Polyphenol-Retaining Decaffeinated Cocoa Powder Obtained by Supercritical Carbon Dioxide Extraction and Its Antioxidant Activity
Abstract
:1. Introduction
2. Experimental Section
2.1. Sample Conditions and SCCO2 Extraction
2.2. Analysis of SCCO2 Extraction Products
2.2.1. Determination of Caffeine and Theobromine Content
2.2.2. Determination of Total Polyphenol Content
2.2.3. Determination of Procyanidin Content
2.2.4. Calculation of the Removal Ratio and the Residual Ratio of Each Ingredient
2.2.5. Evaluation of Antioxidant Activity
2.3. Statistical Analysis
3. Result and Discussion
3.1. Composition of Cocoa Powder before SCCO2 Extraction
| Content (g/100 g) | Calorie (kcal/100 g) | Concentration (mg/g) | ORAC (μmol-TE/g) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Protein | Fat | Carbohydrate | Dietary fiber | Moisture | Mineral | Caffeine | Theobromine | Total polyphenols | Total procyanidins | |||
| Cocoa mass | 14.2 | 55.1 | 25.7 | 15.6 | 1.8 | 3.2 | 655.5 | 1.19 ± 0.02 | 15.17 ± 0.03 | 33.15 ± 0.73 | 10.03 ± 0.16 | NT |
| Cocoa powder | 27.7 | 16.4 | 47.6 | 29.5 | 2.6 | 5.7 | 449.0 | 2.15 ± 0.01 | 29.21 ± 0.13 | 62.32 ± 1.26 | 15.14 ± 0.26 | 1428.9 ± 11.1 |
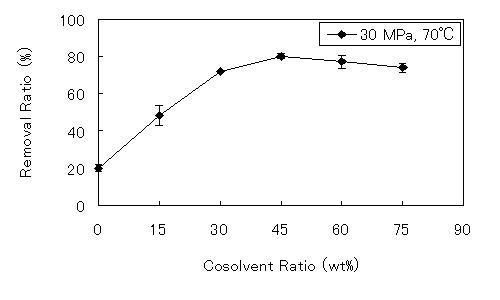
3.2. Effect of Water as a Cosolvent on SCCO2 Extraction
| SCCO2 extraction conditions | Concentration (mg/g) | Removal ratio (%) | Residual ratio (%) | ORAC (μmol-TE/g) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Pressure (MPa) | Temperature (°C) | Cosolvent (wt%) | Caffeine | Theobromine | Total polyphenols | Total procyanidins | Caffeine | Theobromine | Total polyphenols | Total procyanidins | |
| 30 | 70 | 0 | 2.13 ± 0.05 | 34.24 ± 0.42 | 64.61 ± 0.68 | 12.96 ± 0.24 | 20.07 ± 1.79 | 94.56 ± 1.37 | 83.63 ± 1.06 | 85.58 ± 1.60 | 1287.4 ± 26.3 |
| 30 | 70 | 15 | 1.43 ± 0.12 | 35.08 ± 0.65 | 66.36 ± 0.79 | 12.64 ± 0.04 | 48.26 ± 5.47 | 93.52 ± 0.16 | 82.98 ± 2.67 | 83.47 ± 0.26 | 1335.3 ± 42.9 |
| 30 | 70 | 30 | 0.74 ± 0.01 | 33.50 ± 0.28 | 60.97 ± 0.77 | 11.49 ± 0.16 | 71.89 ± 0.15 | 94.32 ± 1.12 | 80.46 ± 0.75 | 75.92 ± 1.04 | 1203.0 ± 53.1 |
| 30 | 70 | 45 | 0.52 ± 0.09 | 33.44 ± 0.08 | 64.17 ± 0.98 | 8.79 ± 0.37 | 80.12 ± 1.05 | 94.12 ± 0.02 | 84.67 ± 1.13 | 58.06 ± 2.43 | 1218.5 ± 50.0 |
| 30 | 70 | 60 | 0.58 ± 0.09 | 33.00 ± 0.51 | 60.21 ± 0.73 | 7.99 ± 0.15 | 77.10 ± 3.55 | 96.02 ± 1.49 | 83.12 ± 1.00 | 52.76 ± 0.97 | 1203.8 ± 14.6 |
| 30 | 70 | 75 | 0.66 ± 0.06 | 31.44 ± 0.13 | 56.77 ± 1.08 | 6.76 ± 0.61 | 74.03 ± 2.50 | 90.98 ± 0.85 | 77.01 ± 1.86 | 44.66 ± 4.02 | 1079.5 ± 75.9 |

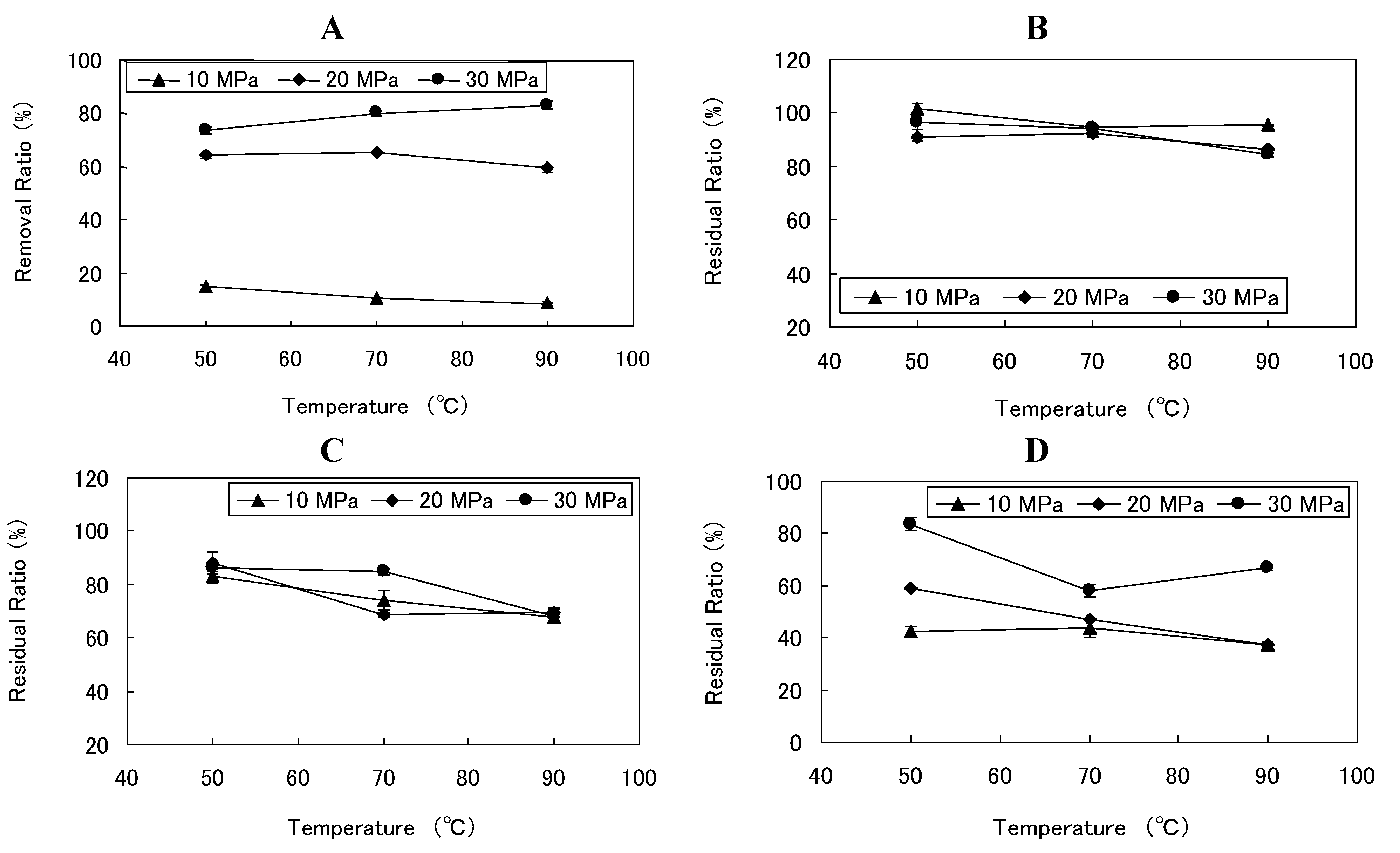
3.3. Effect of Temperature and Pressure on SCCO2 Extraction
| SCCO2 Extraction Conditions | Concentration (mg/g) | Removal ratio (%) | Residual ratio (%) | ORAC (μmol-TE/g) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Pressure (MPa) | Temperature (°C) | Cosolvent (wt%) | Caffeine | Theobromine | Total polyphenols | Total procyanidins | Caffeine | Theobromine | Total polyphenols | Total procyanidins | |
| 10 | 50 | 45 | 1.85 ± 0.01 | 29.99 ± 0.13 | 52.44 ± 2.45 | 6.43 ± 0.28 | 14.91 ± 0.69 | 101.41 ± 2.08 | 83.05 ± 2.54 | 42.25 ± 1.88 | 902.6 ± 12.5 |
| 10 | 70 | 45 | 2.03 ± 0.02 | 29.21 ± 0.23 | 48.73 ± 1.70 | 6.61 ± 0.53 | 10.55 ± 0.15 | 94.72 ± 0.34 | 74.10 ± 3.43 | 43.69 ± 3.52 | 911.6 ± 42.3 |
| 10 | 90 | 45 | 2.02 ± 0.02 | 28.70 ± 0.30 | 43.34 ± 1.08 | 5.64 ± 0.17 | 8.67 ± 0.39 | 95.45 ± 0.34 | 67.55 ± 1.24 | 37.22 ± 1.09 | 555.3 ± 21.2 |
| 20 | 50 | 45 | 0.86 ± 0.00 | 29.65 ± 0.55 | 61.19 ± 0.72 | 8.92 ± 0.07 | 64.19 ± 1.38 | 90.79 ± 1.31 | 87.92 ± 3.94 | 58.92 ± 0.46 | 1001.5 ± 59.6 |
| 20 | 70 | 45 | 0.90 ± 0.01 | 32.43 ± 0.28 | 51.39 ± 0.33 | 7.12 ± 0.06 | 65.41 ± 0.73 | 92.32 ± 1.23 | 68.57 ± 0.77 | 47.01 ± 0.39 | 1006.9 ± 55.9 |
| 20 | 90 | 45 | 0.94 ± 0.04 | 27.14 ± 0.20 | 46.73 ± 1.16 | 5.66 ± 0.02 | 59.56 ± 1.93 | 86.18 ± 0.49 | 69.55 ± 1.60 | 37.40 ± 0.13 | 874.5 ± 38.8 |
| 30 | 50 | 45 | 0.68 ± 0.02 | 33.75 ± 0.00 | 64.28 ± 1.09 | 12.65 ± 0.39 | 73.52 ± 1.47 | 96.66 ± 2.97 | 86.25 ± 1.19 | 83.56 ± 2.58 | 1164.2 ± 34.1 |
| 30 | 70 | 45 | 0.52 ± 0.03 | 33.44 ± 0.08 | 64.17 ± 0.98 | 8.79 ± 0.37 | 80.12 ± 1.05 | 94.12 ± 0.02 | 84.67 ± 1.13 | 58.06 ± 2.43 | 1218.5 ± 50.0 |
| 30 | 90 | 45 | 0.45 ± 0.02 | 30.15 ± 1.41 | 52.33 ± 0.05 | 10.11 ± 0.16 | 82.86 ± 1.53 | 84.29 ± 0.51 | 68.17 ± 2.87 | 66.77 ± 1.06 | 1057.1 ± 6.9 |
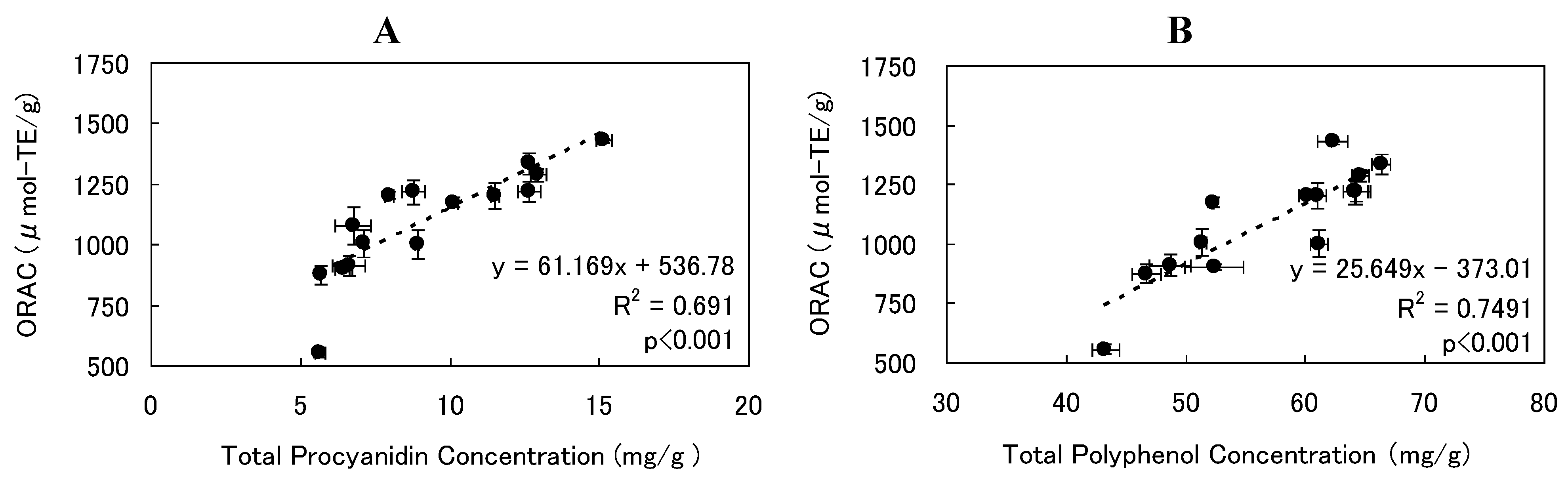
3.4. Effect of SCCO2 Extraction Conditions on ORAC Value

4. Conclusions
Acknowledgments
Conflicts of Interest
References
- Van den Bogaard, B.; Draijer, R.; Westerhof, B.E.; van den Meiracker, A.H.; van Montfrans, G.A.; van den Born, B.J. Effects on peripheral and central blood pressure of cocoa with natural or high-dose theobromine: A randomized, double-blind crossover trial. Hypertension 2010, 56, 839–846. [Google Scholar] [CrossRef]
- Neufingerl, N.; Zebregs, Y.E.; Schuring, E.A.; Trautwein, E.A. Effect of cocoa and theobromine consumption on serum HDL-cholesterol concentrations: A randomized controlled trial. Am. J. Clin. Nutr. 2013, 97, 1201–1209. [Google Scholar] [CrossRef]
- Arteel, G.E.; Schroeder, P.; Sies, H. Reactions of peroxynitrite with cocoa procyanidin oligomers. J. Nutr. 2000, 130, 2100S–2104S. [Google Scholar]
- Corti, R.; Flammer, A.J.; Hollenberg, N.K.; Lüscher, T.F. Cocoa and cardiovascular health. Circulation 2009, 119, 1433–1441. [Google Scholar] [CrossRef]
- Grassi, D.; Desideri, G.; Necozione, S.; Lippi, C.; Casale, R.; Properzi, G.; Blumberg, J.B.; Ferri, C. Blood pressure is reduced and insulin sensitivity increased in glucose-intolerant, hypertensive subjects after 15 days of consuming high-polyphenol dark chocolate. J. Nutr. 2008, 138, 1671–1676. [Google Scholar]
- Ostertag, L.M.; Kroon, P.A.; Wood, S.; Horgan, G.W.; Cienfuegos-Jovellanos, E.; Saha, S.; Duthie, G.G.; de Roos, B. Flavan-3-ol-enriched dark chocolate and white chocolate improve acute measures of platelet function in a gender-specific way—A randomized-controlled human intervention trial. Mol. Nutr. Food Res. 2013, 57, 191–202. [Google Scholar] [CrossRef]
- Schewe, T.; Kühn, H.; Sies, H. Flavonoids of cocoa inhibit recombinant human 5-lipoxygenase. J. Nutr. 2002, 132, 1825–1829. [Google Scholar]
- Yamagishi, M.; Natsume, M.; Osakabe, N.; Okazaki, K.; Furukawa, F.; Imazawa, T.; Nishikawa, A.; Hirose, M. Chemoprevention of lung carcinogenesis by cacao liquor proanthocyanidins in a male rat multi-organ carcinogenesis model. Cancer Lett. 2003, 191, 49–57. [Google Scholar] [CrossRef]
- Pase, M.P.; Scholey, A.B.; Pipingas, A.; Kras, M.; Nolidin, K.; Gibbs, A.; Wesnes, K.; Stough, C. Cocoa polyphenols enhance positive mood states but not cognitive performance: A randomized, placebo-controlled trial. J. Psychopharmacol. 2013, 27, 451–458. [Google Scholar] [CrossRef]
- Sorond, F.A.; Lipsitz, L.A.; Hollenberg, N.K.; Fisher, N.D. Cerebral blood flow response to flavanol-rich cocoa in healthy elderly humans. Neuropsychiatr. Dis. Treat. 2008, 4, 433–440. [Google Scholar]
- Buijsse, B.; Weikert, C.; Drogan, D.; Bergmann, M.; Boeing, H. Chocolate consumption in relation to blood pressure and risk of cardiovascular disease in German adults. Eur. Heart J. 2010, 31, 1616–1623. [Google Scholar] [CrossRef]
- Larsson, S.C.; Virtamo, J.; Wolk, A. Chocolate consumption and risk of stroke: A prospective cohort of men and meta-analysis. Neurology 2012, 79, 1223–1229. [Google Scholar] [CrossRef]
- Mineharu, Y.; Koizumi, A.; Wada, Y.; Iso, H.; Watanabe, Y.; Date, C.; Yamamoto, A.; Kikuchi, S.; Inaba, Y.; Toyoshima, H.; et al. Coffee, green tea, black tea and oolong tea consumption and risk of mortality from cardiovascular disease in Japanese men and women. J. Epidemiol. Community Health 2011, 65, 230–240. [Google Scholar] [CrossRef]
- Lucas, M.; Mirzaei, F.; Pan, A.; Okereke, O.I.; Willett, W.C.; O’Reilly, E.J.; Koenen, K.; Ascherio, A. Coffee, caffeine, and risk of depression among women. Arch. Intern. Med. 2011, 171, 1571–1578. [Google Scholar] [CrossRef]
- Liu, R.; Guo, X.; Park, Y.; Huang, X.; Sinha, R.; Freedman, N.D.; Hollenbeck, A.R.; Blair, A.; Chen, H. Caffeine intake, smoking, and risk of Parkinson disease in men and women. Am. J. Epidemiol. 2012, 175, 1200–1207. [Google Scholar] [CrossRef]
- Mesas, A.E.; Leon-Muñoz, L.M.; Rodriguez-Artalejo, F.; Lopez-Garcia, E. The effect of coffee on blood pressure and cardiovascular disease in hypertensive individuals: A systematic review and meta-analysis. Am. J. Clin. Nutr. 2011, 94, 1113–1126. [Google Scholar] [CrossRef]
- Robertson, D.; Frölich, J.C.; Carr, R.K.; Watson, J.T.; Hollifield, J.W.; Shand, D.G.; Oates, J.A. Effects of caffeine on plasma renin activity, catecholamines and blood pressure. N. Engl. J. Med. 1978, 298, 181–186. [Google Scholar] [CrossRef]
- Karacan, I.; Thornby, J.I.; Anch, M.; Booth, G.H.; Williams, R.L.; Salis, P.J. Dose-related sleep disturbances induced by coffee and caffeine. Clin. Pharmacol. Ther. 1976, 20, 682–689. [Google Scholar]
- Dixon, R.; Hwang, S.; Britton, F.; Sanders, K.; Ward, S. Inhibitory effect of caffeine on pacemaker activity in the oviduct is mediated by cAMP-regulated conductances. Br. J. Pharmacol. 2011, 163, 745–754. [Google Scholar] [CrossRef]
- Weng, X.; Odouli, R.; Li, D.K. Maternal caffeine consumption during pregnancy and the risk of miscarriage: A prospective cohort study. Am. J. Obstet. Gynecol. 2008, 198, 279.e1–279.e8. [Google Scholar] [CrossRef]
- Boylan, S.; Cade, J.E.; Dolby, V.A.; Greenwood, D.C.; Hay, A.W.; Kirk, S.F.; Shires, S.; Simpson, N.; Thomas, J.D.; Walker, J.; et al. Maternal caffeine intake during pregnancy and risk of fetal growth restriction: A large prospective observational study. BMJ 2008, 337, 1–8. [Google Scholar]
- Zoumas, B.L.; Kreiser, W.R.; Martin, R. Theobromine and caffeine content of chocolate products. J. Food Sci. 1980, 45, 314–316. [Google Scholar] [CrossRef]
- Margolis, G.; Chiovini, J.; Pagliaro, F.A. Removal of xanthine stimulants from cocoa. U.K. Patent Application GB2095091 1982.
- Park, H.S.; Lee, H.J.; Shin, M.H.; Lee, K.W.; Lee, H.; Kim, Y.S.; Kim, K.O.; Kim, K.H. Effects of cosolvents on the decaffeination of green tea by supercritical carbon dioxide. Food Chem. 2007, 105, 1011–1017. [Google Scholar] [CrossRef]
- Rahoma, S.M.; Marleny, D.A.S.; Paulo, M. Extraction of caffeine, theobromine, and cocoa butter from Brazilian cocoa beans using supercritical CO2 and ethane. Ind. Eng. Chem. Res. 2002, 41, 6751–6758. [Google Scholar] [CrossRef]
- Moriyasu, T.; Saito, K.; Nakazato, M.; Ishikawa, F.; Fujinuma, K.; Nishima, T.; Tamura, Y. Simultaneous determination of caffeine, theobromine and theophylline in foods by HPLC. Food Hyg. Saf. Sci. 1996, 37, 14–19. [Google Scholar] [CrossRef]
- Ramirez-Sanchez, I.; Maya, L.; Ceballos, G.; Villarreal, F. Fluorescent detection of (−)-epicatechin in microsamples from cacao seeds and cocoa products: Comparison with Folin-Ciocalteu method. J. Food Compost. Anal. 2010, 23, 790–793. [Google Scholar] [CrossRef]
- Kelm, M.A.; Johnson, J.C.; Robbins, R.J.; Hammerstone, J.F.; Schmitz, H.H. High-performance liquid chromatography separation and purification of cacao (Theobroma cacao L.) procyanidins according to degree of polymerization using a diol stationary phase. J. Agric. Food Chem. 2006, 54, 1571–1576. [Google Scholar] [CrossRef]
- Bergmann, W.R.; Barkley, M.D.; Hemingway, R.W.; Mattice, W.L. Heterogeneous fluorescence decay of (4beta->6)- and (4beta->8)-linked dimers of (+)-catechin and (−)-epicatechin as a result of rotational isomerism. J. Am. Chem. Soc. 1987, 109, 6614–6619. [Google Scholar] [CrossRef]
- Singleton, V.L.; Joseph, A.R.J. Colorimetry of total phenolics with phosphomolybdic phosphotungstic acid reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar]
- Takebayashi, J.; Oki, T.; Chen, J.; Sato, M.; Matsumoto, T.; Taku, K.; Tsubota-Utsugi, M.; Watanabe, J.; Ishimi, Y. Estimated average daily intake of antioxidants from typical vegetables consumed in Japan: A preliminary study. Biosci. Biotechnol. Biochem. 2010, 74, 2137–2140. [Google Scholar] [CrossRef]
- Huang, D.; Ou, B.; Hampsch-Woodill, M.; Flanagan, J.A.; Prior, R.L. High-throughput assay of oxygen radical absorbance capacity (ORAC) using a multichannel liquid handling system coupled with a microplate fluorescence reader in 96-well format. J. Agric. Food Chem. 2002, 50, 4437–4444. [Google Scholar] [CrossRef]
- Kreiser, W.R.; Martin, R.A.J. High pressure liquid chromatographic determination of theobromine and caffeine in cocoa and chocolate products. J. Assoc. Off. Anal. Chem. 1978, 61, 1424–1427. [Google Scholar]
- Natsume, M.; Osakabe, N.; Yamagishi, M.; Takizawa, T.; Nakamura, T.; Miyatake, H.; Hatano, T.; Yoshida, T. Analyses of polyphenols in cacao liquor, cocoa, and chocolate by normal-phase and reversed-phase HPLC. Biosci. Biotechnol. Biochem. 2000, 64, 2581–2587. [Google Scholar] [CrossRef]
- Adamson, G.E.; Lazarus, S.A.; Mitchell, A.E.; Prior, R.L.; Cao, G.; Jacobs, P.H.; Kremers, B.G.; Hammerstone, J.F.; Rucker, R.B.; Ritter, K.A.; Schmitz, H.H. HPLC method for the quantification of procyanidins in cocoa and chocolate samples and correlation to total antioxidant capacity. J. Agric. Food Chem. 1999, 47, 4184–4188. [Google Scholar] [CrossRef]
- Kim, W.J.; Kim, J.D.; Kim, J.; Oh, S.G.; Lee, Y.W. Selective caffeine removal from green tea using supercritical carbon dioxide extraction. J. Food Eng. 2008, 89, 303–308. [Google Scholar] [CrossRef]
- Brunner, G. Gas Extraction—An Introduction to Fundamentals of Supercritical Fluids and the Application to Separation Processes; Springer: New York, NY, USA, 1994. [Google Scholar]
- Sabirzyanov, A.N.; Il’in, A.P.; Akhunov, A.R.; Gumerov, F.M. Solubility of water in supercritical carbon dioxide. High Temp. 2002, 40, 203–206. [Google Scholar] [CrossRef]
- Castro, M.D.L.; Valcárcel, M.; Tena, M.T. Analytical supercritical fluid extraction. Springer-Verlag: Berlin, Germany, 1994; pp. 100–108. [Google Scholar]
- Johannsen, M.; Brunner, G. Measurements of solubilities of xanthines in supercritical carbon dioxide + methanol. J. Chem. Eng. Data 1995, 40, 431–434. [Google Scholar] [CrossRef]
- Johannsen, M.; Brunner, G. Solubilities of the xanthines caffeine, theophylline and theobromine in supercritical carbon dioxide. Fluid Phase Equilib. 1994, 95, 215–226. [Google Scholar] [CrossRef]
- Calvo, L.; Muguerza, B.; Cienfuegos-Jovellanos, E. Microbial inactivation and butter extraction in a cocoa derivative using high pressure CO2. J. Supercrit. Fluids 2007, 42, 80–87. [Google Scholar] [CrossRef]
- Brunner, G.; Zwiefelhofer, U.; Simon, A. Abstract Handbook: 2nd High Pressure Chemical Engineering; DECHEMA: Erlangen, Germany, 1990. [Google Scholar]
- Schulmeyr, J.; Igl, N. Supercritical CO2-Extraction: Pressure up to 1000 Bars Open New Horizons. In Proceedings of the 9th Meeting on Supercritical Fluids, Trieste, Italy, 13–16 June 2004.
- Payne, M.J.; Hurst, W.J.; Miller, K.B.; Rank, C.; Stuart, D.A. Impact of fermentation, drying, roasting, and Dutch processing on epicatechin and catechin content of cacao beans and cocoa ingredients. J. Agric. Food Chem. 2010, 58, 10518–10527. [Google Scholar] [CrossRef]
- Stahl, E.; Gerard, D. Solubility behaviour and fractionation of essential oils in dense carbon dioxide. Perfum. Flavorist 1985, 10, 29–37. [Google Scholar]
- Gu, L.; House, S.E.; Wu, X.; Ou, B.; Prior, R.L. Procyanidin and catechin contents and antioxidant capacity of cocoa and chocolate products. J. Agric. Food Chem. 2006, 54, 4057–4061. [Google Scholar] [CrossRef]
- Goto, Y.; Aihara, T.; Okuyama, S.; Kamiwaki, T.; Tsukada, K.; Uzawa, M. Compositional changes of Venezuelan cacao beans during the fermentation. J. Jpn. Soc. Food Sci. Technol. 2002, 49, 731–735. [Google Scholar] [CrossRef]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Kobori, K.; Maruta, Y.; Mineo, S.; Shigematsu, T.; Hirayama, M. Polyphenol-Retaining Decaffeinated Cocoa Powder Obtained by Supercritical Carbon Dioxide Extraction and Its Antioxidant Activity. Foods 2013, 2, 462-477. https://doi.org/10.3390/foods2040462
Kobori K, Maruta Y, Mineo S, Shigematsu T, Hirayama M. Polyphenol-Retaining Decaffeinated Cocoa Powder Obtained by Supercritical Carbon Dioxide Extraction and Its Antioxidant Activity. Foods. 2013; 2(4):462-477. https://doi.org/10.3390/foods2040462
Chicago/Turabian StyleKobori, Kinji, Yuto Maruta, Shigeru Mineo, Toru Shigematsu, and Masao Hirayama. 2013. "Polyphenol-Retaining Decaffeinated Cocoa Powder Obtained by Supercritical Carbon Dioxide Extraction and Its Antioxidant Activity" Foods 2, no. 4: 462-477. https://doi.org/10.3390/foods2040462