Analysis of Fungal Diversity, Physicochemical Properties and Volatile Organic Compounds of Strong-Flavor Daqu from Seven Different Areas
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Collection
2.2. DNA Extraction, PCR Amplification, and Illumina MiSeq Sequencing
2.3. Analysis of Physicochemical Properties
2.4. Detection of Volatile Organic Compounds (VOCs) in Daqu
2.5. Statistical Analysis
3. Results and Discussion
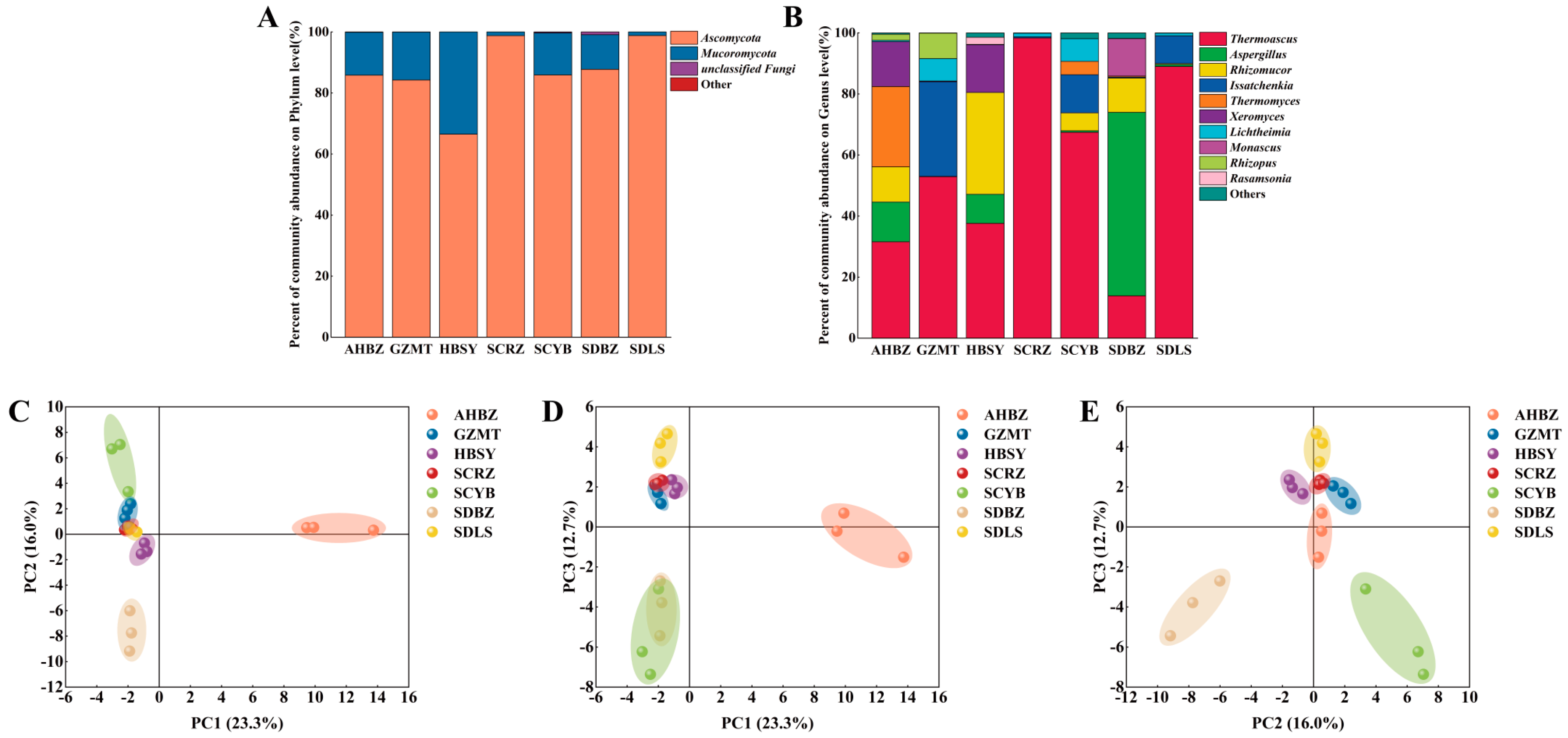
3.1. Microbial Community Structure of Daqu from Different Origins
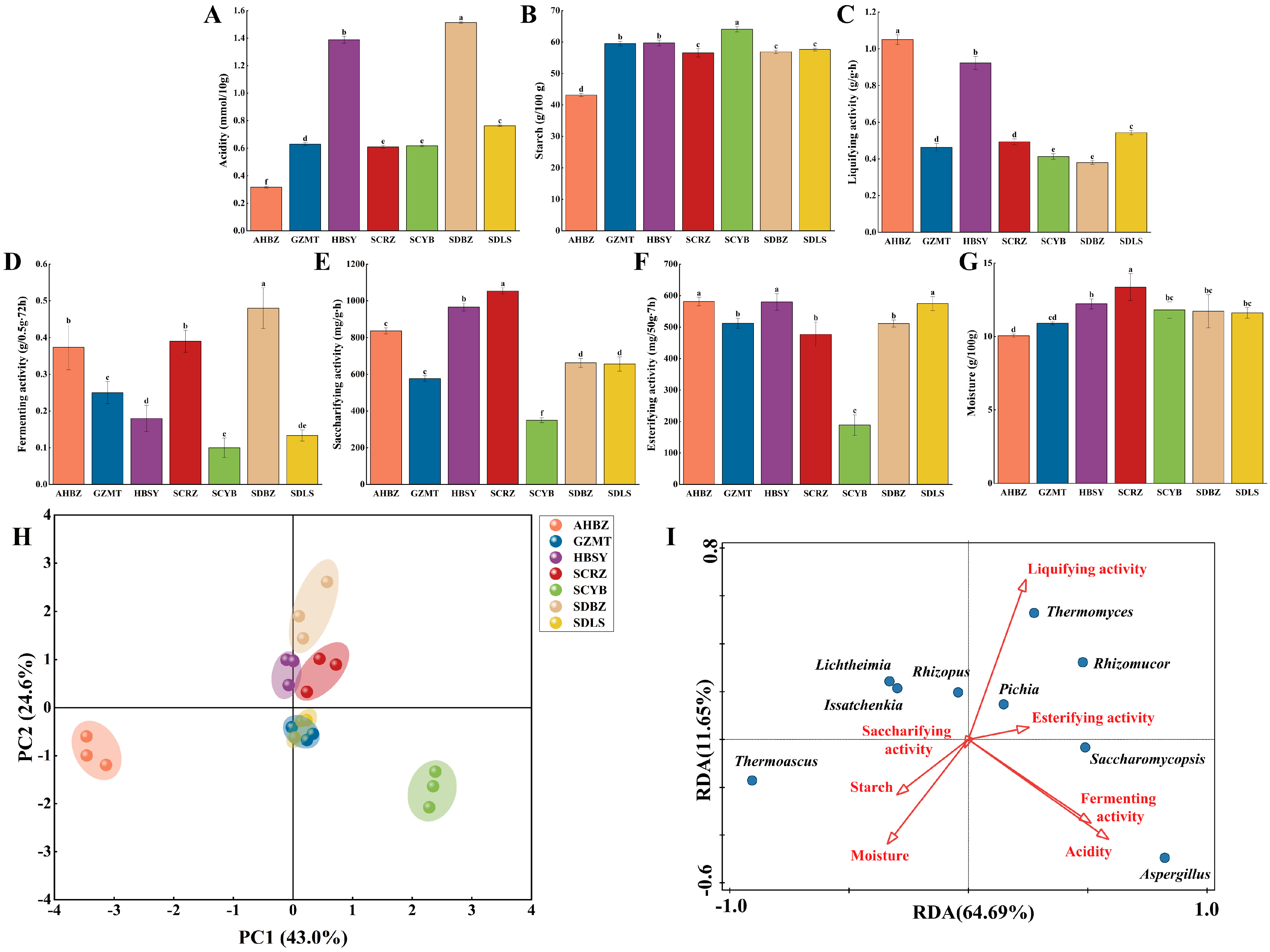
3.2. Physicochemical Properties of Daqu from Different Origins
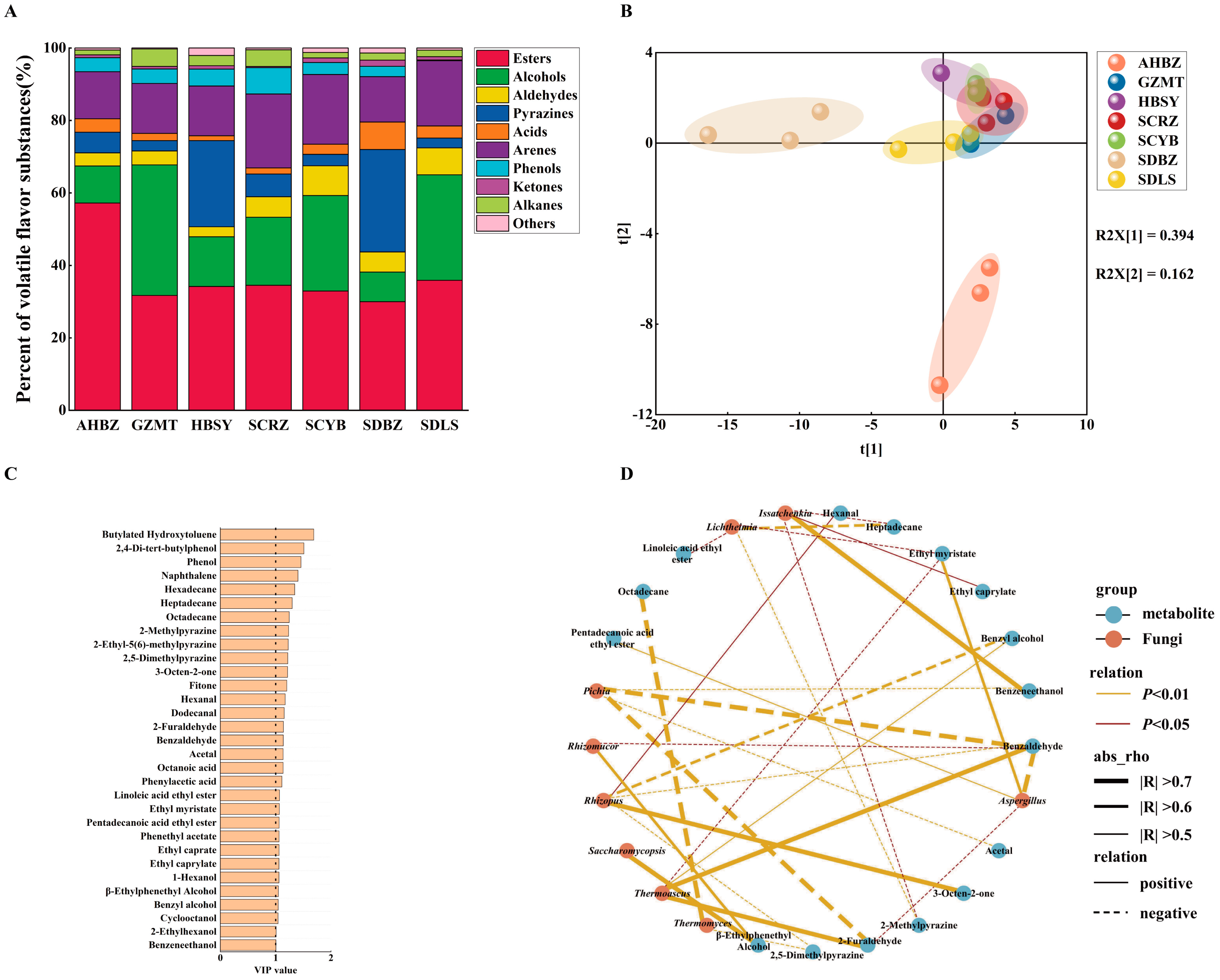
3.3. Volatile Organic Compounds of Daqu from Different Origins
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liu, H.L.; Sun, B.G. Effect of fermentation processing on the flavor of Baijiu. J. Agric. Food Chem. 2018, 66, 5425–5432. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.K.; Tang, Y.M.; Guo, X.J.; Zhao, K.; Penttinen, P.; Tian, X.H.; Zhang, X.Y.; Ren, D.Q.; Zhang, X.P. Structural and functional changes in prokaryotic communities in artificial pit mud during Chinese Baijiu production. mSystems 2020, 5, 17. [Google Scholar] [CrossRef] [PubMed]
- Gou, M.; Wang, H.Z.; Yuan, H.W.; Zhang, W.X.; Tang, Y.Q.; Kida, K. Characterization of the microbial community in three types of fermentation starters used for Chinese liquor production. J. Inst. Brew. 2015, 121, 620–627. [Google Scholar] [CrossRef]
- Wang, H.Y.; Gao, Y.B.; Fan, Q.W.; Xu, Y. Characterization and comparison of microbial community of different typical Chinese liquor Daqus by PCR-DGGE. Lett. Appl. Microbiol. 2011, 53, 134–140. [Google Scholar] [CrossRef] [PubMed]
- Xiao, C.; Yang, Y.; Lu, Z.M.; Chai, L.J.; Zhang, X.J.; Wang, S.T.; Shen, C.H.; Shi, J.S.; Xu, Z.H. Daqu microbiota exhibits species-specific and periodic succession features in Chinese Baijiu fermentation process. Food Microbiol. 2021, 98, 9. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.T.; Cheng, Y.X.; Shi, Q.L.; Ge, X.Y.; Yang, Y.; Huang, Y.G. Metagenomic analyses reveal microbial communities and functional differences between Daqu from seven provinces. Food Res. Int. 2023, 172, 15. [Google Scholar] [CrossRef]
- Zheng, X.W.; Yan, Z.; Nout, M.J.R.; Boekhout, T.; Han, B.Z.; Zwietering, M.H.; Smid, E.J. Characterization of the microbial community in different types of Daqu samples as revealed by 16S rRNA and 26S rRNA gene clone libraries. World J. Microbiol. Biotechnol. 2015, 31, 199–208. [Google Scholar] [CrossRef] [PubMed]
- Du, H.; Wang, X.S.; Zhang, Y.H.; Xu, Y. Exploring the impacts of raw materials and environments on the microbiota in Chinese Daqu starter. Int. J. Food Microbiol. 2019, 297, 32–40. [Google Scholar] [CrossRef]
- Fan, W.; Qian, M.C. Characterization of aroma compounds of Chinese “Wuliangye” and “Jiannanchun” liquors by aroma extract dilution analysis. J. Agric. Food Chem. 2006, 54, 2695–2704. [Google Scholar] [CrossRef]
- QB/T 4257 2011; Ministry of Industry and Information Technology of the People’s Republic of China, General analysis Method of Brewing Daqu (QB/T4257 2011). China Light Industry Press: Beijing, China, 2011.
- Yang, J.G.; Dou, X.; Han, P.J.; Bai, F.Y.; Zhou, J.; Zhang, S.Y.; Qin, H.; Ma, Y.Y. Microbial diversity in Daqu during production of Luzhou-flavored liquor. J. Am. Soc. Brew. Chem. 2017, 75, 136–144. [Google Scholar] [CrossRef]
- McClendon, S.D.; Batth, T.; Petzold, C.J.; Adams, P.D.; Simmons, B.A.; Singer, S.W. Thermoascus aurantiacus is a promising source of enzymes for biomass deconstruction under thermophilic conditions. Biotechnol. Biofuels 2012, 5, 9. [Google Scholar] [CrossRef]
- Zhang, Y.Y.; Zhu, X.Y.; Li, X.Z.; Tao, Y.; Jia, J.; He, X.H. The process-related dynamics of microbial community during a simulated fermentation of Chinese strong-flavored liquor. BMC Microbiol. 2017, 17, 10. [Google Scholar] [CrossRef]
- Li, P.; Aflakpui, F.W.K.; Yu, H.; Luo, L.X.; Lin, W.T. Characterization of activity and microbial diversity of typical types of Daqu for traditional Chinese vinegar. Ann. Microbiol. 2015, 65, 2019–2027. [Google Scholar] [CrossRef]
- Guan, T.W.; Yang, H.; Ou, M.Y.; Zhang, J.X. Storage period affecting dynamic succession of microbiota and quality changes of Strong-flavor Baijiu Daqu. LWT Food Sci. Technol. 2021, 139, 11. [Google Scholar] [CrossRef]
- Jin, G.Y.; Zhu, Y.; Xu, Y. Mystery behind Chinese liquor fermentation. Trends Food Sci. Technol. 2017, 63, 18–28. [Google Scholar] [CrossRef]
- Yan, Z.K.; Feng, Y.F.; Meng, Q.Y.; Wang, X.Z.; Gou, J.Y.; Fang, H.Z.; Du, J.; Duan, K.L. Research on the perspective of microbial diversity and physicochemical properties of Xifeng-Daqu’s culturable microorganisms. Liquor. Mak. 2015, 3, 36–41. [Google Scholar] [CrossRef]
- Hou, X.G.; Hui, M.; Sun, Z.K.; Li, X.S.; Shi, X.; Xiao, R.; Wang, J.F.; Pan, C.M.; Li, R.F. Comparative analysis of the microbiotas and physicochemical properties inside and outside medium-temperature Daqu during the fermentation and storage. Front. Microbiol. 2022, 13, 14. [Google Scholar] [CrossRef]
- Deng, L.; Mao, X.; Liu, D.; Ning, X.Q.; Shen, Y.; Chen, B.; Nie, H.F.; Huang, D.; Luo, H.B. Comparative analysis of physicochemical properties and microbial composition in high-temperature Daqu with different colors. Front. Microbiol. 2020, 11, 13. [Google Scholar] [CrossRef]
- Su, Y.; Yang, L.; Hui, L.; Ge, Y.Y.; Zhang, M.J.; Xin, C.H.; Ling, X.; Chi, C. Bacterial communities during the process of high-temperature Daqu production of roasted sesame-like flavour liquor. J. Inst. Brew. 2015, 121, 440–448. [Google Scholar] [CrossRef]
- He, Z.G.; Yin, Y.C.; Mao, Y.Z.; Zhou, S.X.; Pan, Q.; Wei, D.Z.; Gao, B. Cloning and high-level expression of thermostable α-amylase gene from Rhizomucor pusillus. J. South. Agric. 2014, 45, 165–172. [Google Scholar] [CrossRef]
- Xia, Y.; Zhu, M.; Du, Y.K.; Wu, Z.Y.; Gomi, K.; Zhang, W.X. Metaproteomics reveals protein composition of multiple saccharifying enzymes in Nongxiangxing Daqu and Jiangxiangxing Daqu under different thermophilic temperatures. Int. J. Food Sci. Technol. 2022, 57, 5102–5113. [Google Scholar] [CrossRef]
- Londoño-Hernández, L.; Ramírez-Toro, C.; Ruiz, H.A.; Ascacio-Valdés, J.A.; Aguilar-Gonzalez, M.A.; Rodríguez-Herrera, R.; Aguilar, C.N. Rhizopus oryzae-ancient microbial resource with importance in modern food industry. Int. J. Food Microbiol. 2017, 257, 110–127. [Google Scholar] [CrossRef]
- Jin, Y.; Li, D.Y.; Ai, M.; Tang, Q.X.; Huang, J.; Ding, X.F.; Wu, C.D.; Zhou, R.Q. Correlation between volatile profiles and microbial communities: A metabonomic approach to study Jiang-flavor liquor Daqu. Food Res. Int. 2019, 121, 422–432. [Google Scholar] [CrossRef]
- Wang, Y.; Quan, S.K.; Zhao, Y.J.; Xia, Y.; Zhang, R.; Ran, M.F.; Wu, Z.Y.; Zhang, W.X. The active synergetic microbiota with Aspergillus as the core dominates the metabolic network of ester synthesis in medium-high temperature Daqu. Food Microbiol. 2023, 115, 12. [Google Scholar] [CrossRef]
- Xu, Y.Q.; Zhao, J.R.; Liu, X.; Zhang, C.S.; Zhao, Z.G.; Li, X.T.; Sun, B.G. Flavor mystery of Chinese traditional fermented Baijiu: The great contribution of ester compounds. Food Chem. 2022, 369, 13. [Google Scholar] [CrossRef]
- Shen, S.M.; Liu, J.L.; Luo, R.Q.; Zhang, J.J.; Zhao, D.; Xue, X.X.; Zheng, J.; Qiao, Z.W.; Zhang, Q.; Feng, Z.; et al. Analysis of the influence of microbial community structure on flavor composition of Jiang-Flavor Liquor in different batches of pre-pit fermented grains. Fermentation 2022, 8, 671. [Google Scholar] [CrossRef]
- Chen, S.; Xu, Y.; Qian, M.C. Comparison of the aromatic profile of traditional and modern types of Huang Jiu (Chinese rice wine) by aroma extract dilution analysis and chemical analysis. Flavour Frag. J. 2018, 33, 263–271. [Google Scholar] [CrossRef]
- Zhang, C.L.; Ao, Z.H.; Chui, W.Q.; Shen, C.H.; Tao, W.Y.; Zhang, S.Y. Characterization of volatile compounds from Daqu-a traditional Chinese liquor fermentation starter. Int. J. Food Sci. Technol. 2011, 46, 1591–1599. [Google Scholar] [CrossRef]
- He, F.; Duan, J.W.; Zhao, J.W.; Li, H.H.; Sun, J.Y.; Huang, M.Q.; Sun, B.G. Different distillation stages Baijiu classification by temperature-programmed headspace-gas chromatography-ion mobility spectrometry and gas chromatography-olfactometry-mass spectrometry combined with chemometric strategies. Food Chem. 2021, 365, 10. [Google Scholar] [CrossRef]
- Xia, Y.; Luo, H.B.; Wu, Z.Y.; Zhang, W.X. Microbial diversity in Jiuqu and its fermentation features: Saccharification, alcohol fermentation and flavors generation. Appl. Microbiol. Biotechnol. 2023, 107, 25–41. [Google Scholar] [CrossRef]
- Gao, X.L.; Zhao, X.; Hu, F.; Fu, J.Y.; Zhang, Z.K.; Liu, Z.; Wang, B.; He, R.H.; Ma, H.L.; Ho, C.T. The latest advances on soy sauce research in the past decade: Emphasis on the advances in China. Food Res. Int. 2023, 173, 21. [Google Scholar] [CrossRef]
- Pang, Z.; Hao, J.; Li, W.; Du, B.; Guo, C.; Li, X.; Sun, B. Investigation into spatial profile of microbial community dynamics and flavor metabolites during the bioaugmented solid-state fermentation of Baijiu. Food Biosci. 2023, 56, 103–292. [Google Scholar] [CrossRef]
- Hong, J.X.; Huang, H.; Zhao, D.R.; Sun, J.Y.; Huang, M.Q.; Sun, X.T.; Sun, B.G. Investigation on the key factors associated with flavor quality in northern strong aroma type of Baijiu by flavor matrix. Food Chem. 2023, 426, 11. [Google Scholar] [CrossRef]
- He, M.W.; Jin, Y.; Zhou, R.Q.; Zhao, D.; Zheng, J.; Wu, C.D. Dynamic succession of microbial community in Nongxiangxing Daqu and microbial roles involved in flavor formation. Food Res. Int. 2022, 159, 11. [Google Scholar] [CrossRef]
- Fan, G.S.; Sun, B.G.; Xu, D.; Teng, C.; Fu, Z.L.; Du, Y.H.; Li, X.T. Isolation and identifation of nigh-yield ethyl acetate-producing yeast from Gujinggony Daqu and its fermentation characteristics. J. Am. Soc. Brew. Chem. 2018, 76, 117–124. [Google Scholar] [CrossRef]
- Niu, Y.W.; Kong, J.L.; Xiao, Z.B.; Chen, F.; Ma, N.; Zhu, J.C. Characterization of odor-active compounds of various Chinese “Wuliangye” liquors by gas chromatography-olfactometry, gas chromatography-mass spectrometry and sensory evaluation. Int. J. Food Prop. 2017, 20, 735–745. [Google Scholar] [CrossRef]
- Zhang, C.L.; Ao, Z.H.; Chui, W.Q.; Shen, C.H.; Tao, W.Y.; Zhang, S.Y. Characterization of the aroma-active compounds in Daqu: A tradition Chinese liquor starter. Eur. Food Res. Technol. 2012, 234, 69–76. [Google Scholar] [CrossRef]

| Sobs | Shannon | Simpson | Ace | Chao 1 | Coverage | |
|---|---|---|---|---|---|---|
| AHBZ | 67.33 ± 7.77 a | 1.69 ± 0.17 a | 0.26 ± 0.06 c | 95.00 ± 21.43 a | 85.56 ± 14.49 a | 0.9999 |
| GZMT | 36.33 ± 2.89 c | 1.30 ± 0.20 b | 0.39 ± 0.11 bc | 68.21 ± 11.91 b | 53.83 ± 4.04 b | 0.9999 |
| HBSY | 28.33 ± 8.14 cd | 1.35 ± 0.29 ab | 0.31 ± 0.09 c | 36.25 ± 12.58 c | 33.07 ± 11.68 c | 0.9999 |
| SCRZ | 26.00 ± 5.20 d | 0.30 ± 0.07 c | 0.89 ± 0.03 a | 39.59 ± 2.90 c | 32.78 ± 3.02 c | 0.9999 |
| SCYB | 48.33 ± 5.51 b | 1.22 ± 0.29 b | 0.48 ± 0.12 b | 55.09 ± 6.49 bc | 56.17 ± 11.59 b | 0.9999 |
| SDBZ | 47.33 ± 1.53 b | 1.23 ± 0.10 b | 0.41 ± 0.06 bc | 58.57 ± 2.83 bc | 55.38 ± 4.55 b | 0.9999 |
| SDLS | 32.67 ± 1.15 cd | 0.49 ± 0.12 c | 0.80 ± 0.06 a | 50.23 ± 15.82 bc | 45.58 ± 9.88 bc | 0.9999 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Z.; Yan, X.; Zou, S.; Ji, C.; Dong, L.; Zhang, S.; Liang, H.; Lin, X. Analysis of Fungal Diversity, Physicochemical Properties and Volatile Organic Compounds of Strong-Flavor Daqu from Seven Different Areas. Foods 2024, 13, 1263. https://doi.org/10.3390/foods13081263
Li Z, Yan X, Zou S, Ji C, Dong L, Zhang S, Liang H, Lin X. Analysis of Fungal Diversity, Physicochemical Properties and Volatile Organic Compounds of Strong-Flavor Daqu from Seven Different Areas. Foods. 2024; 13(8):1263. https://doi.org/10.3390/foods13081263
Chicago/Turabian StyleLi, Zhigao, Xu Yan, Sibo Zou, Chaofan Ji, Liang Dong, Sufang Zhang, Huipeng Liang, and Xinping Lin. 2024. "Analysis of Fungal Diversity, Physicochemical Properties and Volatile Organic Compounds of Strong-Flavor Daqu from Seven Different Areas" Foods 13, no. 8: 1263. https://doi.org/10.3390/foods13081263