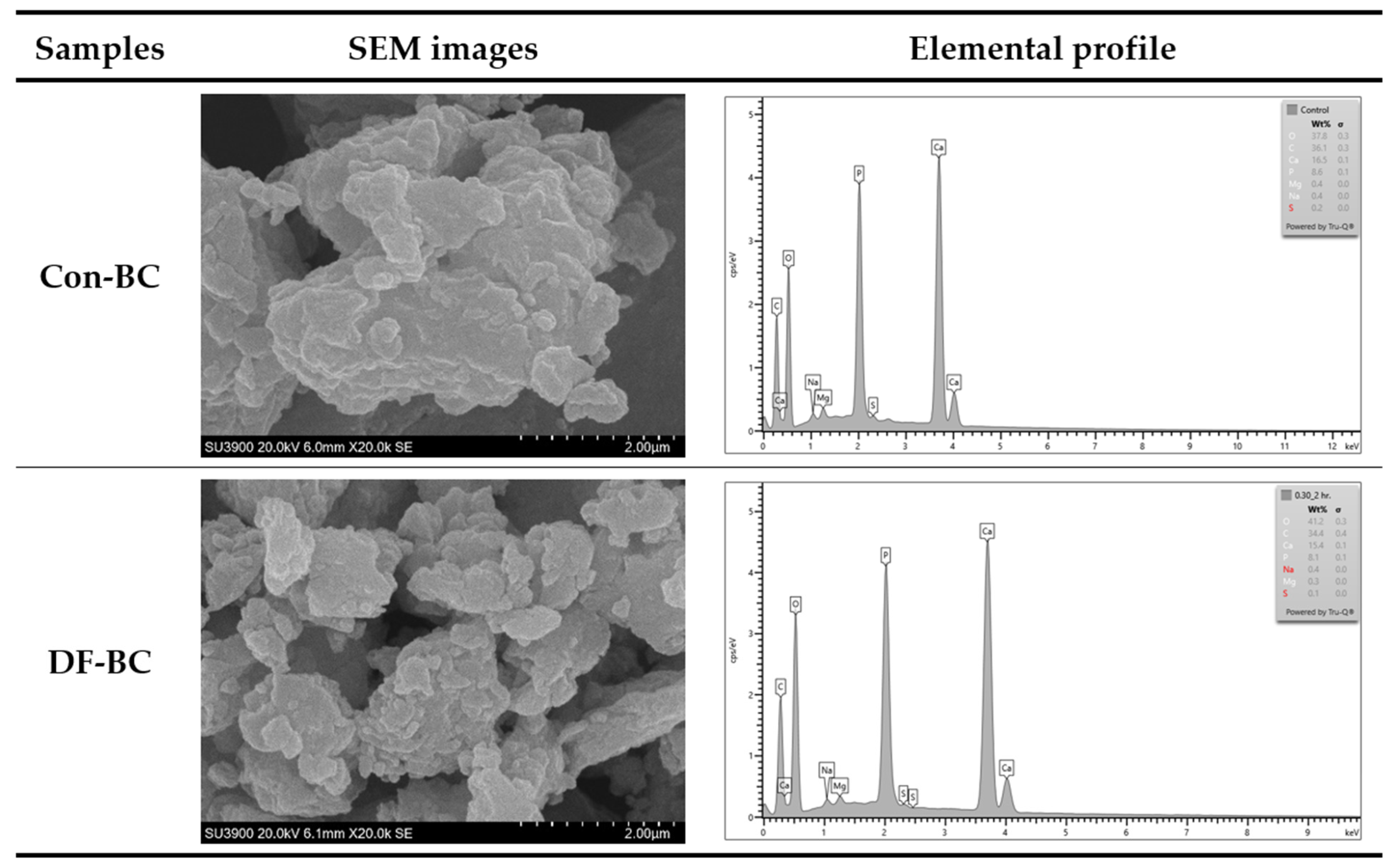
3.3.8. Scanning Electron Microscopy with Energy Dispersive X-ray Spectroscopy (SEM–EDX)
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cashman, K.D. Calcium intake, calcium bioavailability and bone health. Br. J. Nutr. 2002, 87, S169–S177. [Google Scholar] [CrossRef]
- Onakpoya, I.J.; Perry, R.; Zhang, J.; Ernst, E. Efficacy of calcium supplementation for management of overweight and obesity: Systematic review of randomized clinical trials. Nutr. Rev. 2011, 69, 335–343. [Google Scholar] [CrossRef]
- Shkembi, B.; Huppertz, T. Calcium absorption from food products: Food matrix effects. Nutrients 2022, 14, 180. [Google Scholar] [CrossRef] [PubMed]
- Benjakul, S.; Mad-Ali, S.; Senphan, T.; Sookchoo, P. Biocalcium powder from precooked skipjack tuna bone: Production and its characteristics. J. Food Biochem. 2017, 41, e12412. [Google Scholar] [CrossRef]
- Xiao, J.; Li, X.; Min, X.; Sakaguchi, E. Mannitol improves absorption and retention of calcium and magnesium in growing rats. Nutrition 2013, 29, 325–331. [Google Scholar] [CrossRef] [PubMed]
- Bellmann, C.; Tipping, A.; Sumaila, U.R. Global trade in fish and fishery products: An overview. Mar. Policy 2016, 69, 181–188. [Google Scholar] [CrossRef]
- Debeer, J.; Nolte, F.; Lord, C.W.; Colley, J.; Correa Gonzalez, G.; Cox, J.; Bell, J.W. Processing tuna, Scombridae, for canning: A review. Mar. Fish. Rev. 2021, 83, 1–44. [Google Scholar] [CrossRef]
- Gamarro, E.G.; Orawattanamateekul, W.; Sentina, J.; Gopal, T.K.S. By-Products of Tuna Processing; Food and Agriculture Organization of the United Nations: Rome, Italy, 2013; Volume 112, p. 48. [Google Scholar]
- Wijayanti, I.; Benjakul, S.; Sookchoo, P. Effect of high pressure heating on physical and chemical characteristics of Asian sea bass (Lates calcarifer) backbone. J. Food Sci. Technol. 2021, 58, 3120–3129. [Google Scholar] [CrossRef] [PubMed]
- Pudtikajorn, K.; Sae-leaw, T.; Yesilsu, A.F.; Sookchoo, P.; Benjakul, S. Process development and characteristics of biocalcium from skipjack tuna (Katsuwonus pelamis) eyeball scleral cartilage. Waste Biomass Valorization 2023, 14, 2909–2922. [Google Scholar] [CrossRef]
- Kang, Z.-L.; Zhang, X.-h.; Li, X.; Song, Z.-j.; Ma, H.-j.; Lu, F.; Zhu, M.-m.; Zhao, S.-m.; Wang, Z.-r. The effects of sodium chloride on proteins aggregation, conformation and gel properties of pork myofibrillar protein Running Head: Relationship aggregation, conformation and gel properties. J. Food Sci. Technol. 2021, 58, 2258–2264. [Google Scholar] [CrossRef]
- Sae-leaw, T.; Benjakul, S. Lipase from liver of seabass (Lates calcarifer): Characteristics and the use for defatting of fish skin. Food Chem. 2018, 240, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Gornall, A.G.; Bardawill, C.J.; David, M.M. Determination of serum proteins by means of the biuret reaction. J. Biol. Chem. 1949, 177, 751–766. [Google Scholar] [CrossRef] [PubMed]
- Bergman, I.; Loxley, R. Two improved and simplified methods for the spectrophotometric determination of hydroxyproline. Anal. Chem. 1963, 35, 1961–1965. [Google Scholar] [CrossRef]
- Bligh, E.G.; Dyer, W.J. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 1959, 37, 911–917. [Google Scholar] [CrossRef] [PubMed]
- AOAC. Official Methods of Analysis; Association of Official Analytical Chemists: Washington, DC, USA, 2002. [Google Scholar]
- Feist, B.; Mikula, B. Preconcentration of heavy metals on activated carbon and their determination in fruits by inductively coupled plasma optical emission spectrometry. Food Chem. 2014, 147, 302–306. [Google Scholar] [CrossRef] [PubMed]
- Ichimura, Y. Thermal denaturation of proteins II. Appl. Brief Hitachi High-Tech Sci. Corporat. TA 1991, 54, 1–2. [Google Scholar]
- Cui, F.-Z.; Li, Y.; Ge, J. Self-assembly of mineralized collagen composites. Mater. Sci. Eng. R Rep. 2007, 57, 1–27. [Google Scholar] [CrossRef]
- Zhang, M.; Zhu, S.; Li, Q.; Xue, D.; Jiang, S.; Han, Y.; Li, C. Effect of thermal processing on the conformational and digestive properties of myosin. Foods 2023, 12, 1249. [Google Scholar] [CrossRef] [PubMed]
- de Vrieze, E.; Heijnen, L.; Metz, J.R.; Flik, G. Evidence for a hydroxyapatite precursor in regenerating cyprinid scales. J. Appl. Ichthyol. 2012, 28, 388–392. [Google Scholar] [CrossRef]
- Swamiappan, S.; Ganesan, S.; Sekar, V.; Devaraj, S.; Subramanian, A.; Ponnusamy, V.K.; Kathirvel, P. Effective removal of cationic methylene blue dye using nano-hydroxyapatite synthesized from fish scale bio-waste. Int. J. Appl. Ceram. Technol. 2021, 18, 902–912. [Google Scholar] [CrossRef]
- Barralet, J.; Knowles, J.C.; Best, S.; Bonfield, W. Thermal decomposition of synthesised carbonate hydroxyapatite. J. Mater. Sci. Mater. Med. 2002, 13, 529–533. [Google Scholar] [CrossRef] [PubMed]
- Dorozhkin, S.V. Calcium orthophosphates. J. Mater. Sci. 2007, 42, 1061–1095. [Google Scholar] [CrossRef]
- Toppe, J.; Albrektsen, S.; Hope, B.; Aksnes, A. Chemical composition, mineral content and amino acid and lipid profiles in bones from various fish species. Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 2007, 146, 395–401. [Google Scholar] [CrossRef] [PubMed]
- Onwulata, C. Encapsulated and Powdered Foods; CRC Press: Boca Raton, FL, USA, 2005. [Google Scholar]
- Londoño-Restrepo, S.M.; Jeronimo-Cruz, R.; Millán-Malo, B.M.; Rivera-Muñoz, E.M.; Rodriguez-García, M.E. Effect of the nano crystal size on the X-ray diffraction patterns of biogenic hydroxyapatite from human, bovine, and porcine bones. Sci. Rep. 2019, 9, 5915. [Google Scholar] [CrossRef]
- Newbury, D.E.; Ritchie, N.W.M. Performing elemental microanalysis with high accuracy and high precision by scanning electron microscopy/silicon drift detector energy-dispersive X-ray spectrometry (SEM/SDD-EDS). J. Mater. Sci. 2015, 50, 493–518. [Google Scholar] [CrossRef]

| Parameters | Samples | |
|---|---|---|
| Con-BC | DF-BC | |
| Yield (%, dry weight basis) | 86.56 ± 2.54 * a | 73.01 ± 2.95 b |
| Hydroxyproline (mg/g dry sample) | 12.86 ± 1.00 b | 15.21 ± 0.33 a |
| Moisture content (%, wet weight basis) | 5.33 ± 0.04 a | 3.08 ± 0.01 b |
| Protein content (%, dry weight basis) | 27.77 ± 0.16 a | 24.57 ± 0.05 b |
| Fat content (%, dry weight basis) | 5.46 ± 0.03 a | 3.76 ± 0.17 b |
| Ash content (%, dry weight basis) | 58.66 ± 0.33 a | 61.43 ± 0.01 b |
| Calcium (Ca) content (%, dry weight basis) | 23.66 ± 0.60 b | 26.18 ± 0.17 a |
| Phosphorus (P) content (%, dry weight basis) | 14.07 ± 0.08 b | 14.51 ± 0.19 a |
| Ca/P mole ratio | 1.30 | 1.39 |
| L* | 84.44 ± 0.78 a | 83.21 ± 0.70 b |
| a* | 1.51 ± 0.34 a | 1.90 ± 0.27 a |
| b* | 15.24 ± 1.29 a | 15.54 ± 1.38 a |
| ΔE* | 17.19 ± 1.54 a | 18.13 ± 1.56 a |
| Mean particle size (μm) | 15.33 ± 0.40 a | 14.03 ± 0.23 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Benjakul, S.; Pomtong, S.; Chedosama, A.; Saetang, J.; Sookchoo, P.; Nilsuwan, K. Chemical Compositions and Characteristics of Biocalcium from Pre-Cooked Tuna Bone as Influenced by Sodium Chloride Pretreatment and Defatting by Asian Seabass Lipase. Foods 2024, 13, 1261. https://doi.org/10.3390/foods13081261
Benjakul S, Pomtong S, Chedosama A, Saetang J, Sookchoo P, Nilsuwan K. Chemical Compositions and Characteristics of Biocalcium from Pre-Cooked Tuna Bone as Influenced by Sodium Chloride Pretreatment and Defatting by Asian Seabass Lipase. Foods. 2024; 13(8):1261. https://doi.org/10.3390/foods13081261
Chicago/Turabian StyleBenjakul, Soottawat, Saowakon Pomtong, Afeefah Chedosama, Jirakrit Saetang, Pornsatit Sookchoo, and Krisana Nilsuwan. 2024. "Chemical Compositions and Characteristics of Biocalcium from Pre-Cooked Tuna Bone as Influenced by Sodium Chloride Pretreatment and Defatting by Asian Seabass Lipase" Foods 13, no. 8: 1261. https://doi.org/10.3390/foods13081261





