Ultrasonic-Assisted Extraction of Dictyophora rubrovolvata Volva Proteins: Process Optimization, Structural Characterization, Intermolecular Forces, and Functional Properties
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Optimization of UAE Process of DPVP
2.2.1. Extraction Method and Determination of Proteins Extraction Rate
2.2.2. Single-Factor and Orthogonal Experiments Design
2.3. Determinations of the Physicochemical Properties
2.3.1. Microstructures
2.3.2. Turbidity
2.3.3. Particle Size Distribution and Zeta Potential
2.3.4. Thermal Properties
2.4. Characterization of the Structures
2.4.1. Amino Acid Profile
2.4.2. Molecular Weight Distribution
2.4.3. Secondary Structure
2.4.4. Surface Hydrophobicity (H0)
2.4.5. Free Sulfhydryl (SH) Content
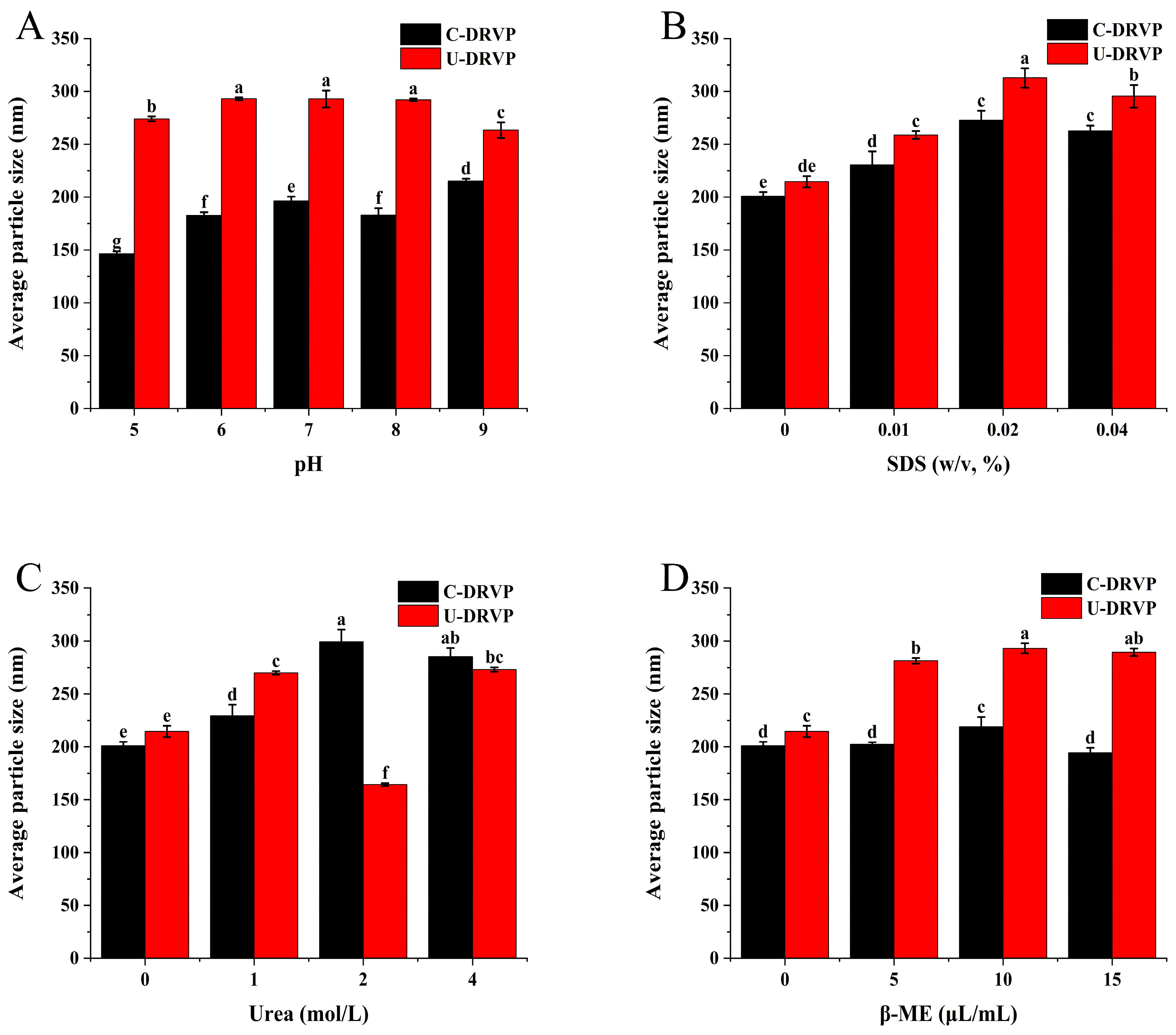
2.5. Proteins Aggregates Dissociation Test
2.6. Measurements of Functional Properties
2.6.1. Solubility
2.6.2. Water Holding Capacity (WHC) and Oil Holding Capacity (OHC)
2.6.3. Emulsion Activity Index (EAI) and Emulsion Stability Index (ESI)
2.6.4. Foaming Capacity (FC) and Foaming Stability (FS)
2.7. Statistical Analysis
3. Results and Discussion
3.1. Optimization of the Extraction Process of DRVPs
3.2. Physical and Chemical Properties of U-DRVP
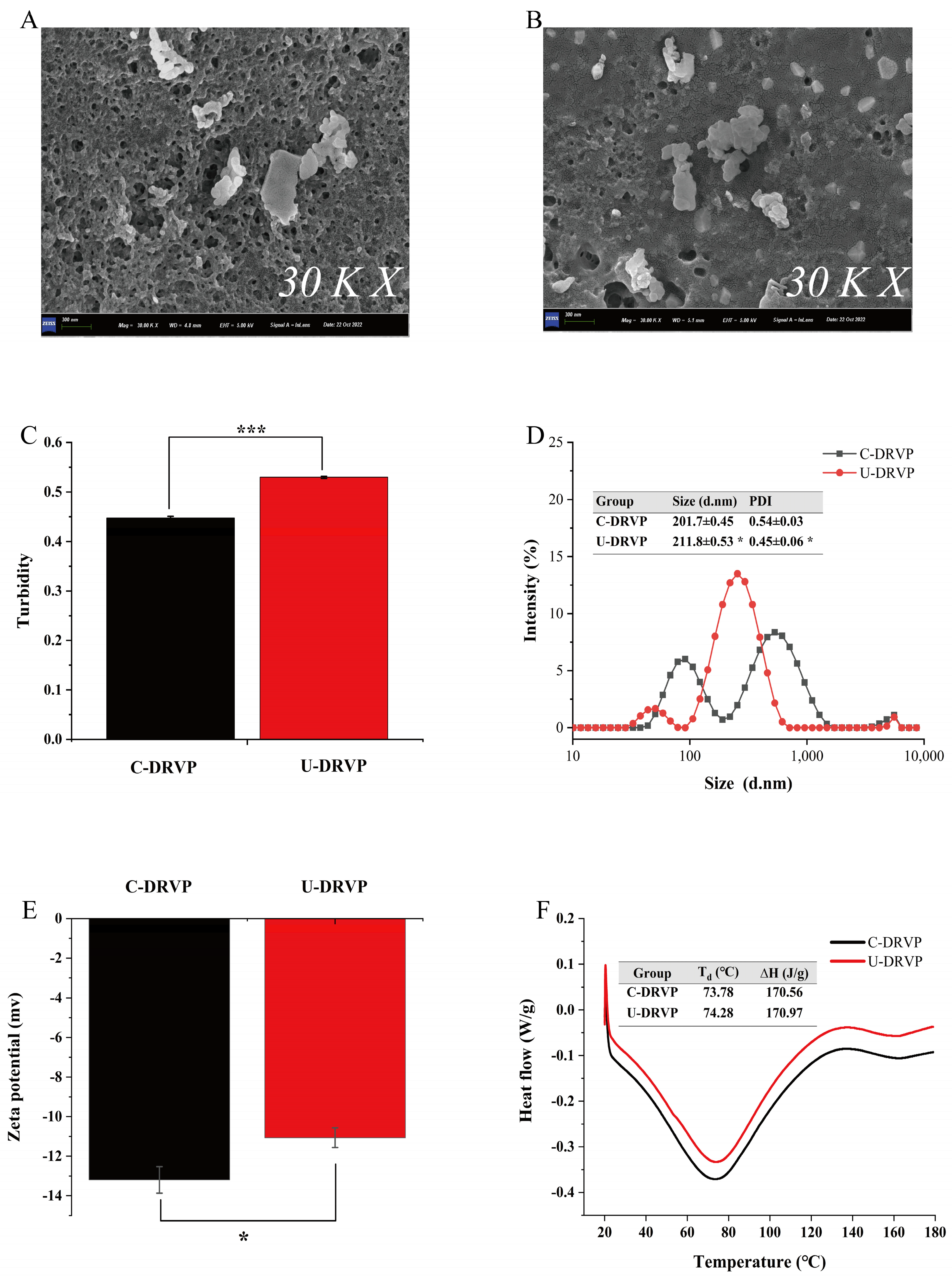
3.2.1. SEM Analysis
3.2.2. Turbidity, Particle Size Distribution, and Zeta-Potential Analysis
3.2.3. Differential Scanning Calorimetry (DSC) Analysis
3.3. Changes in the Structure of U-DRVPs
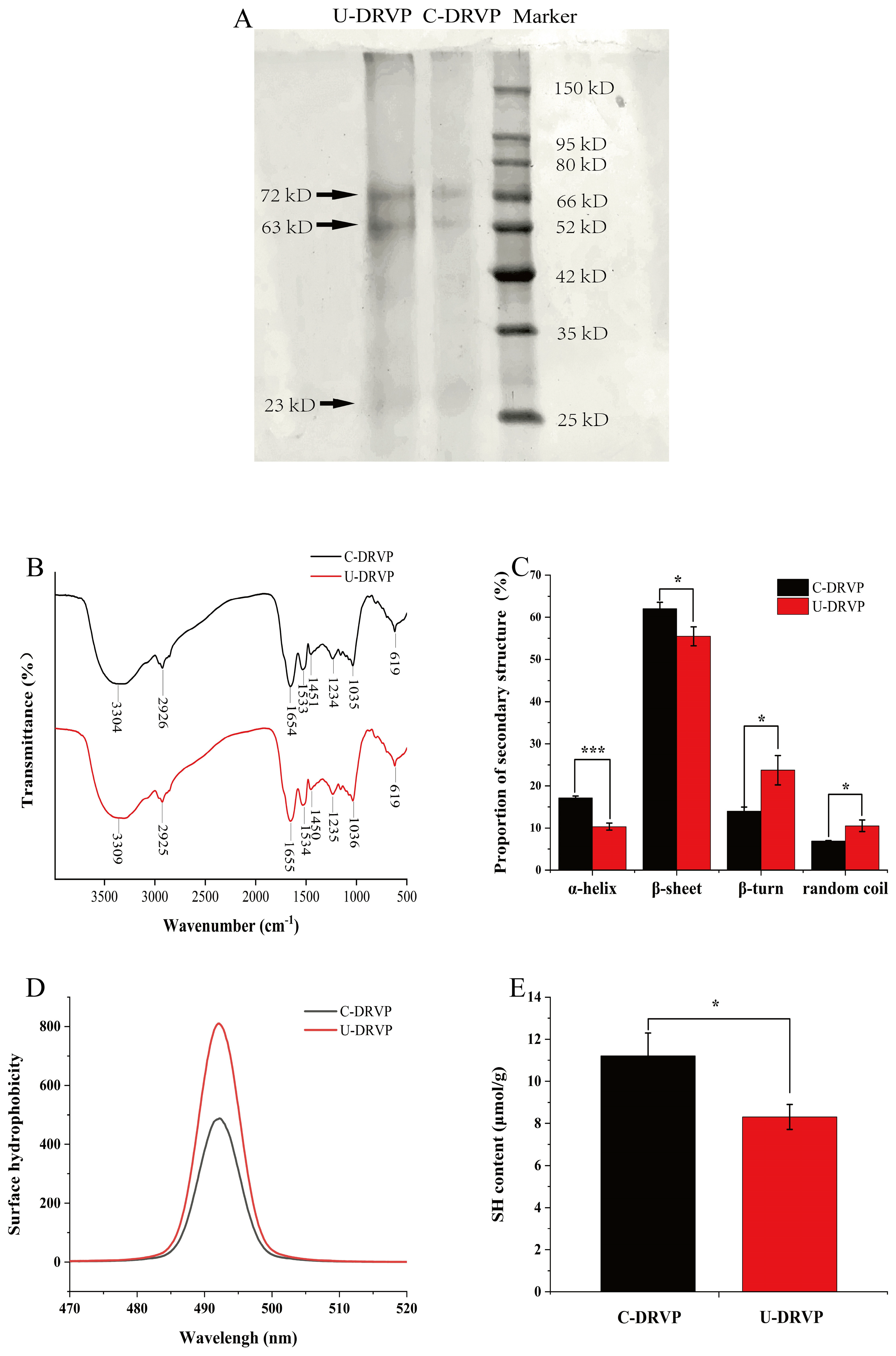
3.3.1. Primary Structure
3.3.2. Secondary Structure
3.3.3. Tertiary Structure
3.4. Intermolecular Forces Analysis
3.4.1. Electrostatic Interactions
3.4.2. Hydrophobic Interaction
3.4.3. Hydrogen Bonding
3.4.4. Disulfide Bonding
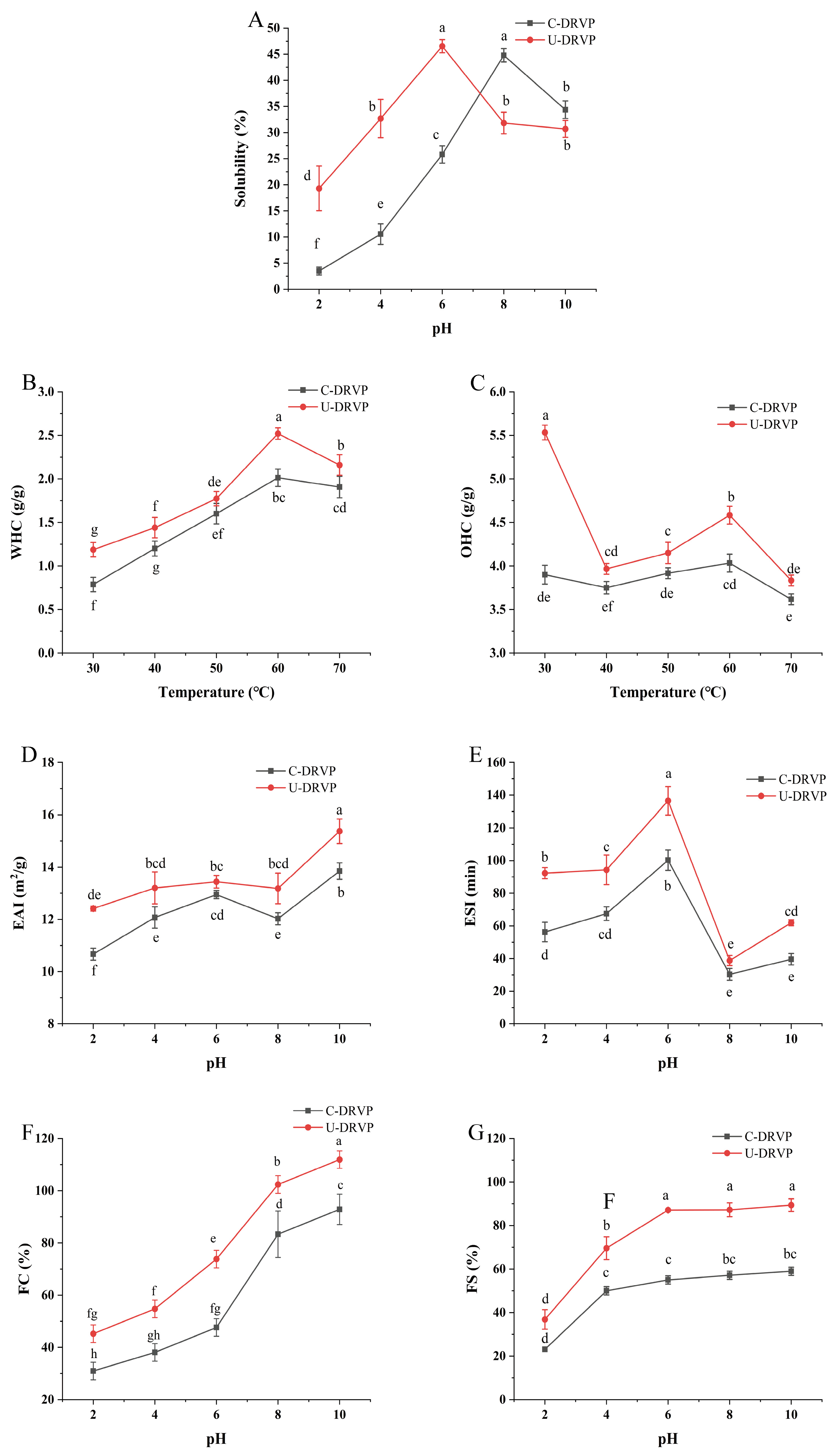
3.5. Functional Properties of DRVPs
3.5.1. Solubility
3.5.2. WHC and OHC
3.5.3. Emulsifying Properties
3.5.4. Foaming Properties
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Colgrave, M.L.; Dominik, S.; Tobin, A.B.; Stockmann, R.; Simon, C.; Howitt, C.A.; Belobrajdic, D.P.; Paull, C.; Vanhercke, T. Perspectives on future protein production. J. Agric. Food Chem. 2021, 69, 15076–15083. [Google Scholar] [CrossRef]
- Zhuang, Y.; Sun, L. Nutritional characteristics of proteins from the volva and pileus in cultivated mushroom Dictyophora rubrovolvata. Int. J. Food Sci. Nutr. 2011, 62, 392–396. [Google Scholar] [CrossRef]
- Bao, K.; Song, M.; Wang, S.; Li, T.; Wang, J.; Cheng, X.; Wang, L.; Wang, S.; Wen, T.; Zhu, Z. Isolation, purification, characterization and immunomodulatory effects of polysaccharides from Dictyophora rubrovalvata waste. Ind. Crops Prod. 2023, 206, 117754. [Google Scholar] [CrossRef]
- Zhang, Y.; Xun, H.; Gao, Q.; Qi, F.; Sun, J.; Tang, F. Chemical constituents of the mushroom Dictyophora indusiata and their anti-inflammatory activities. Molecules 2023, 28, 2760. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Lei, Y.; Qi, S.; Fan, M.; Zheng, S.; Huang, Q.; Lu, X. Ultrasonic-microwave-assisted extraction for enhancing antioxidant activity of Dictyophora indusiata polysaccharides: The difference mechanisms between single and combined assisted extraction. Ultrason. Sonochem. 2023, 95, 106356. [Google Scholar] [CrossRef]
- Guo, Q.; Liang, S.; Xiao, Z.; Ge, C. Research progress on extraction technology and biological activity of polysaccharides from edible fungi: A review. Food Rev. Int. 2023, 39, 4909–4940. [Google Scholar] [CrossRef]
- Riad, N.; Zahi, M.R.; Trovato, E.; Bouzidi, N.; Daghbouche, Y.; Utczás, M.; Mondello, L.; El Hattab, M. Chemical screening and antibacterial activity of essential oil and volatile fraction of Dictyopteris polypodioides. Microchem. J. 2020, 152, 104415. [Google Scholar] [CrossRef]
- Chen, J.; Chen, X.; Zhou, G.; Xu, X. Ultrasound: A reliable method for regulating food component interactions in protein-based food matrices. Trends Food Sci. Technol. 2022, 128, 316–330. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, Y.; Huang, M.; Hayat, K.; Kurtz, N.C.; Wu, X.; Ahmad, M.; Zheng, F. Ultrasound-assisted alkaline proteinase extraction enhances the yield of pecan protein and modifies its functional properties. Ultrason. Sonochem. 2021, 80, 105789. [Google Scholar] [CrossRef]
- Chen, L.; Chen, J.; Ren, J.; Zhao, M. Effects of ultrasound pretreatment on the enzymatic hydrolysis of soy protein isolates and on the emulsifying properties of hydrolysates. J. Agric. Food Chem. 2011, 59, 2600–2609. [Google Scholar] [CrossRef]
- Xie, Y.; Yang, F.; Zhao, K.; Zhang, W.; Liu, Q.; Yuan, Y. Regulation of protein flexibility and promoting the cod protein gel formation using ultrasound treatment. J. Agric. Food Chem. 2023, 71, 18601–18612. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Chen, J.; Wu, K.; Yu, L. Improved low pH emulsification properties of glycated peanut protein isolate by ultrasound Maillard reaction. J. Agric. Food Chem. 2016, 64, 5531–5538. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Wang, Y.; Yan, Y.; Li, Z. Ultrasonic-alkali method for synergistic breakdown of excess sludge for protein extraction. J. Clean. Prod. 2021, 295, 126288. [Google Scholar] [CrossRef]
- Cui, Q.; Ni, X.; Zeng, L.; Tu, Z.; Li, J.; Sun, K.; Chen, X.; Li, X. Optimization of protein extraction and decoloration conditions for tea residues. Hortic. Plant J. 2017, 3, 172–176. [Google Scholar] [CrossRef]
- Huang, D.; Li, W.; Li, G.; Zhang, W.; Chen, H.; Jiang, Y.; Li, D. Effect of high-intensity ultrasound on the physicochemical properties of Tenebrio Molitor protein. Food Hydrocoll. 2023, 134, 108056. [Google Scholar] [CrossRef]
- Zhong, Z.; Xiong, Y.L. Thermosonication-induced structural changes and solution properties of mung bean protein. Ultrason. Sonochem. 2020, 62, 104908. [Google Scholar] [CrossRef] [PubMed]
- Eze, O.F.; Chatzifragkou, A.; Charalampopoulos, D. Properties of protein isolates extracted by ultrasonication from soybean residue (okara). Food Chem. 2022, 368, 130837. [Google Scholar] [CrossRef]
- Li, K.; Fu, L.; Zhao, Y.Y.; Xue, S.W.; Wang, P.; Xu, X.L.; Bai, Y.H. Use of high-intensity ultrasound to improve emulsifying properties of chicken myofibrillar protein and enhance the rheological properties and stability of the emulsion. Food Hydrocoll. 2020, 98, 105275. [Google Scholar] [CrossRef]
- Li, X.; Luo, T.; Wang, L.; Song, H.; Wang, F.; Weng, Z.; Zhou, J.; Xiang, X.; Xiong, L.; Shen, X. Emulsifying properties of wheat germ protein: Effect of different ultrasonic treatment. Ultrason. Sonochem. 2023, 98, 106479. [Google Scholar] [CrossRef]
- Wang, R.; Zhang, L.; Chi, Y.; Chi, Y. Forces involved in freeze-induced egg yolk gelation: Effects of various bond dissociation reagents on gel properties and protein structure changes. Food Chem. 2022, 371, 131190. [Google Scholar] [CrossRef]
- Alavi, F.; Chen, L.; Emam-Djomeh, Z. Effect of ultrasound-assisted alkaline treatment on functional property modifications of faba bean protein. Food Chem. 2021, 354, 129494. [Google Scholar] [CrossRef]
- Li, W.; Yang, H.; Coldea, T.E.; Zhao, H. Modification of structural and functional characteristics of brewer’s spent grain protein by ultrasound assisted extraction. LWT-Food Sci. Technol. 2021, 139, 110582. [Google Scholar] [CrossRef]
- Gordalina, M.; Pinheiro, H.M.; Mateus, M.; da Fonseca, M.M.R.; Cesário, M.T. Macroalgae as protein sources—A review on protein bioactivity, extraction, purification and characterization. Appl. Sci. 2021, 11, 7969. [Google Scholar] [CrossRef]
- Zhao, F.; Liu, X.; Ding, X.; Dong, H.; Wang, W. Effects of high-intensity ultrasound pretreatment on structure, properties, and enzymolysis of soy protein isolate. Molecules 2019, 24, 3637. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Wang, J.; Li, Y.; Wang, Z.; Liang, J.; Wang, R.; Chen, Y.; Ma, W.; Qi, B.; Zhang, M. Effects of ultrasound on the structure and physical properties of black bean protein isolates. Food Res. Int. 2014, 62, 595–601. [Google Scholar] [CrossRef]
- Lv, S.; Taha, A.; Hu, H.; Lu, Q.; Pan, S. Effects of ultrasonic-assisted extraction on the physicochemical properties of different walnut proteins. Molecules 2019, 24, 4260. [Google Scholar] [CrossRef] [PubMed]
- Figueroa-González, J.J.; Lobato-Calleros, C.; Vernon-Carter, E.J.; Aguirre-Mandujano, E.; Alvarez-Ramirez, J.; Martínez-Velasco, A. Modifying the structure, physicochemical properties, and foaming ability of amaranth protein by dual pH-shifting and ultrasound treatments. LWT-Food Sci. Technol. 2022, 153, 112561. [Google Scholar] [CrossRef]
- Wang, F.; Zhang, Y.; Xu, L.; Ma, H. An efficient ultrasound-assisted extraction method of pea protein and its effect on protein functional properties and biological activities. LWT-Food Sci. Technol. 2020, 127, 109348. [Google Scholar] [CrossRef]
- Arzeni, C.; Martínez, K.; Zema, P.; Arias, A.; Pérez, O.E.; Pilosof, A.M.R. Comparative study of high intensity ultrasound effects on food proteins functionality. J. Food Eng. 2012, 108, 463–472. [Google Scholar] [CrossRef]
- Kang, S.; Zhang, J.; Guo, X.; Lei, Y.; Yang, M. Effects of ultrasonic treatment on the structure, functional properties of chickpea protein isolate and its digestibility in vitro. Foods 2022, 11, 880. [Google Scholar] [CrossRef]
- Sun, N.; Wang, Y.; Bao, Z.; Cui, P.; Wang, S.; Lin, S. Calcium binding to herring egg phosphopeptides: Binding characteristics, conformational structure and intermolecular forces. Food Chem. 2020, 310, 125867. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.; Li, Q.; Li, D.; Sun, C.; Bao, Y.; Li, F.; Jiang, S. Optimizing the extraction of protein from broken rice using response surface methodology and comparing the protein functional properties. J. Cereal Sci. 2023, 113, 130726. [Google Scholar] [CrossRef]
- Loushigam, G.; Shanmugam, A. Modifications to functional and biological properties of proteins of cowpea pulse crop by ultrasound-assisted extraction. Ultrason. Sonochem. 2023, 97, 106448. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Qi, B.; Zhang, S.; Li, Y. Effects of ultrasonic treatment on the structural and functional properties of cactus (Opuntia ficus-indica) seed protein. Ultrason. Sonochem. 2023, 97, 106465. [Google Scholar] [CrossRef] [PubMed]
- Gani, A.; Ashraf, Z.; Noor, N.; Ahmed Wani, I. Ultrasonication as an innovative approach to tailor the apple seed proteins into nanosize: Effect on protein structural and functional properties. Ultrason. Sonochem. 2022, 86, 106010. [Google Scholar] [CrossRef] [PubMed]
- Shi, R.; He, Y.; Wang, Q.; Cai, J.; Gantumur, M.; Jiang, Z. Insight into the physicochemical characteristics, functionalities and digestion behavior of protein isolate derived from Lactarius volemus (L.volemus): Impacts of microwave-assisted extraction. Food Chem. 2024, 431, 137070. [Google Scholar] [CrossRef] [PubMed]
- Fang, B.; Chang, L.; Ohm, J.B.; Chen, B.; Rao, J. Structural, functional properties, and volatile profile of hemp protein isolate as affected by extraction method: Alkaline extraction–isoelectric precipitation vs salt extraction. Food Chem. 2023, 405, 135001. [Google Scholar] [CrossRef] [PubMed]
- Görgüç, A.; Bircan, C.; Yılmaz, F.M. Sesame bran as an unexploited by-product: Effect of enzyme and ultrasound-assisted extraction on the recovery of protein and antioxidant compounds. Food Chem. 2019, 283, 637–645. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.S.; Dias, F.F.G.; Pham, T.T.K.; Barile, D.; de Moura Bell, J.M.L.N. A sequential fractionation approach to understanding the physicochemical and functional properties of aqueous and enzyme-assisted aqueous extracted black bean proteins. Food Hydrocoll. 2024, 146, 109250. [Google Scholar] [CrossRef]
- Karabulut, G.; Yemiş, O. Modification of hemp seed protein isolate (Cannabis sativa L.) by high-intensity ultrasound treatment. Part 1: Functional properties. Food Chem. 2022, 375, 131843. [Google Scholar] [CrossRef]

| Amino Acids | Absolute Quantities (mg/g) | Relative Quantities (%) | ||
|---|---|---|---|---|
| C-DRVPs | U-DRVPs | C-DRVPs | U-DRVPs | |
| Histidine (His) | 16.98 b | 18.79 a | 2.48 A | 2.50 A |
| Isoleucine (Ile) | 39.04 b | 43.01 a | 5.71 A | 5.72 A |
| Leucine (Leu) | 53.96 b | 60.54 a | 7.89 B | 8.05 A |
| Lysine (Lys) | 24.16 b | 27.65 a | 3.53 B | 3.68 A |
| Methionine (Met) | 9.06 b | 9.89 a | 1.33 A | 1.31 A |
| Phenylalanine (Phe) | 47.28 b | 51.68 a | 6.91 A | 6.87 A |
| Threonine (Thr) | 52.64 b | 56.99 a | 7.70 A | 7.58 B |
| Valine (Val) | 43.74 b | 47.98 a | 6.39 A | 6.38 A |
| Aspartic acid (Asp) | 85.52 b | 93.15 a | 12.51 A | 12.39 B |
| Glutamic acid (Glu) | 77.70 b | 86.48 a | 11.36 A | 11.50 A |
| Serine (Ser) | 46.66 b | 50.81 a | 6.82 A | 6.76 B |
| Cystine (Cys) | 2.26 b | 2.44 a | 0.33 A | 0.33 A |
| Glycine (Gly) | 40.54 b | 44.51 a | 5.93 A | 5.92 A |
| Tyrosine (Tyr) | 27.02 b | 29.76 a | 3.95 A | 3.96 A |
| Arginine (Arg) | 26.24 b | 29.42 a | 3.84 B | 3.91 A |
| Alanine (Ala) | 44.86 b | 49.66 a | 6.56 A | 6.60 A |
| Proline (Pro) | 46.24 b | 49.15 a | 6.76 A | 6.54 B |
| HAA | 324.72 b | 356.41 a | 49.25 A | 49.05 B |
| EAA | 286.86 b | 316.53 a | 41.94 B | 42.09 A |
| NCAA | 163.22 b | 179.63 a | 23.87 A | 23.89 A |
| PCAA | 67.38 b | 75.85 a | 9.85 B | 10.09 A |
| Total | 683.90 b | 751.89 a | 100.00 A | 100.00 A |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Wei, S.; Xiong, Q.; Meng, L.; Li, Y.; Ge, Y.; Guo, M.; Luo, H.; Lin, D. Ultrasonic-Assisted Extraction of Dictyophora rubrovolvata Volva Proteins: Process Optimization, Structural Characterization, Intermolecular Forces, and Functional Properties. Foods 2024, 13, 1265. https://doi.org/10.3390/foods13081265
Zhang Y, Wei S, Xiong Q, Meng L, Li Y, Ge Y, Guo M, Luo H, Lin D. Ultrasonic-Assisted Extraction of Dictyophora rubrovolvata Volva Proteins: Process Optimization, Structural Characterization, Intermolecular Forces, and Functional Properties. Foods. 2024; 13(8):1265. https://doi.org/10.3390/foods13081265
Chicago/Turabian StyleZhang, Yongqing, Shinan Wei, Qinqin Xiong, Lingshuai Meng, Ying Li, Yonghui Ge, Ming Guo, Heng Luo, and Dong Lin. 2024. "Ultrasonic-Assisted Extraction of Dictyophora rubrovolvata Volva Proteins: Process Optimization, Structural Characterization, Intermolecular Forces, and Functional Properties" Foods 13, no. 8: 1265. https://doi.org/10.3390/foods13081265