Purification of Recombinant Peanut Allergen Ara h 1 and Comparison of IgE Binding to the Natural Protein
Abstract
:1. Introduction

2. Experimental Section
2.1. Plasmid Constructions and Protein Expression
2.2. Purification of Mature rAra h 1
2.3. Purification of Core rAra h 1
2.4. Gel Electrophoresis and Western Blots
2.5. Secondary Structure Determination
2.6. Patient Sera
2.7. Western and Spot Blot Analysis with Patient Sera
3. Results and Discussion
3.1. Expression and Purification of Mature rAra h 1

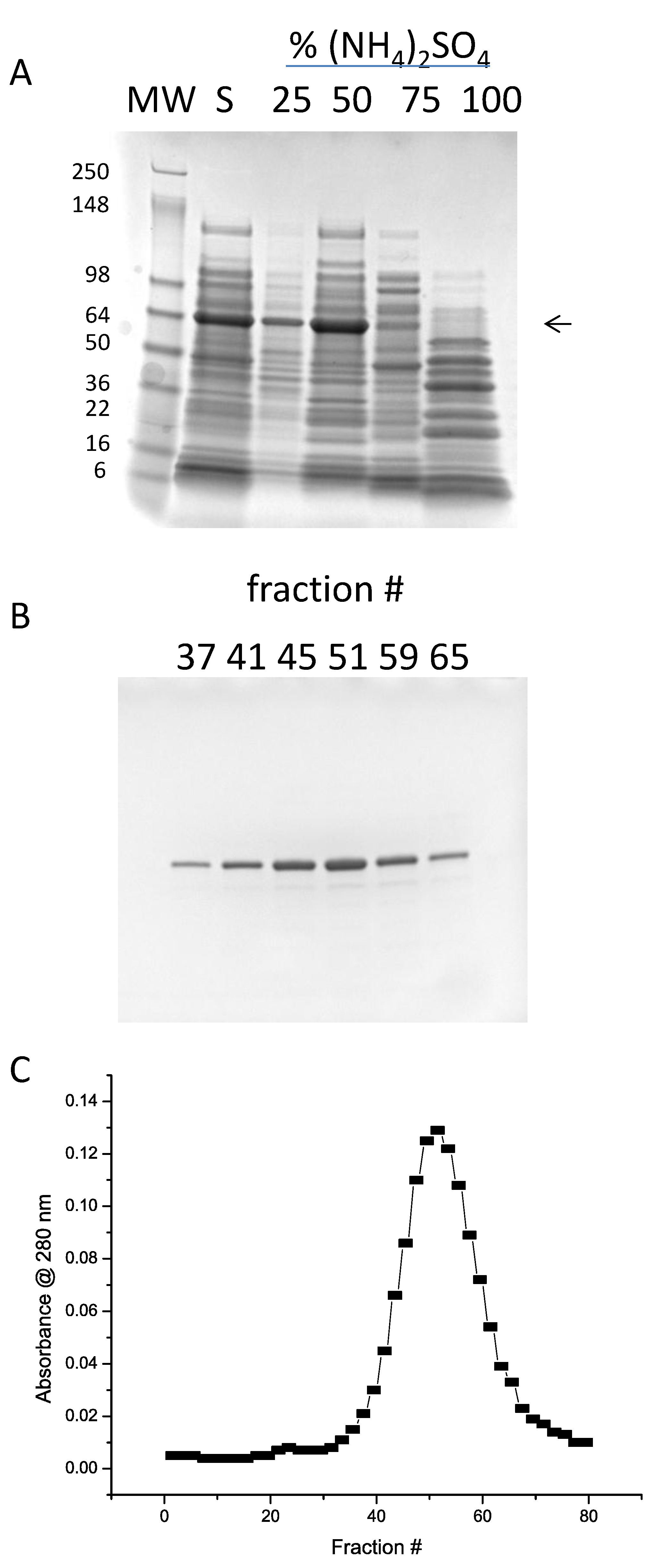
3.2. Expression and Purification of Core rAra h 1



3.3. Secondary Structure Analysis of Recombinant Mature and Core Ara h 1

3.4. Western and Spot Blot Analysis

4. Conclusions
Acknowledgements
Author Contributions
Conflicts of Interest
References
- Table 13 Peanut Area, Yield, and Production. Available online: http://www.apps.fas.usda.gov/psdonline/psdreport.aspx?hidReportRetrievalName=BVS&hidReportRetrievalID=918&hidReportRetrievalTemplateID=1#ancor (accessed on 10 December 2014).
- Sicherer, S.H.; Munoz-Furlong, A.; Godbold, J.H.; Sampson, H.A. US prevalence of self-reported peanut, tree nut, and sesame allergy: 11-year follow-up. J. Allergy Clin. Immunol. 2010, 125, 1322–1326. [Google Scholar]
- Rabjohn, P.; Burks, A.W.; Sampson, H.A.; Bannon, G.A. Mutational analysis of the IgE-binding epitopes of the peanut allergen, Ara h 3: A member of the glycinin family of seed-storage proteins. J. Allergy Clin. Immun. 1999, 103, S101–S101. [Google Scholar]
- Shin, D.S.; Compadre, C.M.; Maleki, S.J.; Kopper, R.A.; Sampson, H.; Huang, S.K.; Burks, A.W.; Bannon, G.A. Biochemical and structural analysis of the IgE binding sites on Ara h1, an abundant and highly allergenic peanut protein. J. Biol. Chem. 1998, 273, 13753–13759. [Google Scholar]
- Stanley, J.S.; King, N.; Burks, A.W.; Huang, S.K.; Sampson, H.; Cockrell, G.; Helm, R.M.; West, C.M.; Bannon, G.A. Identification and mutational analysis of the immunodominant IgE binding epitopes of the major peanut allergen Ara h 2. Arch. Biochem. Biophys. 1997, 342, 244–253. [Google Scholar]
- Maleki, S.J.; Chung, S.Y.; Champagne, E.T.; Raufman, J.P. The effects of roasting on the allergenic properties of peanut proteins. J. Allergy Clin. Immunol. 2000, 106, 763–768. [Google Scholar]
- Cong, Y.J.; Lou, F.; Xue, W.T.; Li, L.F.; Chen, M.H. Characterisation of the IgE-binding immunodominant epitopes on Ara h1. Food Agric. Immunol. 2008, 19, 175–185. [Google Scholar]
- Shreffler, W.G.; Beyer, K.; Chu, T.H.; Burks, A.W.; Sampson, H.A. Microarray immunoassay: Association of clinical history, in vitro IgE function, and heterogeneity of allergenic peanut epitopes. J. Allergy Clin. Immunol 2004, 113, 776–782. [Google Scholar]
- Stanley, J.S.; Helm, R.M.; Cockrell, G.; Burks, A.W.; Bannon, G.A. Peanut hypersensitivity. IgE binding characteristics of a recombinant Ara h I protein. Adv. Exp. Med. Biol. 1996, 409, 213–216. [Google Scholar]
- Wichers, H.J.; de Beijer, T.; Savalkoul, H.F.J.; van Amerongen, A. The major peanut allergen Ara h 1 and its cleaved-off N-terminal peptide; possible implications for peanut allergen detection. J. Agric. Food Chem. 2004, 52, 4903–4907. [Google Scholar]
- Burks, A.W.; Shin, D.; Cockrell, G.; Stanley, J.S.; Helm, R.M.; Bannon, G.A. Mapping and mutational analysis of the IgE-binding epitopes on Ara h 1, a legume vicilin proteins and a major allergen in peanut hypersensitivity. Eur. J. Biochem. 1997, 245, 334–339. [Google Scholar]
- Cabanos, C.; Urabe, H.; Masuda, T.; Tandang-Silvas, M.R.; Utsumi, S.; Mikami, B.; Maruyama, N. Crystallization and preliminary X-ray analysis of the major peanut allergen Ara h 1 core region. Acta Crystallogr. Sect. F Struct. Biol. Cryst. Commun. 2010, 66, 1071–1073. [Google Scholar] [Green Version]
- Cabanos, C.; Urabe, H.; Tandang-Silvas, M.R.; Utsumi, S.; Mikami, B.; Maruyama, N. Crystal structure of the major peanut allergen Ara h 1. Mol. Immunol. 2011, 49, 115–123. [Google Scholar]
- Chruszcz, M.; Maleki, S.J.; Majorek, K.A.; Demas, M.; Bublin, M.; Solberg, R.; Hurlburt, B.K.; Ruan, S.; Mattison, C.P.; Breiteneder, H.; Minor, W. Structural and immunologic characterization of Ara h 1, a major peanut allergen. J. Biol. Chem. 2011, 286, 39318–39327. [Google Scholar]
- Maleki, S.J.; Kopper, R.A.; Shin, D.S.; Park, C.W.; Compadre, C.M.; Sampson, H.; Burks, A.W.; Bannon, G.A. Structure of the major peanut allergen Ara h 1 may protect IgE-binding epitopes from degradation. J. Immunol. 2000, 164, 5844–5849. [Google Scholar]
- Pollastri, G.; Przybylski, D.; Rost, B.; Baldi, P. Improving the prediction of protein secondary structure in three and eight classes using recurrent neural networks and profiles. Proteins 2002, 47, 228–235. [Google Scholar]
- Rost, B.; Sander, C. Improved prediction of protein secondary structure by use of sequence profiles and neural networks. Proc. Natl. Acad. Sci. USA 1993, 90, 7558–7562. [Google Scholar]
- Rost, B.; Sander, C. Secondary structure prediction of all-helical proteins in two states. Protein Eng. 1993, 6, 831–836. [Google Scholar]
- Sreerama, N.; Woody, R.W. A self-consistent method for the analysis of protein secondary structure from circular dichroism. Anal. Biochem. 1993, 209, 32–44. [Google Scholar]
- Sreerama, N.; Woody, R.W. Protein secondary structure from circular dichroism spectroscopy. Combining variable selection principle and cluster analysis with neural network, ridge regression and self-consistent methods. J. Mol. Biol. 1994, 242, 497–507. [Google Scholar]
- Van Boxtel, E.L.; Koppelman, S.J.; van den Broek, L.A.M.; Gruppen, H. Determination of pepsin-susceptible and pepsin-resistant epitopes in native and heat-treated peanut allergen Ara h 1. J. Agric. Food Chem. 2008, 56, 2223–2230. [Google Scholar]
- Andrade, M.A.; Chacon, P.; Merelo, J.J.; Moran, F. Evaluation of secondary structure of proteins from UV circular dichroism spectra using an unsupervised learning neural network. Protein Eng. 1993, 6, 383–390. [Google Scholar]
- Mueller, G.A.; Maleki, S.J.; Johnson, K.; Hurlburt, B.K.; Cheng, H.; Ruan, S.; Nesbit, J.B.; Pomes, A.; Edwards, L.L.; Schorzman, A. Identification of Maillard reaction products on peanut allergens that influence binding to the receptor for advanced glycation end products. Allergy 2013, 68, 1546–1554. [Google Scholar]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hurlburt, B.K.; McBride, J.K.; Nesbit, J.B.; Ruan, S.; Maleki, S.J. Purification of Recombinant Peanut Allergen Ara h 1 and Comparison of IgE Binding to the Natural Protein. Foods 2014, 3, 642-657. https://doi.org/10.3390/foods3040642
Hurlburt BK, McBride JK, Nesbit JB, Ruan S, Maleki SJ. Purification of Recombinant Peanut Allergen Ara h 1 and Comparison of IgE Binding to the Natural Protein. Foods. 2014; 3(4):642-657. https://doi.org/10.3390/foods3040642
Chicago/Turabian StyleHurlburt, Barry K., Jane K. McBride, Jacqueline B. Nesbit, Sanbao Ruan, and Soheila J. Maleki. 2014. "Purification of Recombinant Peanut Allergen Ara h 1 and Comparison of IgE Binding to the Natural Protein" Foods 3, no. 4: 642-657. https://doi.org/10.3390/foods3040642
APA StyleHurlburt, B. K., McBride, J. K., Nesbit, J. B., Ruan, S., & Maleki, S. J. (2014). Purification of Recombinant Peanut Allergen Ara h 1 and Comparison of IgE Binding to the Natural Protein. Foods, 3(4), 642-657. https://doi.org/10.3390/foods3040642



