Contemporary Views of the Extraction, Health Benefits, and Industrial Integration of Rice Bran Oil: A Prominent Ingredient for Holistic Human Health
Abstract
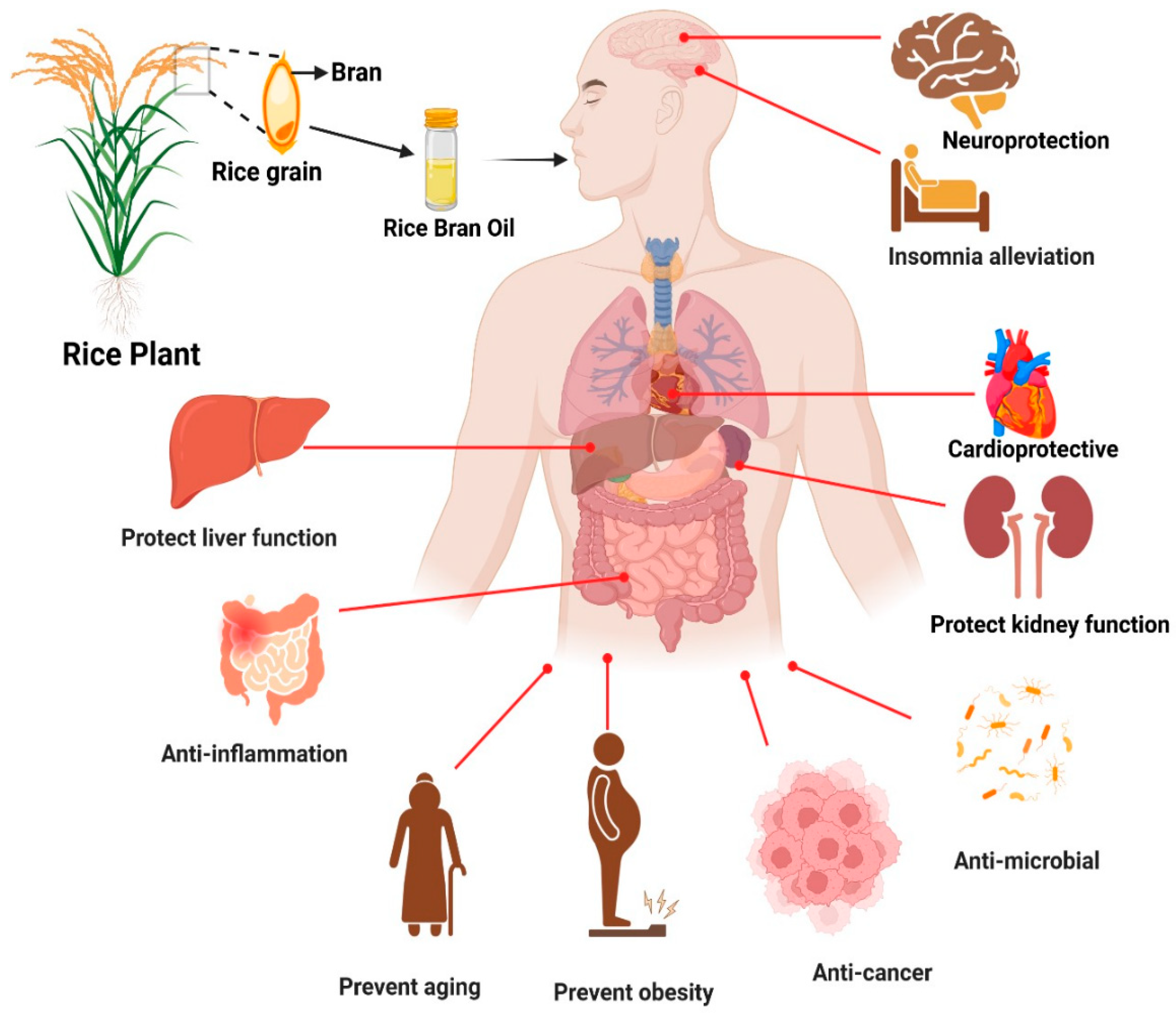
:1. Introduction
2. Stabilization of Rice Bran
2.1. Stabilization Techniques of Rice Bran Oil
2.1.1. Microwave Heating
2.1.2. Extrusion
2.1.3. Dry Heating
2.1.4. Infrared Heating
2.1.5. Low-Temperature Treatment
2.1.6. Ohmic Heating
2.1.7. Biological Treatment
2.1.8. Moisture Heating
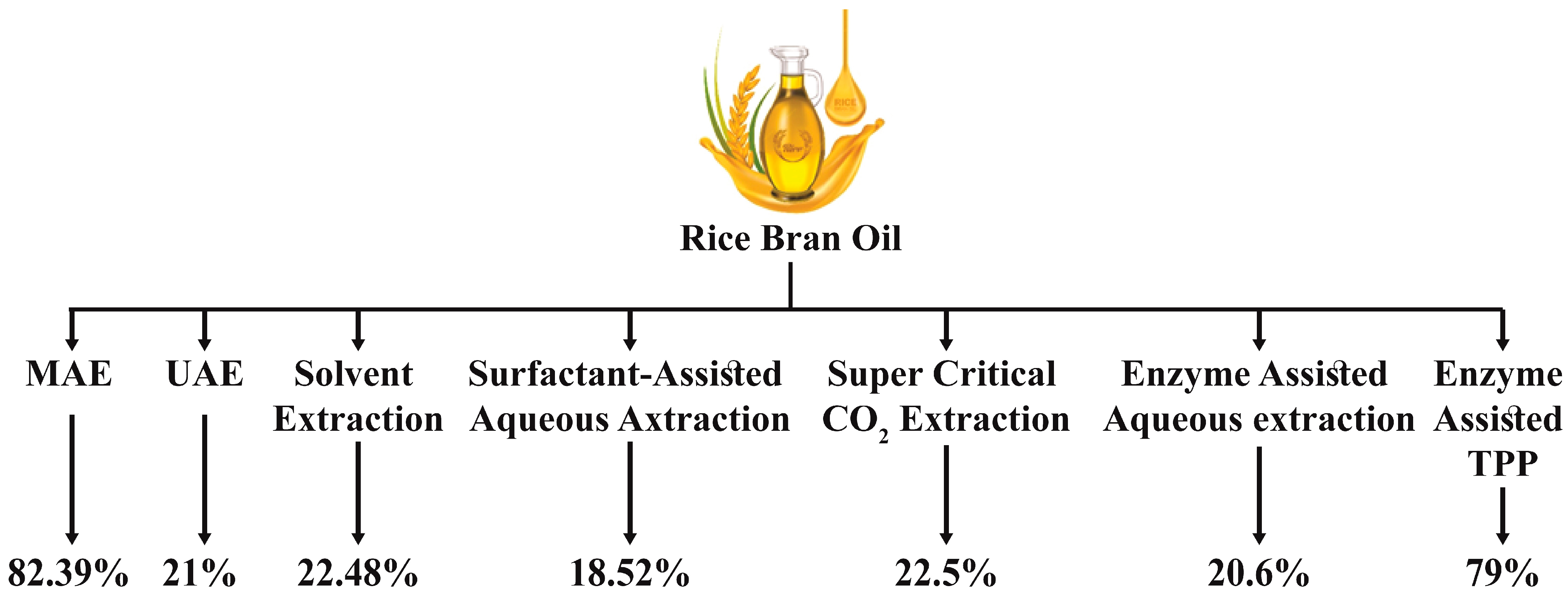
3. Extraction of Rice Bran Oil
3.1. Mechanical Pressing (Cold Pressing)
3.1.1. Soxhlet Extraction
3.1.2. Supercritical Fluid Extraction
3.1.3. Sub-Critical Fluids Extraction
3.1.4. Assisting Enzymes Aqueous Extraction
3.1.5. Microwave-Assisted Aqueous Extraction
3.1.6. Ultrasound-Assisted Extraction
3.1.7. Solvent Extraction
3.1.8. Surfactant-Assisted Aqueous Extraction
3.1.9. Enzyme Assisted Three Phase Partitioning
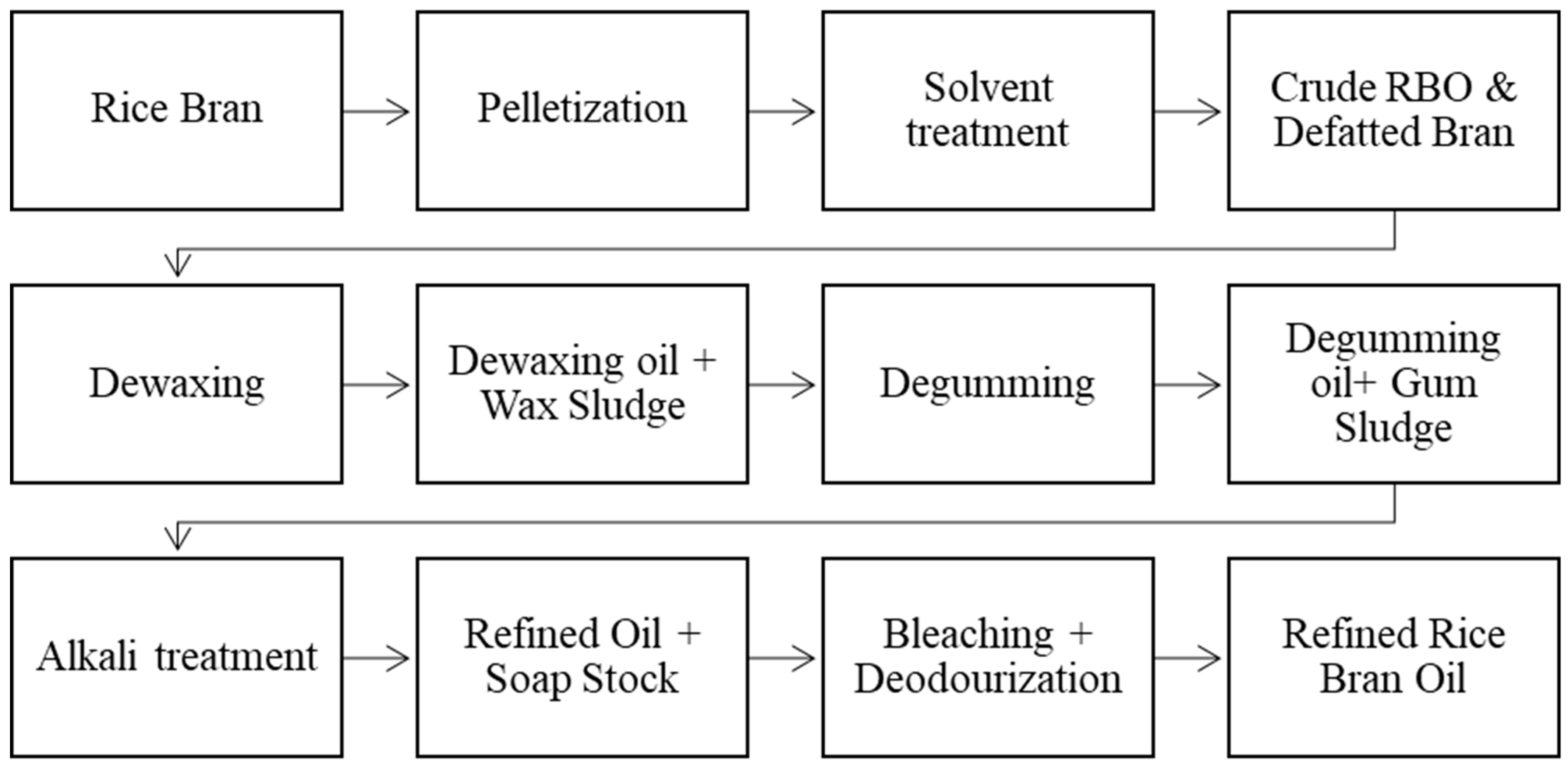
4. Refining of Rice Bran Oil
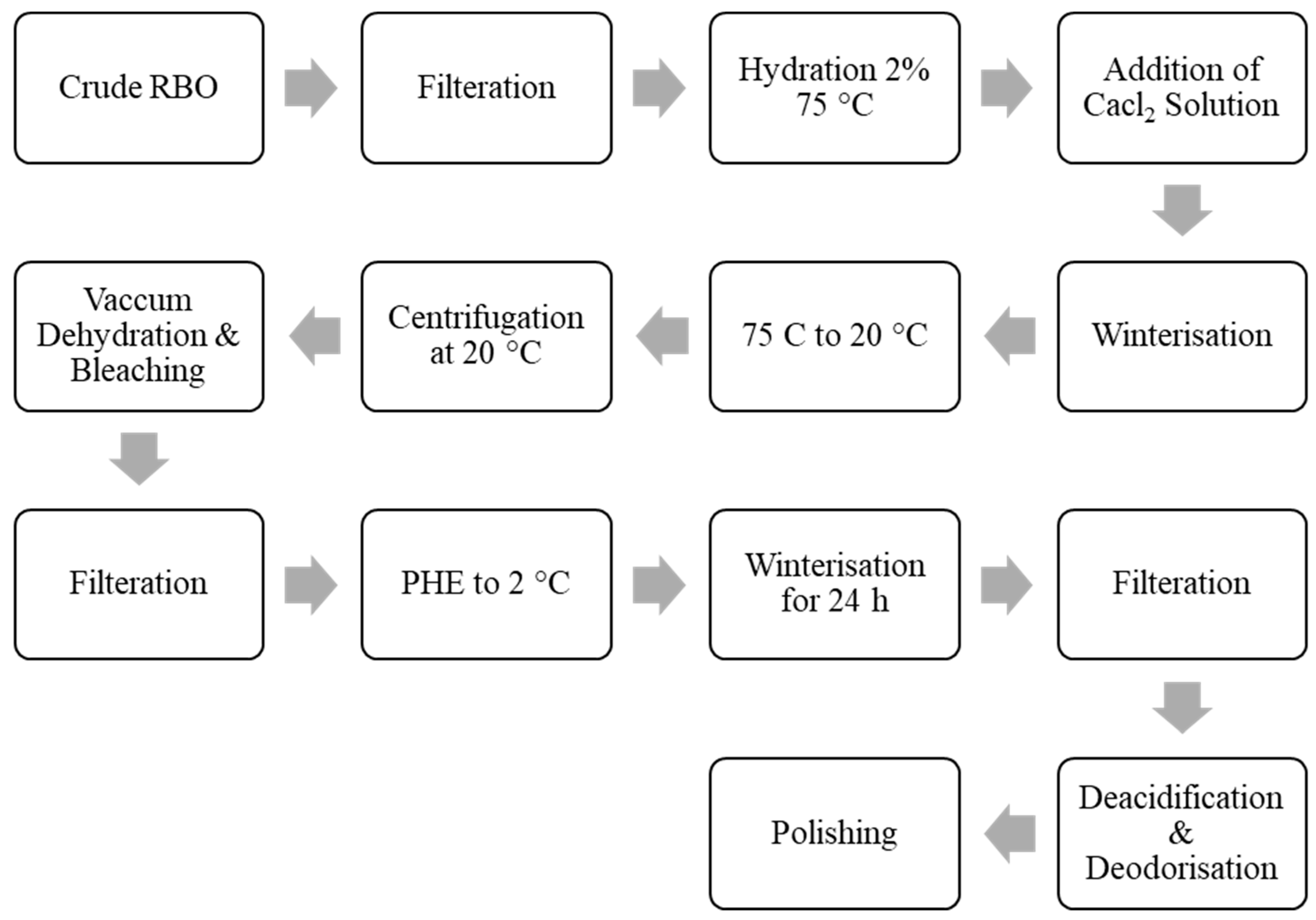
4.1. Winterization
4.2. Bleaching, Hydrogenation, and Deodorization
4.3. Oxidative Stability
4.4. Electrostatic Filtration
5. Composition of Rice Bran Oil
5.1. Fatty Acids
5.2. γ-Oryzanol in Rice Bran Oil
5.3. Vitamin E in Rice Bran Oil
5.4. Protein
5.5. Enzymes
6. Applications of Rice Bran Oil
6.1. Cooking
6.2. Deep Frying
6.3. Flavor
6.4. Functional Foods Applications
6.5. Health Benefits of Rice Bran Oil
6.5.1. Antihyperlipidemic
6.5.2. Antioxidant Property of RBO
6.5.3. Anti-Diabetic Effects of Rice Bran Oil
6.5.4. Antibacterial Activity of Rice Bran Oil
6.5.5. Anticancer Effects of Rice Bran Oil
6.5.6. Colorectal Cancer
6.5.7. Regulating Immune Response
6.5.8. The Antihypertension Activity of Rice Bran Oil
6.5.9. Antiaging Effects of Rice Bran Oil
6.5.10. Other Cosmetic Applications
6.5.11. Neuroprotective Effects of Rice Bran Oil
6.5.12. Insomnia Alleviation
7. Industrial Applications
7.1. Stabilization of Fats, Frying Oils and Fried Products
7.2. Stabilization and Development of Other Food Products
7.3. Poly Hydroxy Alkenoates
7.4. Biodiesels
7.5. Miscellaneous Industrial Applications
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nagendra Prasad, M.N.; Sanjay, K.R.; Shravya Khatokar, M.; Vismaya, M.N.; Nanjunda Swamy, S. Health Benefits of Rice Bran—A Review. J. Nutr. Food Sci. 2011, 1, 1000108. [Google Scholar] [CrossRef]
- Verma, D.K.; Srivastav, P.P. Bioactive compounds of rice (Oryza sativa L.): Review on paradigm and its potential benefit in human health. Trends Food Sci. Technol. 2020, 97, 355–365. [Google Scholar] [CrossRef]
- Tong, C.; Gao, H.; Luo, S.; Liu, L.; Bao, J. Impact of Postharvest Operations on Rice Grain Quality: A Review. Compr. Rev. Food Sci. Food Saf. 2019, 18, 626–640. [Google Scholar] [CrossRef] [PubMed]
- Muthayya, S.; Sugimoto, J.D.; Montgomery, S.; Maberly, G.F. An overview of global rice production, supply, trade, and consumption. Ann. N. Y. Acad. Sci. 2014, 1324, 7–14. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, J.; Yuan, W.; Zhang, R.; Xi, X. Multiple leveling for paddy field preparation with double axis rotary tillage accelerates rice growth and economic benefits. Agriculture 2021, 11, 1223. [Google Scholar] [CrossRef]
- Sapwarobol, S.; Saphyakhajorn, W.; Astina, J. Biological Functions and Activities of Rice Bran as a Functional Ingredient: A Review. Nutr. Metab. Insights 2021, 14, 388211058559. [Google Scholar] [CrossRef]
- Gopala Krishna, A.G.; Hemakumar, K.H.; Khatoon, S. Study on the composition of rice bran oil and its higher free fatty acids value. JAOCS J. Am. Oil Chem. Soc. 2006, 83, 117–120. [Google Scholar] [CrossRef]
- Duangsi, R.; Krongyut, W. Stabilization of Rice Bran by Infrared Radiation Heating for Increased Resilience and Quality of Rice Bran Oil Production. Prev. Nutr. Food Sci. 2023, 28, 189–199. [Google Scholar] [CrossRef]
- Punia, S.; Kumar, M.; Siroha, A.K.; Purewal, S.S. Rice Bran Oil: Emerging Trends in Extraction, Health Benefit, and Its Industrial Application. Rice Sci. 2021, 28, 217–232. [Google Scholar] [CrossRef]
- Dyer, J.M.; Mullen, R.T. Engineering plant oils as high-value industrial feedstocks for biorefining: The need for underpinning cell biology research. Physiol. Plant. 2008, 132, 11–22. [Google Scholar] [CrossRef]
- Wancura, J.H.C.; Brondani, M.; Vezaro, F.D.; Martins-Vieira, J.C.; Moreira, B.P.; dos Santos, M.S.N.; Abaide, E.R.; de Castilhos, F.; Mayer, F.D. Motivations to produce biofuels from rice bran: An overview involving a recent panorama. Ind. Crops Prod. 2023, 203, 11717. [Google Scholar] [CrossRef]
- Neeharika, T.S.V.R.; Anjaneyulu, B.; Rani, K.N.P.; Sandeepa, K.; Satyannarayana, S. Mathematical Modelling for Sustainable Extraction of Oil from Rice Bran, Safflower Seeds and Flaxseeds Employing Supercritical Carbon Dioxide at Pilot Scale: An Insight to Comprehensive Physico-Chemical Analysis. Sep. Purif. Technol. 2024, 342, 127007. [Google Scholar] [CrossRef]
- Eng, H.Y.; Mohd Rozalli, N.H. Rice bran and its constituents: Introduction and potential food uses. Int. J. Food Sci. Technol. 2022, 57, 4041–4051. [Google Scholar] [CrossRef]
- Manzoor, A.; Pandey, V.K.; Dar, A.H.; Fayaz, U.; Dash, K.K.; Shams, R.; Ahmad, S.; Bashir, I.; Fayaz, J.; Singh, P.; et al. Rice bran: Nutritional, phytochemical, and pharmacological profile and its contribution to human health promotion. Food Chem. Adv. 2023, 2, 100296. [Google Scholar] [CrossRef]
- Lakkakula, N.R.; Lima, M.; Walker, T. Rice bran stabilization and rice bran oil extraction using ohmic heating. Bioresour. Technol. 2004, 92, 157–161. [Google Scholar] [CrossRef] [PubMed]
- El-Beltagi, H.S.; Mohamed, H.I.; Aldaej, M.I.; Al-Khayri, J.M.; Rezk, A.A.; Al-Mssallem, M.Q.; Sattar, M.N.; Ramadan, K.M.A. Production and antioxidant activity of secondary metabolites in Hassawi rice (Oryza sativa L.) cell suspension under salicylic acid, yeast extract, and pectin elicitation. Vitr. Cell. Dev. Biol.-Plant 2022, 58, 615–629. [Google Scholar] [CrossRef]
- Nordin, N.N.A.M.; Karim, R.; Ghazali, H.M.; Adzahan, N.M.; Sultan, M.T. Effects of various stabilization techniques on the nutritional quality and antioxidant potential of brewer’s rice. J. Eng. Sci. Technol. 2014, 9, 347–363. [Google Scholar]
- Patil, S.S.; Kar, A.; Mohapatra, D. Stabilization of rice bran using microwave: Process optimization and storage studies. Food Bioprod. Process. 2016, 99, 204–211. [Google Scholar] [CrossRef]
- Sahini, M.G.; Mutegoa, E. Extraction, phytochemistry, nutritional, and therapeutical potentials of rice bran oil: A review. Phytomed. Plus 2023, 3, 100453. [Google Scholar] [CrossRef]
- Wang, T.; Khir, R.; Pan, Z.; Yuan, Q. Simultaneous rough rice drying and rice bran stabilization using infrared radiation heating. LWT 2017, 78, 281–288. [Google Scholar] [CrossRef]
- Amarasinghe, B.M.W.P.K.; Kumarasiri, M.P.M.; Gangodavilage, N.C. Effect of method of stabilization on aqueous extraction of rice bran oil. Food Bioprod. Process. 2009, 87, 108–114. [Google Scholar] [CrossRef]
- Dhingra, D.; Chopra, S.; Rai, D.R. Stabilization of Raw Rice Bran using Ohmic Heating. Agric. Res. 2012, 1, 392–398. [Google Scholar] [CrossRef]
- S Vallabha, V.; Indira, T.N.; Jyothi Lakshmi, A.; Radha, C.; Tiku, P.K. Enzymatic process of rice bran: A stabilized functional food with nutraceuticals and nutrients. J. Food Sci. Technol. 2015, 52, 8252–8259. [Google Scholar] [CrossRef] [PubMed]
- Pradeep, K.; Kuttappa, M.A.; Prasana, K.R. Probiotics and oral health: An update. S. Afr. Dent. J. 2014, 69, 20–24. [Google Scholar]
- Chakrabarti, P.P.; Jala, R.C.R. Processing Technology of Rice Bran Oil. In Rice Bran and Rice Bran Oil; Elsevier: Amsterdam, The Netherlands, 2019; pp. 55–95. [Google Scholar]
- Yılmaz Tuncel, N. Stabilization of Rice Bran: A Review. Foods 2023, 12, 1924. [Google Scholar] [CrossRef]
- Yu, C.W.; Hu, Q.R.; Wang, H.W.; Deng, Z.Y. Comparison of 11 rice bran stabilization methods by analyzing lipase activities. J. Food Process. Preserv. 2020, 44, e14370. [Google Scholar] [CrossRef]
- Dubey, B.N. Comparative Study on the Rice Bran Stabilization Processes: A Review. Res. Dev. Mater. Sci. 2019, 11, 2. [Google Scholar] [CrossRef]
- Liu, Y.Q.; Strappe, P.; Zhou, Z.K.; Blanchard, C. Impact on the nutritional attributes of rice bran following various stabilization procedures. Crit. Rev. Food Sci. Nutr. 2019, 59, 2458–2466. [Google Scholar] [CrossRef] [PubMed]
- Yan, W.; Liu, Q.; Wang, Y.; Tao, T.; Liu, B.; Liu, J.; Ding, C. Inhibition of Lipid and Aroma Deterioration in Rice Bran by Infrared Heating. Food Bioprocess Technol. 2020, 13, 1677–1687. [Google Scholar] [CrossRef]
- Kim, S.M.; Chung, H.J.; Lim, S.T. Effect of various heat treatments on rancidity and some bioactive compounds of rice bran. J. Cereal Sci. 2014, 60, 243–248. [Google Scholar] [CrossRef]
- Tan, X.W.; Kobayashi, K.; Shen, L.; Inagaki, J.; Ide, M.; San, H.S.; Matsuura, E. Antioxidative attributes of rice bran extracts in ameliorative effects of atherosclerosis-associated risk factors. Heliyon 2020, 6, e05743. [Google Scholar] [CrossRef]
- Kubo, M.T.; Siguemoto, É.S.; Funcia, E.S.; Augusto, P.E.; Curet, S.; Boillereaux, L.; Sastry, S.K.; Gut, J.A. Non-thermal effects of microwave and ohmic processing on microbial and enzyme inactivation: A critical review. Curr. Opin. Food Sci. 2020, 35, 36–48. [Google Scholar] [CrossRef]
- Head, D.; Cenkowski, S.; Arntfield, S.; Henderson, K. Storage stability of oat groats processed commercially and with superheated steam. LWT 2011, 44, 261–268. [Google Scholar] [CrossRef]
- Kumar, P.; Yadav, D.; Kumar, P.; Panesar, P.S.; Bunkar, D.S.; Mishra, D.; Chopra, H.K. Comparative Study on Conventional, Ultrasonication and Microwave Assisted Extraction of γ-Oryzanol from Rice Bran. J. Food. Sci. Technol. 2016, 53, 2047–2053. [Google Scholar] [CrossRef]
- Pandey, R.; Shrivastava, S.L. Comparative Evaluation of Rice Bran Oil Obtained with Two-Step Microwave Assisted Extraction and Conventional Solvent Extraction. J. Food Eng. 2018, 218, 106–114. [Google Scholar] [CrossRef]
- Amarasinghe, B.M.W.P.K.; Gangodavilage, N.C. Rice Bran Oil Extraction in Sri Lanka Data for Process Equipment Design. Food Bioprod. Process. 2004, 82, 54–59. [Google Scholar] [CrossRef]
- Trevisani Juchen, P.; Nolasco Araujo, M.; Hamerski, F.; Corazza, M.L.; Pedersen Voll, F.A. Extraction of Parboiled Rice Bran Oil with Supercritical CO2 and Ethanol as Co-Solvent: Kinetics and Characterization. Ind. Crops Prod. 2019, 139, 111506. [Google Scholar] [CrossRef]
- Nadar, S.S.; Rao, P.; Rathod, V.K. Enzyme Assisted Extraction of Biomolecules as an Approach to Novel Extraction Technology: A Review. Food Res. Int. 2018, 108, 309–330. [Google Scholar] [CrossRef]
- Pourali, O.; Salak Asghari, F.; Yoshida, H. Simultaneous Rice Bran Oil Stabilization and Extraction Using Sub-Critical Water Medium. J. Food Eng. 2009, 95, 510–516. [Google Scholar] [CrossRef]
- Rohman, A. Rice Bran Oil’s Role in Health and Cooking. In Wheat and Rice in Disease Prevention and Health; Academic Press: Cambridge, MA, USA, 2014; pp. 481–490. ISBN 9780124017160. [Google Scholar]
- Khoei, M.; Chekin, F. The ultrasound-assisted aqueous extraction of rice bran oil. Food Chem. 2016, 194, 503–507. [Google Scholar] [CrossRef]
- Liu, S.X.; Mamidipally, P.K. Quality comparison of rice bran oil extracted with d-limonene and hexane. Cereal Chem. 2005, 82, 209–215. [Google Scholar] [CrossRef]
- Sereewatthanawut, I.; Baptista, I.I.R.; Boam, A.T.; Hodgson, A.; Livingston, A.G. Nanofiltration process for the nutritional enrichment and refining of rice bran oil. J. Food Eng. 2011, 102, 16–24. [Google Scholar] [CrossRef]
- Ribas, F.B.T.; Gasparetto, H.; Salau, N.P.G. Sustainable extraction of rice bran Oil: Assessing renewable solvents, kinetics, and thermodynamics. Chem. Eng. Res. Des. 2023, 197, 342–354. [Google Scholar] [CrossRef]
- Byun, J.I.; Shin, Y.Y.; Chung, S.E.; Shin, W.C. Safety and efficacy of gamma-aminobutyric acid from fermented rice germ in patients with insomnia symptoms: A randomized, double-blind trial. J. Clin. Neurol. 2018, 14, 291–295. [Google Scholar] [CrossRef] [PubMed]
- Zou, Y.; Ju, X.; Chen, W.; Yuan, J.; Wang, Z.; Aluko, R.E.; He, R. Rice bran attenuated obesity via alleviating dyslipidemia, browning of white adipocytes and modulating gut microbiota in high-fat diet-induced obese mice. Food Funct. 2020, 11, 2406–2417. [Google Scholar] [CrossRef] [PubMed]
- Umadevi, M.; Pushpa, R.; Sampathkumar, K.P.; Bhowmik, D. Rice-Traditional Medicinal Plant in India. J. Pharmacogn. Phytochem. 2012, 1, 6–12. [Google Scholar]
- Gonzalez-Rivera, J.; Duce, C.; Campanella, B.; Bernazzani, L.; Ferrari, C.; Tanzini, E. In situ microwave assisted extraction of clove buds to isolate essential oil, polyphenols, and lignocellulosic compounds. Ind. Crops Prod. 2021, 161, 11320. [Google Scholar] [CrossRef]
- Thanonkaew, A.; Wongyai, S.; Decker, E.A.; McClements, D.J. Formation, antioxidant property and oxidative stability of cold pressed rice bran oil emulsion. J. Food Sci. Technol. 2015, 52, 6520–6528. [Google Scholar] [CrossRef]
- Garba, U.; Singanusong, R.; Jiamyangyeun, S.; Thongsook, T. Extraction and utilisation of rice bran oil. A review. Riv. Ital. Delle Sostanze Grasse 2019, 96, 161–170. [Google Scholar]
- Sivakanthan, S.; Fawzia, S.; Mundree, S.; Madhujith, T.; Karim, A. Optimization and Characterization of New Oleogels Developed Based on Sesame Oil and Rice Bran Oil. Food Hydrocoll. 2023, 142, 108839. [Google Scholar] [CrossRef]
- Santos, K.A.; Frohlich, P.C.; Hoscheid, J.; Tiuman, T.S.; Gonçalves, J.E.; Cardozo-Filho, L.; da Silva, E.A. Candeia (Eremanthus erythroppapus) Oil Extraction Using Supercritical CO2 with Ethanol and Ethyl Acetate Cosolvents. J. Supercrit. Fluids 2017, 128, 323–330. [Google Scholar] [CrossRef]
- Punia, S.; Kumar, M.; Sandhu, K.S.; Whiteside, W.S. Rice-Bran Oil: An Emerging Source of Functional Oil. J. Food Process. Preserv. 2021, 45, 4. [Google Scholar] [CrossRef]
- Loypimai, P.; Moongngarm, A.; Chottanom, P. Impact of stabilization and extraction methods on chemical quality and bioactive compounds of rice bran oil. Emir. J. Food Agric. 2015, 27, 849–856. [Google Scholar] [CrossRef]
- Soquetta, M.B.; Terra, L.d.M.; Bastos, C.P. Green technologies for the extraction of bioactive compounds in fruits and vegetables. CYTA-J. Food 2018, 16, 400–412. [Google Scholar] [CrossRef]
- Shukla, H.S.; Pratap, A. Comparative studies between conventional and microwave assisted extraction for rice bran oil. J. Oleo Sci. 2017, 66, 973–979. [Google Scholar] [CrossRef] [PubMed]
- Geow, C.H.; Tan, M.C.; Yeap, S.P.; Chin, N.L. A Review on Extraction Techniques and Its Future Applications in Industry. Eur. J. Lipid Sci. Technol. 2021, 123, 2000302. [Google Scholar] [CrossRef]
- Liu, Y.; Gong, G.; Zhang, J.; Jia, S.; Li, F.; Wang, Y.; Wu, S. Response surface optimization of ultrasound-assisted enzymatic extraction polysaccharides from Lycium barbarum. Carbohydr. Polym. 2014, 110, 278–284. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Padilla, L.P.; Franke, L.; Xu, X.Q.; Juliano, P. Improved extraction of avocado oil by application of sono-physical processes. Ultrason. Sonochem. 2018, 40, 720–726. [Google Scholar] [CrossRef]
- Capellini, M.C.; Giacomini, V.; Cuevas, M.S.; Rodrigues, C.E.C. Rice bran oil extraction using alcoholic solvents: Physicochemical characterization of oil and protein fraction functionality. Ind. Crops Prod. 2017, 104, 133–143. [Google Scholar] [CrossRef]
- Hasanah, Y.M.; Raharjo, S.; Pranoto, Y.; Ningrum, A. The optimization of oil extraction by surfactant-assisted aqueous extraction process of rice bran (Oryza sativa L.) using Box-Behnken design. Food Res. 2023, 7, 219–225. [Google Scholar] [CrossRef]
- Ghosh, M. Review on recent trends in rice bran oil processing. JAOCS J. Am. Oil Chem. Soc. 2007, 84, 315–324. [Google Scholar] [CrossRef]
- Terigar, B.G.; Balasubramanian, S.; Sabliov, C.M.; Lima, M.; Boldor, D. Soybean and rice bran oil extraction in a continuous microwave system: From laboratory- to pilot-scale. J. Food Eng. 2011, 104, 208–217. [Google Scholar] [CrossRef]
- Liu, C.; Xi, X.; Liu, Y.; Lu, Y.; Che, F.; Gu, Y.; Yu, Y.; Li, H.; Liu, J.; Wei, Y. Isolation of Four Major Compounds of γ-Oryzanol from Rice Bran Oil by Ionic Liquids Modified High-Speed Countercurrent Chromatography and Antimicrobial Activity and Neuroprotective Effect of Cycloartenyl Ferulate In Vitro. Chromatographia 2021, 84, 635–644. [Google Scholar] [CrossRef]
- Pestana-Bauer, V.R.; Zambiazi, R.C.; Mendonça, C.R.B.; Beneito-Cambra, M.; Ramis-Ramos, G. γ-Oryzanol and tocopherol contents in residues of rice bran oil refining. Food Chem. 2012, 134, 1479–1483. [Google Scholar] [CrossRef] [PubMed]
- Strieder, M.M.; Pinheiro, C.P.; Borba, V.S.; Pohndorf, R.S.; Cadaval, T.R.S.; Pinto, L.A.A. Bleaching optimization and winterization step evaluation in the refinement of rice bran oil. Sep. Purif. Technol. 2017, 175, 72–78. [Google Scholar] [CrossRef]
- Orthoefer, F.T. Rice Bran Oil. In Bailey’s Industrial Oil and Fat Products; Wiley: Hoboken, NJ, USA, 2005. [Google Scholar]
- Piloto-Rodríguez, R.; Díaz-Domínguez, Y. Production process, methods of extraction, and refining technologies of unconventional seed oils. In Multiple Biological Activities of Unconventional Seed Oils; Academic Press: Cambridge, MA, USA, 2022; pp. 413–430. ISBN 9780128241356. [Google Scholar]
- Chew, S.C.; Teng, S.K. Bioactive Phytochemicals from Rice Bran Oil Processing By-Products; Springer: Cham, Switzerland, 2023; pp. 65–103. [Google Scholar]
- Bakota, E.L.; Dunn, R.O.; Liu, S.X. Heavy metals screening of rice bran oils and its relation to composition. Eur. J. Lipid Sci. Technol. 2015, 117, 1452–1462. [Google Scholar] [CrossRef]
- Srivastava, S.; Pandey, V.K.; Singh, R.; Dar, A.H.; Bashir, I. Recent insights on electrostatic filtration and its potential applications in food industry. Trends Food Sci. Technol. 2023, 136, 239–250. [Google Scholar] [CrossRef]
- Wang, H.; Geng, H.; Chen, J.; Wang, X.; Li, D.; Wang, T.; Yu, D.; Wang, L. Three phase partitioning for simultaneous extraction of oil, protein and polysaccharide from rice bran. Innov. Food Sci. Emerg. Technol. 2020, 65, 10244. [Google Scholar] [CrossRef]
- Rathna Priya, T.S.; Eliazer Nelson, A.R.L.; Ravichandran, K.; Antony, U. Nutritional and functional properties of coloured rice varieties of South India: A review. J. Ethn. Foods 2019, 6, 11. [Google Scholar] [CrossRef]
- Tsuchiya, Y.; Yoshida, T. Pelletization of brown coal and rice bran in Indonesia: Characteristics of the mixture pellets including safety during transportation. Fuel Process. Technol. 2017, 156, 68–71. [Google Scholar] [CrossRef]
- Mishra, R.; Sharma, H.K. Effect of frying conditions on the physico-chemical properties of rice bran oil and its blended oil. J. Food Sci. Technol. 2014, 51, 1076–1084. [Google Scholar] [CrossRef] [PubMed]
- Mezouari, S.; Eichner, K. Comparative study on the stability of crude and refined rice bran oil during long-term storage at room temperature. Eur. J. Lipid Sci. Technol. 2007, 109, 198–205. [Google Scholar] [CrossRef]
- Hamid, A.A.; Dek, M.S.P.; Tan, C.P.; Zainudin, M.A.M.; Fang, E.K.W. Changes of major antioxidant compounds and radical scavenging activity of palm oil and rice bran oil during deep-frying. Antioxidants 2014, 3, 502–515. [Google Scholar] [CrossRef] [PubMed]
- Pal, Y.P.; Pratap, A.P. Rice bran oil: A versatile source for edible and industrial applications. J. Oleo Sci. 2017, 66, 551–556. [Google Scholar] [CrossRef]
- Oluremi, O.I.; Solomon, A.O.; Saheed, A.A. Fatty acids, metal composition and physico-chemical parameters of Igbemo Ekiti rice bran oil. Environ. Chem. 2013, 5, 39–46. [Google Scholar]
- Li, Q.; Tang, X.; Lu, S.; Wu, J. Composition and tocopherol, fatty acid, and phytosterol contents in micro-endosperm ultra-high oil corn. Grasas Y Aceites 2019, 70, 3. [Google Scholar] [CrossRef]
- Jennings, B.H.; Akoh, C.C. Effectiveness of natural versus synthetic antioxidants in a rice bran oil-based structured lipid. Food Chem. 2009, 114, 1456–1461. [Google Scholar] [CrossRef]
- Kerdsiri, J.; Wisuitiprot, W.; Boonnoun, P.; Chantakul, R.; Netsopa, S.; Nuengchamnong, N.; Waranuch, N. Effect of extraction methods on biological activities of Thai rice bran extracts. Songklanakarin J. Sci. Technol. 2020, 42, 1007–1015. [Google Scholar]
- Ramazani, E.; Akaberi, M.; Emami, S.A.; Tayarani-Najaran, Z. Pharmacological and biological effects of alpha-bisabolol: An updated review of the molecular mechanisms. Life Sci. 2022, 304, 2299–2316. [Google Scholar] [CrossRef]
- Fujiwara, Y. Preventive effect of polyunsaturated fatty acid and vitamin e in rice bran oil on lifestyle-related diseases. J. Nutr. Sci. Vitaminol. 2019, 65, S34–S37. [Google Scholar] [CrossRef]
- Endo, Y.; Nakagawa, K. Differences in the compositions of vitamin E tocochromanol (tocopherol and tocotrienol) in rice bran oils produced in Japan and other countries. J. Oleo Sci. 2021, 70, 503–507. [Google Scholar] [CrossRef] [PubMed]
- Rahmania, H.; Kato, S.; Sawada, K.; Hayashi, C.; Hashimoto, H.; Nakajima, S.; Otoki, Y.; Ito, J.; Nakagawa, K. Revealing the thermal oxidation stability and its mechanism of rice bran oil. Sci. Rep. 2020, 10, 1. [Google Scholar] [CrossRef] [PubMed]
- Jahani, M.; Alizadeh, M.; Pirozifard, M.; Qudsevali, A. Optimization of enzymatic degumming process for rice bran oil using response surface methodology. LWT 2008, 41, 1892–1898. [Google Scholar] [CrossRef]
- Xu, L.; Zhang, Y.; Zivkovic, V.; Zheng, M. Deacidification of high-acid rice bran oil by the tandem continuous-flow enzymatic reactors. Food Chem. 2022, 393, 13344. [Google Scholar] [CrossRef]
- Wang, W.; Guo, J.; Zhang, J.; Peng, J.; Liu, T.; Xin, Z. Isolation, identification and antioxidant activity of bound phenolic compounds present in rice bran. Food Chem. 2015, 171, 40–49. [Google Scholar] [CrossRef]
- Ti, H.; Li, Q.; Zhang, R.; Zhang, M.; Deng, Y.; Wei, Z.; Chi, J.; Zhang, Y. Free and bound phenolic profiles and antioxidant activity of milled fractions of different indica rice varieties cultivated in southern China. Food Chem. 2014, 159, 166–174. [Google Scholar] [CrossRef] [PubMed]
- Suttiarporn, P.; Chumpolsri, W.; Mahatheeranont, S.; Luangkamin, S.; Teepsawang, S.; Leardkamolkarn, V. Structures of phytosterols and triterpenoids with potential anti-cancer activity in bran of black non-glutinous rice. Nutrients 2015, 7, 1672–1687. [Google Scholar] [CrossRef]
- Verardo, V.; Gómez-Caravaca, A.M.; Marconi, E.; Segura-Carretero, A.; Garrido-Frenich, A.; Fernández-Gutiérrez, A. Determination of lipophilic and hydrophilic bioactive compounds in raw and parboiled rice bran. RSC Adv. 2016, 6, 50786–50796. [Google Scholar] [CrossRef]
- Akihisa, T.; Yasukawa, K.; Yamaura, M.; Ukiya, M.; Kimura, Y.; Shimizu, N.; Arai, K. Triterpene Alcohol and Sterol Ferulates from Rice Bran and Their Anti- Inflammatory Effects. J Agric Food Chem 2000, 48, 2313–2319. [Google Scholar] [CrossRef]
- Wang, Y. Applications of rice bran oil. In Rice Bran and Rice Bran Oil: Chemistry, Processing and Utilization; Elsevier: Amsterdam, The Netherlands, 2019; pp. 159–168. ISBN 9780128128282. [Google Scholar]
- Farhoosh, R.; Esmaeilzadeh Kenari, R. Anti-rancidity effects of sesame and rice bran oils on canola oil during deep frying. JAOCS J. Am. Oil Chem. Soc. 2009, 86, 539–544. [Google Scholar] [CrossRef]
- Alauddin, M.; Islam, J.; Shirakawa, H.; Koseki, T.; Ardiansyahc, K.M.; Komaia, M. Rice Bran as a Functional Food: An Overview of the Conversion of Rice Bran into a Superfood/Functional Food. In Superfood and Functional Food-An Overview of Their Processing and Utilization; Books on Demand: Paris, France, 2017. [Google Scholar]
- Gul, K.; Yousuf, B.; Singh, A.K.; Singh, P.; Wani, A.A. Rice bran: Nutritional values and its emerging potential for development of functional food—A review. Bioact. Carbohydr. Diet. Fibre 2015, 6, 24–30. [Google Scholar] [CrossRef]
- Liang, Y.; Gao, Y.; Lin, Q.; Luo, F.; Wu, W.; Lu, Q.; Liu, Y. A review of the research progress on the bioactive ingredients and physiological activities of rice bran oil. Eur. Food Res. Technol. 2014, 238, 169–176. [Google Scholar] [CrossRef]
- Jan-on, G.; Sangartit, W.; Pakdeechote, P.; Kukongviriyapan, V.; Sattayasai, J.; Senaphan, K.; Kukongviriyapan, U. Virgin rice bran oil alleviates hypertension through the upregulation of eNOS and reduction of oxidative stress and inflammation in L-NAME–induced hypertensive rats. Nutrition 2020, 69, 5. [Google Scholar] [CrossRef]
- Bumrungpert, A.; Chongsuwat, R.; Phosat, C.; Butacnum, A. Rice Bran Oil Containing Gamma-Oryzanol Improves Lipid Profiles and Antioxidant Status in Hyperlipidemic Subjects: A Randomized Double-Blind Controlled Trial. J. Altern. Complement. Med. 2019, 25, 353–358. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Yu, S.; Park, H.J.; Jung, J.; Go, G.W.; Kim, W. Rice bran oil ameliorates inflammatory responses by enhancing mitochondrial respiration in murine macrophages. PLoS ONE 2019, 14, e0222857. [Google Scholar] [CrossRef]
- Park, T.; Kim, J.Y.; Cho, H.; Moon, H.C.; Kim, B.J.; Park, J.H.; Hong, D.; Park, J.; Choi, I.S. Artificial Spores: Immunoprotective Nanocoating of Red Blood Cells with Supramolecular Ferric Ion-Tannic Acid Complex. Polymers 2017, 9, 140. [Google Scholar] [CrossRef] [PubMed]
- Stewart, J.; McCallin, T.; Martinez, J.; Chacko, S.; Yusuf, S. Hyperlipidemia. Pediatr. Rev. 2020, 41, 393–402. [Google Scholar] [CrossRef]
- Nie, Y.; Luo, F.; Wang, L.; Yang, T.; Shi, L.; Li, X.; Shen, J.; Xu, W.; Guo, T.; Lin, Q. Anti-hyperlipidemic effect of rice bran polysaccharide and its potential mechanism in high-fat diet mice. Food Funct. 2017, 8, 4028–4041. [Google Scholar] [CrossRef] [PubMed]
- Zavoshy, R.; Noroozi, M.; Jahanihashemi, H. Effect of low calorie diet with rice bran oil on cardiovascular risk factors in hyperlipidemic patients. J. Res. Med. Sci. 2012, 17, 626–631. [Google Scholar]
- Ranjbar-Zahedani, M.; Alinejad, N.; Zadeh, S.M.A.; Mazloom, Z. Comparison of the effects of edible oils: Rice bran, grape seed, and canola on serum lipid profile and paraoxonase activity in hyperlipidemic rats. Int. Cardiovasc. Res. J. 2015, 9, 28–33. [Google Scholar]
- Ali, A.; Devarajan, S. Nutritional and health benefits of rice bran oil. In Brown Rice; Springer: Berlin/Heidelberg, Germany, 2017; pp. 135–158. [Google Scholar]
- Maurya, N.K.; Arya, P.; Sengar, N.S. Hypolipidemic effect of rice bran oil on chronic renal failure (undergoing hemodialysis) patients. Plant Arch. 2020, 20, 3285–3289. [Google Scholar]
- Gunawan, S.; Vali, S.R.; Ju, Y.H. Purification and identification of rice bran oil fatty acid steryl and wax esters. JAOCS J. Am. Oil Chem. Soc. 2006, 83, 449–456. [Google Scholar] [CrossRef]
- Chen, Y.; Xiang, Y.C.; Jakeman, L.B.; Chen, L.; Stokes, B.T.; Wolpaw, J.R. Operant Conditioning of H-Reflex Can Correct a Locomotor Abnormality after Spinal Cord Injury in Rats. J. Neurosci. 2006, 26, 12537–12543. [Google Scholar] [CrossRef]
- Lai, O.M.; Jacoby, J.J.; Leong, W.F.; Lai, W.T. Nutritional studies of rice bran oil. In Rice Bran and Rice Bran Oil, Chemistry, Processing and Utilization; Elsevier: Amsterdam, The Netherlands, 2019; pp. 19–54. [Google Scholar]
- Wijarnprecha, K.; Aryusuk, K.; Santiwattana, P.; Sonwai, S.; Rousseau, D. Structure and rheology of oleogels made from rice bran wax and rice bran oil. Food Res. Int. 2018, 112, 199–208. [Google Scholar] [CrossRef]
- Xu, L.; Hao, X.; Chen, L.; Qu, W.; Duan, S.; Wang, Q.; Yang, Q.; Wu, J.; Gong, Z.; Dai, H. Electrochemical Immunosensor Based on Antibody-Oriented Probe for the Detection of AFB1 in Rice Bran Oil. J. Food Compos. Anal. 2024, 130, 106198. [Google Scholar] [CrossRef]
- Joshi, M.; Kaur, R.; Kanwar, P.; Dhiman, G.; Sharma, G.; Lata, S.; Tilak, K.; Gupta, N.; Mishra, T. To Evaluate Antioxidant Activity of Γ –Oryzanol Extracted from Rice Bran Oil. Int. J. Life Sc. Pharma Res. 2016, 6, 17–25. [Google Scholar]
- Mingyai, S.; Kettawan, A.; Srikaeo, K.; Singanusong, R. Physicochemical and antioxidant properties of rice bran oils produced from colored rice using different extraction methods. J. Oleo Sci. 2017, 66, 565–572. [Google Scholar] [CrossRef]
- Sivamaruthi, B.S.; Kesika, P.; Chaiyasut, C. A comprehensive review on functional properties of fermented rice bran. Pharmacogn. Rev. 2018, 12, 218–224. [Google Scholar] [CrossRef]
- Ghatak, S.B.; Panchal, S.S. Anti-diabetic activity of oryzanol and its relationship with the anti-oxidant property. Int. J. Diabetes Dev. Ctries. 2012, 32, 185–192. [Google Scholar] [CrossRef]
- Yamamoto, S.; Ogawa, S.; Yamamoto, Y.; Hara, S. Thermal-oxidative stability of commercial rice bran oil. Food Sci. Technol. Res. 2020, 26, 681–685. [Google Scholar] [CrossRef]
- Liu, R.; Xu, Y.; Chang, M.; Tang, L.; Lu, M.; Liu, R.; Jin, Q.; Wang, X. Antioxidant interaction of α-tocopherol, γ-oryzanol and phytosterol in rice bran oil. Food Chem. 2021, 343, 12843. [Google Scholar] [CrossRef]
- Kanwar, K.; Kaushal, S.; Kumar, H.; Gupta, G.; Khari, M. BC DCN: A new edge centrality measure to identify and rank critical edges pertaining to SIR diffusion in complex networks. Soc. Netw. Anal. Min. 2022, 12, 49. [Google Scholar] [CrossRef]
- Andriani, R.; Subroto, T.; Ishmayana, S.; Kurnia, D. Enhancement Methods of Antioxidant Capacity in Rice Bran: A Review. Foods 2022, 11, 2994. [Google Scholar] [CrossRef]
- Al-Okbi, S.Y.; Mohamed, D.A.; Hamed, T.E.; Al-Siedy, E.S.K. Rice bran as source of nutraceuticals for management of cardiovascular diseases, cardio-renal syndrome and hepatic cancer. J. HerbMed Pharmacol. 2020, 9, 68–74. [Google Scholar] [CrossRef]
- Yao, W.; Gong, Y.; Li, L.; Hu, X.; You, L. The effects of dietary fibers from rice bran and wheat bran on gut microbiota: An overview. Food Chem. X 2022, 13, 2. [Google Scholar] [CrossRef]
- Zhao, L.; Fang, X.; Marshall, M.R.; Chung, S.; Pan, M.H. Regulation of obesity and metabolic complications by gamma and delta tocotrienols. Molecules 2016, 21, 344. [Google Scholar] [CrossRef]
- Lang, E. In out-of-hospital cardiac arrest, mechanical CPR did not improve survival compared with manual CPR. Ann. Intern. Med. 2014, 160, 4. [Google Scholar] [CrossRef]
- Lu, M.; Zhang, T.; Jiang, Z.; Guo, Y.; Qiu, F.; Liu, R.; Zhang, L.; Chang, M.; Liu, R.; Jin, Q.; et al. Physical properties and cellular antioxidant activity of vegetable oil emulsions with different chain lengths and saturation of triglycerides. LWT 2020, 121, 10894. [Google Scholar] [CrossRef]
- Alauddin, M.; Rahman, S.; Islam, J.; Shirakawa, H.; Komai, M.; Howlader, M.Z.H. Development of rice bran functional food and evaluation of its healthful properties. In Rice Bran and Rice Bran Oil: Chemistry, Processing and Utilization; Elsevier: Amsterdam, The Netherlands, 2019; pp. 183–206. ISBN 9780128128282. [Google Scholar]
- Mukai, T.; Kuno, H.; Kobayashi, R.; Kamai, J.; Azukisawa, S.; Kawaura, K. Review of Biliary Drainage in the Management for Preoperative and Unresectable Malignant Distal Biliary Obstructions. J. Kanazawa Med. Univ. 2022, 47, 7–12. [Google Scholar]
- Udayakumar, G.P.; Muthusamy, S.; Selvaganesh, B.; Sivarajasekar, N.; Rambabu, K.; Banat, F.; Sivamani, S.; Sivakumar, N.; Hosseini-Bandegharaei, A.; Show, P.L. Biopolymers and Composites: Properties, Characterization and Their Applications in Food, Medical and Pharmaceutical Industries. J. Environ. Chem. Eng. 2021, 9, 4. [Google Scholar] [CrossRef]
- Yu, Y.; Zhang, J.; Wang, J.; Sun, B. The Anti-Cancer Activity and Potential Clinical Application of Rice Bran Extracts and Fermentation Products. RSC Adv. 2019, 9, 18060–18069. [Google Scholar] [CrossRef]
- Slimani, N.; Deharveng, G.; Southgate, D.A.T.; Biessy, C.; Chajès, V.; van Bakel, M.M.E.; Boutron-Ruault, M.C.; McTaggart, A.; Grioni, S.; Verkaik-Kloosterman, J.; et al. Contribution of Highly Industrially Processed Foods to the Nutrient Intakes and Patterns of Middle-Aged Populations in the European Prospective Investigation into Cancer and Nutrition Study. Eur. J. Clin. Nutr. 2009, 63, S206–S225. [Google Scholar] [CrossRef]
- Sierra, S.; Lara-Villoslada, F.; Olivares, M.; Jiménez, J.; Boza, J.; Xaus, J. Increased immune response in mice consuming rice bran oil. Eur. J. Nutr. 2005, 44, 509–516. [Google Scholar] [CrossRef]
- Lv, X.; Chen, S.; Wang, Y. Advances in Understanding the Physiological and Molecular Responses of Sugar Beet to Salt Stress. Front. Plant Sci. 2019, 10, 1431. [Google Scholar] [CrossRef]
- Lee, W.J. Vitamin C in Human Health and Disease: Effects, Mechanisms of Action, and New Guidance on Intake; Springer: Berlin/Heidelberg, Germany, 2019; ISBN 9402417133. [Google Scholar]
- Laffite, A.; Kilunga, P.I.; Kayembe, J.M.; Devarajan, N.; Mulaji, C.K.; Giuliani, G.; Slaveykova, V.I.; Poté, J. Hospital effluents are one of several sources of metal, antibiotic resistance genes, and bacterial markers disseminated in sub-saharan urban rivers. Front. Microbiol. 2016, 7, 1128. [Google Scholar] [CrossRef]
- Haddadpour, F.; Kamani, M.M.; Mokhtari, A.; Mahdavi, M. Federated Learning with Compression: Unified Analysis and Sharp Guarantees. In Proceedings of the 24th International Conference on Artificial Intelligence and Statistics, Proceedings of Machine Learning Research, Virtual Event, 13–15 April 2021; Volume 130, pp. 2350–2358. [Google Scholar]
- Bernardi, D.S.; Pereira, T.A.; Maciel, N.R.; Bortoloto, J.; Viera, G.S.; Oliveira, G.C.; Rocha-Filho, P.A. Formation and stability of oil-in-water nanoemulsions containing rice bran oil: In vitro and in vivo assessments. J. Nanobiotechnol. 2011, 9, 44. [Google Scholar] [CrossRef]
- Manosroi, A.; Chutoprapat, R.; Abe, M.; Manosroi, W.; Manosroi, J. Transdermal absorption enhancement of rice bran bioactive compounds entrapped in niosomes. AAPS PharmSciTech 2012, 13, 323–335. [Google Scholar] [CrossRef]
- Rigo, R.; Santos, P.; Frontuto, V. Landscape heterogeneity, basis risk and the feasibility of index insurance: An analysis of rice in upland regions of Southeast Asia. Food Policy 2022, 108, 7. [Google Scholar] [CrossRef]
- Khat-udomkiri, N.; Sivamaruthi, B.S.; Sirilun, S.; Lailerd, N.; Peerajan, S.; Chaiyasut, C. Optimization of alkaline pretreatment and enzymatic hydrolysis for the extraction of xylooligosaccharide from rice husk. AMB Express 2018, 8, 115. [Google Scholar] [CrossRef] [PubMed]
- Godber, J.S. Rice Bran Oil. In Gourmet and Health-Promoting Specialty Oils; Elsevier: Amsterdam, The Netherlands, 2009; Volume 2, pp. 377–408. ISBN 9780128043516. [Google Scholar]
- Kumari, A.; Das, A.; Devanna, B.N.; Thakur, S.; Singh, P.K.; Singh, N.K.; Sharma, T.R. Mining of rice blast resistance gene Pi54 shows effect of single nucleotide polymorphisms on phenotypic expression of the alleles. Eur. J. Plant Pathol. 2013, 137, 55–65. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, L.; Wu, Z.; Zhang, P.; Liu, R.; Chang, M.; Wang, X. The dopaminergic neuroprotective effects of different phytosterols identified in rice bran and rice bran oil. Food Funct. 2021, 12, 10538–10549. [Google Scholar] [CrossRef]
- Lee, H.K.; Jang, J.Y.; Yoo, H.S.; Seong, Y.H. Neuroprotective Effect of 1,3-Dipalmitoyl-2-Oleoylglycerol Derived from Rice Bran Oil against Cerebral Ischemia-Reperfusion Injury in Rats. Nutrients 2022, 14, 1380. [Google Scholar] [CrossRef]
- Jang, J.Y.; Lee, H.K.; Yoo, H.S.; Seong, Y.H. Protective effect of rice bran oil against β-amyloid protein-induced memory impairment and neuronal death in mice. Nat. Prod. Sci. 2020, 26, 221–229. [Google Scholar] [CrossRef]
- Ruen-Ngam, D.; Thawai, C.; Sukonthamat, S.; Khuwaranyu, K. Effect of subcritical solvent extraction conditions on amount of γ-oryzanol and γ-tocopherol in dawk pa-yom rice bran oil. Curr. Appl. Sci. Technol. 2021, 21, 151–161. [Google Scholar]
- Samad, N. Rice bran oil prevents neuroleptic-induced extrapyramidal symptoms in rats: Possible antioxidant mechanisms. J. Food Drug Anal. 2015, 23, 370–375. [Google Scholar] [CrossRef]
- Fukui, K. Neuroprotective and anti-obesity effects of tocotrienols. J. Nutr. Sci. Vitaminol. 2019, 65, S185–S187. [Google Scholar] [CrossRef]
- Abd El Fattah, M.A.; Abdelhamid, Y.A.; Elyamany, M.F.; Badary, O.A.; Heikal, O.A. Rice Bran Extract Protected against LPS-Induced Neuroinflammation in Mice through Targeting PPAR-γ Nuclear Receptor. Mol. Neurobiol. 2021, 58, 1504–1516. [Google Scholar] [CrossRef]
- Zeng, Y.; Yang, J.; Du, J.; Pu, X.; Yang, X.; Yang, S.; Yang, T. Strategies of Functional Foods Promote Sleep in Human Being. Curr. Signal Transduct. Ther. 2015, 9, 148–155. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Chen, H.; Cheng, W.; Yang, K.; Cai, L.; He, L.; Du, L.; Liu, Y.; Liu, A.; Zeng, Z.; et al. Impact of arabinoxylan on characteristics, stability and lipid oxidation of oil-in-water emulsions: Arabinoxylan from wheat bran, corn bran, rice bran, and rye bran. Food Chem. 2021, 358, 12981. [Google Scholar] [CrossRef] [PubMed]
- Vali, S.R.; Ju, Y.H.; Kaimal, T.N.B.; Chern, Y.T. A process for the preparation of food-grade rice bran wax and the determination of its composition. JAOCS J. Am. Oil Chem. Soc. 2005, 82, 57–64. [Google Scholar] [CrossRef]
- Costa, J.M.; Strieder, M.M.; Saldaña, M.D.A.; Rostagno, M.A.; Forster-Carneiro, T. Recent Advances in the Processing of Agri-food By-products by Subcritical Water. Food Bioprocess Technol. 2023, 12, 2705–2724. [Google Scholar] [CrossRef]
- Zullaikah, S.; Utomo, A.T.; Yasmin, M.; Ong, L.K.; Ju, Y.H. Ecofuel conversion technology of inedible lipid feedstocks to renewable fuel. In Advances in Eco-Fuels for a Sustainable Environment; Woodhead Publishing: Sawston, UK, 2018; pp. 237–276. ISBN 9780081027288. [Google Scholar]
- Mahdi, H.I.; Ramlee, N.N.; da Silva Duarte, J.L.; Cheng, Y.S.; Selvasembian, R.; Amir, F.; de Oliveira, L.H.; Wan Azelee, N.I.; Meili, L.; Rangasamy, G. A comprehensive review on nanocatalysts and nanobiocatalysts for biodiesel production in Indonesia, Malaysia, Brazil and USA. Chemosphere 2023, 319, 138003. [Google Scholar] [CrossRef]
- Issara, U.; Rawdkuen, S. Rice bran: A potential of main ingredient in healthy beverage. Int. Food Res. J. 2016, 23, 2306–2318. [Google Scholar]
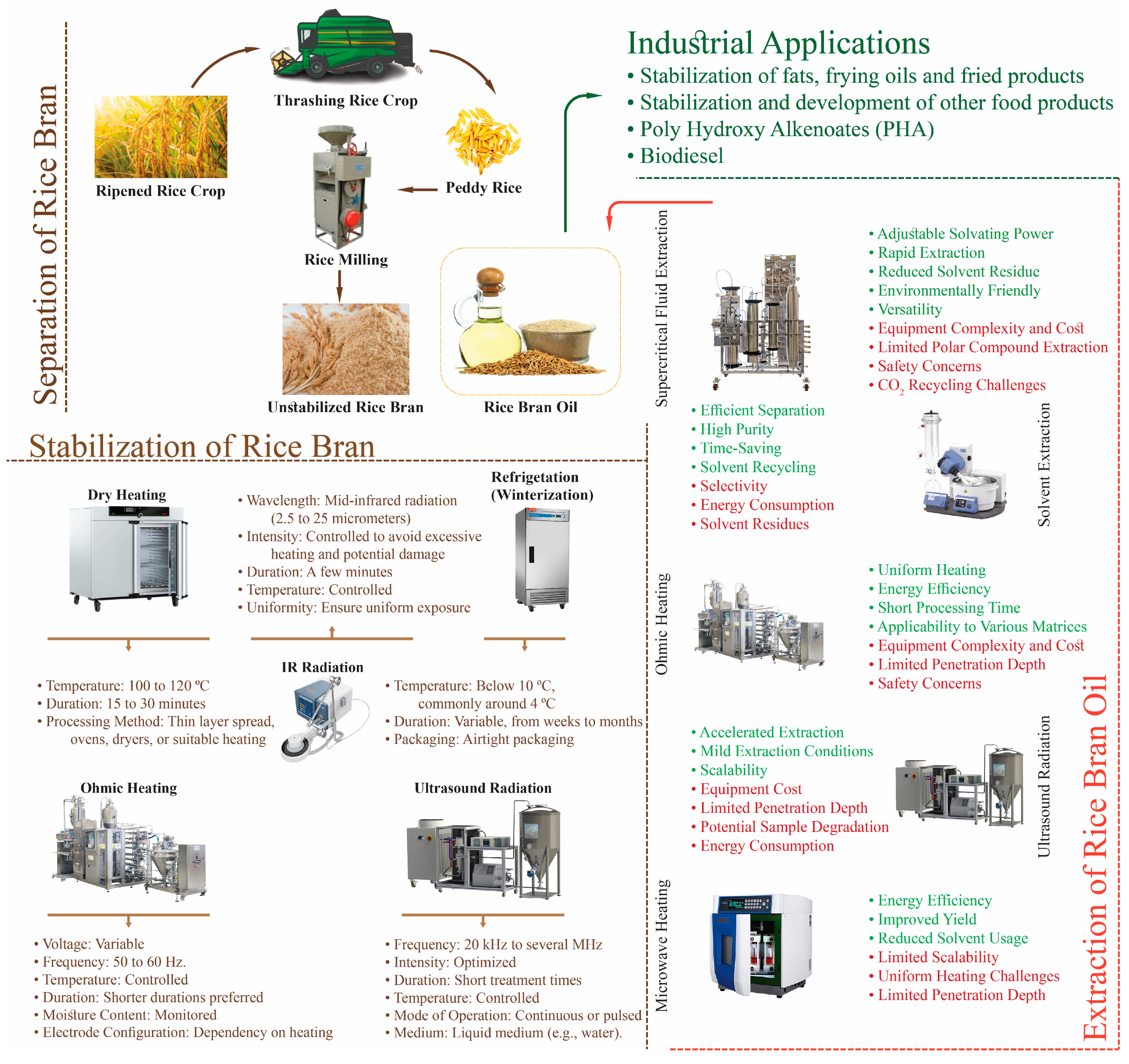


| Methods | Mode of Action | Disadvantages | Advantages | References |
|---|---|---|---|---|
| Microwave heating | Heat energy from the microwave is converted to denature the lipase enzymes. | Expensive and unsuitable for remote regions. Decreased fiber level. | Simple penetration, simultaneous heating, quick heating, and even heating. Improved bioavailability of vital compounds. | [17,18] |
| Extrusion | High temperature, pressure, and shear force all contribute to inactivation. | Because strong extrusion conditions might damage nutrients, operational conditions must be strictly controlled. | High output, quick processing time, effective antioxidants, and essential nutrients are maintained throughout inactivation. | [19] |
| Dry heating | Temperature range: 100–130 °C. Hot air reduces moisture content, resulting in lipase deactivation. | Water reabsorption has the potential to lessen the inhibitory impact. Only suitable for small-scale applications | Simple method. The nutritional potentials of the extracts were preserved. | [20] |
| Infrared heating | Radiation enters the substance and is transformed into heat. | Radiation penetration is limited, resulting in less uniform heating. Radiation may have an impact on the stability of vital nutritional components. | Versatile and quick reaction Lipase deactivation is efficacious. | [20] |
| Low-temperature treatment | Bran preservation at a low temperature (0 °C) regulates lipase activity. | Incomplete suppression owing to activity resumption at room temperature Only suitable for small-scale use. Maintaining a low temperature is wasteful in terms of cost. | Does not influence nutritional availability. | [21] |
| Ohmic heating | An alternating electric current was transmitted through the rice bran, which acted as electrical resistance, creating heat and inactivating the lipase enzyme. | An electric field can influence the metabolic processes. | Heating that is consistent Lipase enzyme inactivation is effective. Increased accessibility to essential molecules like γ-oryzanol and tocopherols. | [22] |
| Biological treatment | Break down the hydrolytic enzyme lipase with another enzyme, for example, protease. | The enzymes are expensive to obtain. | Allow for the specific targeting of enzyme activity. Essential nutritional components are preserved. | [23] |
| Moisture heating | Hot steam inactivates lipase. | Polyphenol concentration has been reduced. | Inactivation efficiency, extended storage period, consistent heating, quick heating, nutritious components preserved | [24] |
| Techniques | Parameter | Optimized Condition | Finding and Yield | References |
|---|---|---|---|---|
| Ultrasound-assisted extraction (UAE) | Time Solvent used Frequency Temperature Solid to liquid ratio | 60 min Petroleum ether, Hexane and Methanol 24 kHz 38 °C 1:3 | Compared to the usual extraction approach, ultrasound treatment considerably improves methanol’s γ-oryzanol extraction performance. Extraction efficiency was 96% of the total oil content available in rice bran. | [35] |
| Microwave-assisted extraction (MAE) | Time Power Solvent used Solid to liquid ratio | 30, 60, 90 and 120 s per step 300, 500, 700, and 900 W Hexane 1:1 | MAE is a potential method for extracting RBO with higher oil purity, increased oil production, and reduced extraction duration. Extraction efficiency with microwave is 80% of the total oil content available in rice bran. | [36] |
| Soxhlet extraction | Temperature Solvent | 65 °C Hexane | The extraction of solvents Hexane as a solvent is an efficient approach for RBO extraction. The extraction efficiency is 80–90% of the total oil content available in rice bran, while the solvent is n-hexane. | [37] |
| Supercritical CO2 extraction | Time Pressure Temperature Solvent used Solid to liquid ratio | 30 min 100, 150, or 200 bar 40 °C, 60 °C or 80 °C Ethanol 0:1, 0.5:1, 1:1, 2:1 | The goal of supercritical CO2 may be to reduce operational expenses while increasing oil output. The extraction efficiency of rice bran oil is 25–26% by weight basis. | [38] |
| Enzyme-assisted three-phase partitioning | Three phases | t-butanol (top phase), protein (middle phase), and ammonium sulfate (lower phase) | This technique was initially used to separate proteins, enzymes, and lipids but is now used to extract bioactive such as oils, oleoresins, and polysaccharides from plant sources. Rice bran extraction efficiency is 79% of total oil content using Proteases and Protizyme. | [39] |
| Sub-critical water extraction | Time of ultrasound Temperature Solvent used Solid to liquid ratio | 10–20 min 180–240 °C Deionized water 1:6 | Subcritical water extraction is a low-impact approach for lipase deactivation and RBO stabilization. The extraction efficiency of rice bran oil is 249 mg/g on a dry matter basis at 240 °C in pure water. | [40] |
| Solvent extraction | Temperature Time Solvent used Solid to liquid Ratio | 60 °C, 40 °C 10 min Hexane, Isopropanol 2:1, 3:1 | The solvent extraction process in the industry can achieve a high yield and recovery rate of as much as 99%. | [19] |
| Micronutrient | Amount % | Advantage |
|---|---|---|
| Oryzanol | 1.2–1.7 | Increase good (HDL) cholesterol and decrease. bad (LDL) cholesterol, treats nerve imbalance and menopause disorder, anti-aging effects, antidandruff and anti-itching agent |
| Tocotrienol | 0.025–0.17 | Cholesterol reduction, reversing. atherosclerosis, anti-cancer (breast, liver) tumor suppression, antioxidant |
| Tocopherol | 0.02–0.08 | Antioxidant, free radical scavenger, reduce risk of cardiovascular diseases, arthritis, cancer and cataracts, anti-tumor activities. |
| Squalene | 0.3–0.4 | Antioxidant |
| Secondary Metabolite | Biological Potentials | References |
|---|---|---|
| Trans-ferulic acid | Antioxidant | [91] |
| Cis-ferulic acid | Antioxidant | [91] |
| Vanillic aldehyde | Antioxidant | [91] |
| Caffeic acid | Antioxidant | [92] |
| Chlorogenic acid | Antioxidant | [92] |
| Gallic acid | Antioxidant | [92] |
| Syringic acid | Antioxidant | [92] |
| Tricin | DPPH radical scavenging activity | |
| Gramisterol | Anti-cancer activity | [93] |
| Lupeol | Anti-cancer activity | [93] |
| 24-methylene cycloartenol | Lowering postprandial hyperglycemia. | |
| 24-methyl cholesterol cis-ferulate | Anti-inflammatory activity | [94] |
| Stigmastanol cis-ferulate | Anti-inflammatory activity | [95] |
| Cycloeucalenol | Anti-cancer activity | [93] |
| Amount Ingested of RBO | Model | Results | References |
|---|---|---|---|
| 400 mg/kg-Tocotrienols | Mice | Gamma-tocotrienol of RBO has the potential to reduce pancreatic. Tumor growth by inhibiting the NF-KB-mediated inflammatory. microenvironment | [101] |
| 30 mL | A randomized double-blind control trial | Gamma-oryzanol-rich RBO may improve cardiovascular. disease risk factors by decreasing LDL-C levels and increasing antioxidant potential in hyperlipidemic issues | [102] |
| 2 mL/kg | Hypertensive rats | γ-oryzanol-rich RBO provides a protective mechanism against oxidative stress and hypertension | |
| 50 g/100 g | Male mice | RBO regulates inflammatory responses in murine macrophages. by upregulating mitochondrial respiration | [103] |
| 100 mg/kg | Male Kunming mice | γ-oryzanol protects against ethanol-induced liver injury, which might be due to its alleviation of oxidative stress and inhibition of apoptosis, possibly inhibiting MAPK. Signaling pathways mediated mitochondrial signaling pathway activation. Rice bran contains immune system-boosting components. including phytosterols, sterol ins and gamma-oryzanol, omega-3 acids, phytonutrients, minerals, etc. | [104] |
| 100 mg kg−1 day−1 | Mice | γ-oryzanol, a component of RBO, shows an anti-allergic effect to inhibit the allergy by reducing the action of NF-KB |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tufail, T.; Ain, H.B.U.; Chen, J.; Virk, M.S.; Ahmed, Z.; Ashraf, J.; Shahid, N.U.A.; Xu, B. Contemporary Views of the Extraction, Health Benefits, and Industrial Integration of Rice Bran Oil: A Prominent Ingredient for Holistic Human Health. Foods 2024, 13, 1305. https://doi.org/10.3390/foods13091305
Tufail T, Ain HBU, Chen J, Virk MS, Ahmed Z, Ashraf J, Shahid NUA, Xu B. Contemporary Views of the Extraction, Health Benefits, and Industrial Integration of Rice Bran Oil: A Prominent Ingredient for Holistic Human Health. Foods. 2024; 13(9):1305. https://doi.org/10.3390/foods13091305
Chicago/Turabian StyleTufail, Tabussam, Huma Bader Ul Ain, Jin Chen, Muhammad Safiullah Virk, Zahoor Ahmed, Jawad Ashraf, Noor Ul Ain Shahid, and Bin Xu. 2024. "Contemporary Views of the Extraction, Health Benefits, and Industrial Integration of Rice Bran Oil: A Prominent Ingredient for Holistic Human Health" Foods 13, no. 9: 1305. https://doi.org/10.3390/foods13091305





