Development and Physico-Chemical Characterization of a Shea Butter-Containing Lipid Nutrition Supplement for Sub-Saharan Africa
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ingredients, Chemicals, and Materials
2.2. Recipe Formulation
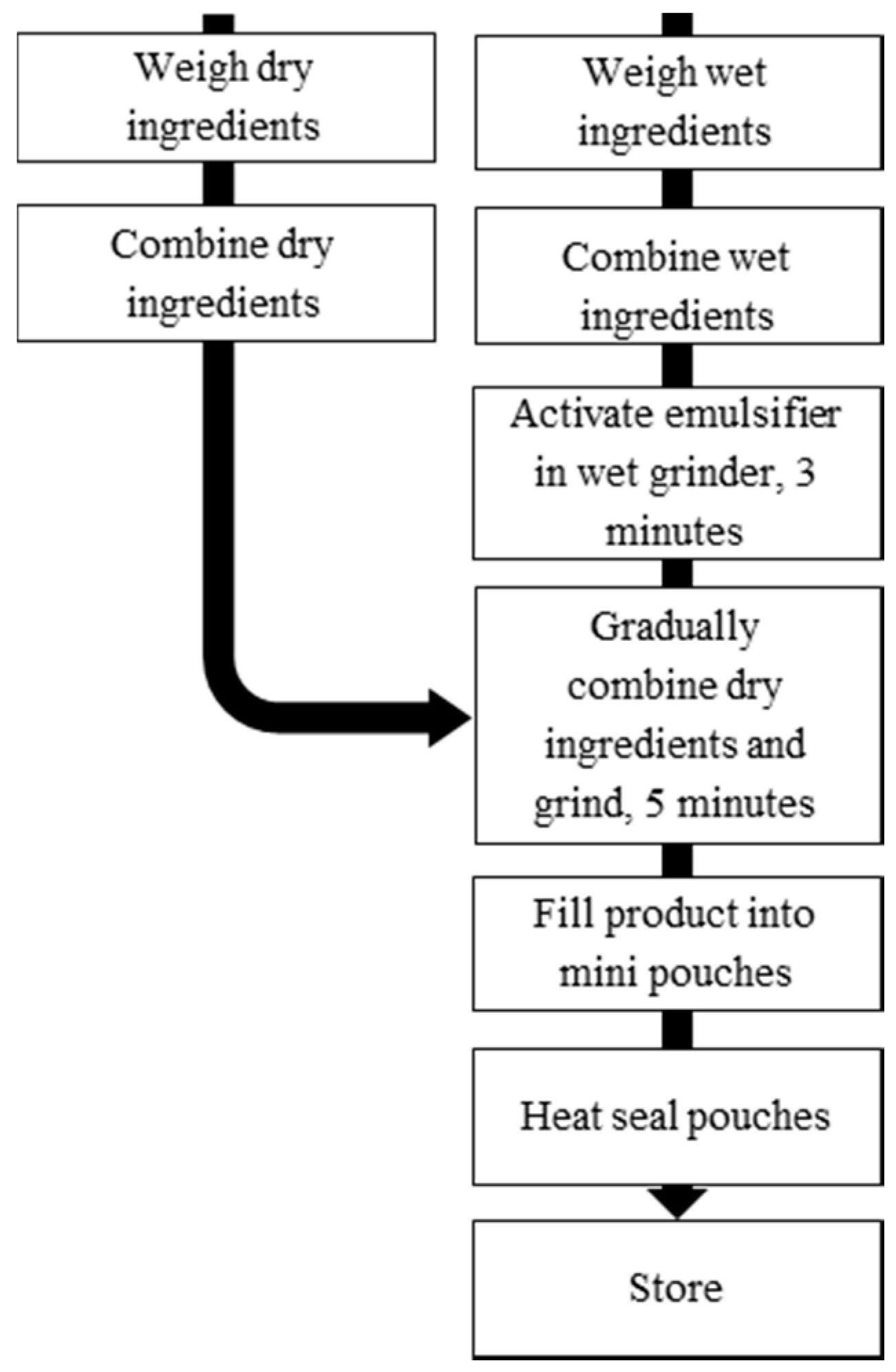
2.3. Production and Storage of LNS
2.4. Water Activity
2.5. Peroxide Value
2.6. Moisture Content
2.7. Lipid Separation
2.8. Texture Analysis
2.9. Vitamin C
2.10. Data Analysis
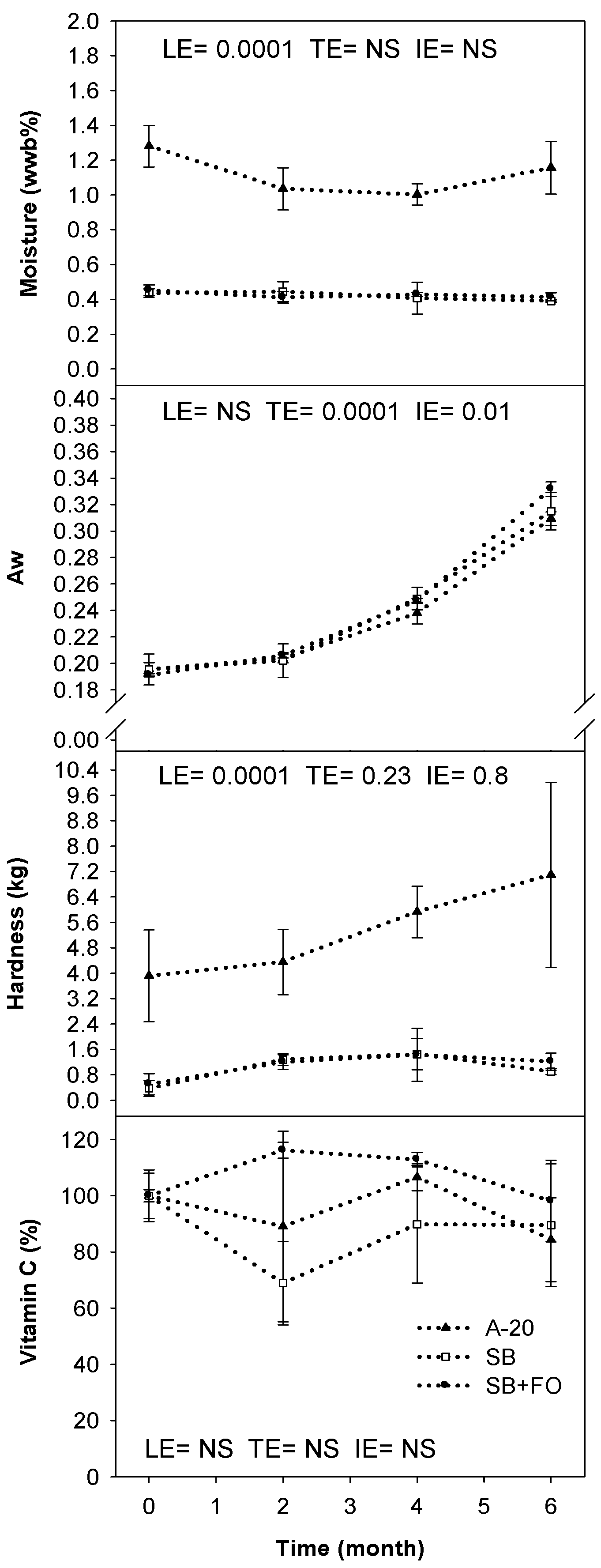
3. Results
4. Discussion
4.1. Reformulation of LNS
4.2. Characterization and Stability of LNS Formulas
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Black, R.E.; Victora, C.G.; Walker, S.P.; Bhutta, Z.A.; Christian, P.; De Onis, M.; Ezzati, M.; Grantham-Mcgregor, S.; Katz, J.; Martorell, R.; et al. Maternal and child undernutrition and overweight in low-income and middle-income countries. Lancet 2013, 382, 427–451. [Google Scholar] [CrossRef]
- UNICEF/WHO/World Bank Levels and trends in child malnutrition. UNICEF-WHO-World Bank Jt. Child Malnutrition Estim. 2016; 2016, 1–8.
- Collins, S.; Dent, N.; Binns, P.; Bahwere, P.; Sadler, K.; Hallam, A. Management of severe acute malnutrition in children. Lancet 2006, 368, 1992–2000. [Google Scholar] [CrossRef]
- Diop, E.H.I.; Dossou, N.I.; Ndour, M.M.; Briend, A.; Wade, S. Comparison of the efficacy of a solid ready-to-use food and a liquid, milk-based diet for the rehabilitation of severely malnourished children: A randomized trial. Am. J. Clin. Nutr. 2003, 78, 302–307. [Google Scholar]
- Manary, M.J.; Ndekha, M.J.; Ashorn, P.; Maleta, K.; Briend, A. Home based therapy for severe malnutrition with ready-to-use food. Arch. Dis. Child. 2004, 89, 557–561. [Google Scholar] [CrossRef] [PubMed]
- Ciliberto, M.A.; Sandige, H.; Ndekha, M.J.; Ashorn, P.; Briend, A.; Ciliberto, H.M.; Manary, M.J. Comparison of home-based therapy with ready-to-use therapeutic food with standard therapy in the treatment of malnourished Malawian children: A controlled, clinical effectiveness trial. Am. J. Clin. Nutr. 2005, 81, 864–870. [Google Scholar] [PubMed]
- World Food Program Specialized Nutritious Foods Sheet. Available online: http://documents.wfp.org/stellent/groups/public/documents/communications/wfp255508.pdf?_ga=2.31802750.1942645254.1495212710-1806367991.1495212710 (accessed on 2 July 2017).
- Briend, A.; Lacsala, R.; Prudhon, C.; Mounier, B.; Grellety, Y.; Golden, M.H. Ready-to-use therapeutic food for treatment of marasmus. Lancet 1999, 353, 1767–1768. [Google Scholar] [CrossRef]
- Briend, A. Highly nutrient-dense spreads: A new approach to delivering multiple micronutrients to high-risk groups. Br. J. Nutr. 2001, 85 (Suppl. 2), S175–S179. [Google Scholar] [CrossRef] [PubMed]
- Adu-Afarwuah, S.; Lartey, A.; Brown, K.H.; Zlotkin, S.; Briend, A.; Dewey, K.G. Home fortification of complementary foods with micronutrient supplements is well accepted and has positive effects on infant iron status in Ghana. Am. J. Clin. Nutr. 2008, 87, 929–938. [Google Scholar] [PubMed]
- Phuka, J.C.; Maleta, K.; Thakwalakwa, C.; Cheung, Y.B.; Briend, A.; Manary, M.J.; Ashorn, P. Complementary feeding with fortified spread and incidence of severe stunting in 6- to 18-month-old rural Malawians. Arch. Pediatr. Adolesc. Med. 2008, 162, 619–626. [Google Scholar] [CrossRef] [PubMed]
- Matsungo, T.M.; Kruger, H.S.; Smuts, C.M.; Faber, M. Lipid-based nutrient supplements and linear growth in children under 2 years: A review. Proc. Nutr. Soc. 2017, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Enserink, M. Patents: A Recipe for Problems? Science 2008, 322, 38. [Google Scholar] [CrossRef] [PubMed]
- Institute of Medicine. High-Energy, Nutrient-Dense Emergency Relief Food Product; National Academies Press (US): Washington, DC, USA, 2002. [Google Scholar]
- Briggs, J.; Maguire, P.; Sherman, P.; Davis, B.; Barrett, A.; Mahon, J.; Doucette, J. Final Report on Development of an Emergency Food Product: Prouct and Packaging Specifications, Shelf Life Study and Drop Test; USAID: Natick, MA, USA, 2007.
- Phuka, J.; Ashorn, U.; Ashorn, P.; Zeilani, M.; Cheung, Y.B.; Dewey, K.G.; Manary, M.; Maleta, K. Acceptability of three novel lipid-based nutrient supplements among Malawian infants and their caregivers. Matern. Child Nutr. 2011, 7, 368–377. [Google Scholar] [CrossRef] [PubMed]
- Ali, E.; Zachariah, R.; Shams, Z.; Manzi, M.; Akter, T.; Alders, P.; Allaouna, M.; Delchevalerie, P.; Harries, A.D. Peanut-based ready-to-use therapeutic food: How acceptable and tolerated is it among malnourished pregnant and lactating women in Bangladesh? Matern. Child Nutr. 2015, 11, 1028–1035. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, J.-C.; Liu, L.; Zeilani, M.; Ickes, S.; Trehan, I.; Maleta, K.; Craig, C.; Thakwalakwa, C.; Singh, L.; Brenna, J.T.; et al. High-Oleic Ready-to-Use Therapeutic Food Maintains Docosahexaenoic Acid Status in Severe Malnutrition. J. Pediatr. Gastroenterol. Nutr. 2015, 61, 138–143. [Google Scholar] [CrossRef] [PubMed]
- Jones, K.D.J.; Ali, R.; Khasira, M.A.; Odera, D.; West, A.L.; Koster, G.; Akomo, P.; Talbert, A.W.A.; Goss, V.M.; Ngari, M.; et al. Ready-to-use therapeutic food with elevated n-3 polyunsaturated fatty acid content, with or without fish oil, to treat severe acute malnutrition: A randomized controlled trial. BMC Med. 2015, 13, 93. [Google Scholar] [CrossRef] [PubMed]
- Brenna, J.T.; Akomo, P.; Bahwere, P.; Berkley, J.A.; Calder, P.C.; Jones, K.D.; Liu, L.; Manary, M.; Trehan, I.; Briend, A. Balancing omega-6 and omega-3 fatty acids in ready-to-use therapeutic foods (RUTF). BMC Med. 2015, 13, 117. [Google Scholar] [CrossRef] [PubMed]
- National Nutrient Database for Standard Reference Release 28. Available online: https://ndb.nal.usda.gov/ndb/search/list?ds=Standard+Reference (accessed on 19 September 2016).
- Chaparro, C.M.; Dewey, K.G. Use of lipid-based nutrient supplements (LNS) to improve the nutrient adequacy of general food distribution rations for vulnerable sub-groups in emergency settings. Matern. Child Nutr. 2010, 6, 1–69. [Google Scholar] [CrossRef] [PubMed]
- Caron, O. RUTF Product Specifications. Available online: http://www.unicef.org/supply/files/5._Common_RUTF_Specifications_and_Labelling.pdf (accessed on 19 September 2016).
- United States Department of Agriculture. Agricultural Marketing Service Commercial Item Description: Emergency Food Products, Ready-to-Eat Meal (mEal Replacements). 2009. Available online: https://www.ams.usda.gov/sites/default/files/media/CID%20Emergency%20Food%20Products%2C%20Ready-to-Eat%20%28Meal%20Replacements%29.pdf (accessed on 19 September 2016).
- United States Department of Agriculture. Commodity Requirements EFP2 Emergency Food Product for Use in Export Program. 2009. Available online: https://www.fsa.usda.gov/Internet/FSA_File/efp2.pdf (accessed on 19 September 2016).
- Water Activity Method 32.004–32.009. In Official Methods of Analysis of AOAC International; AOAC International: Gaithersburg, MD, USA, 1995.
- Crowe, T.D.; White, P.J. Adaptation of the AOCS official method for measuring hydroperoxides from small-scale oil samples. J. Am. Oil Chem. Soc. 2001, 78, 1267–1269. [Google Scholar] [CrossRef]
- Peroxide Value of Oils and Fats Method 965.33. In Official Methods of Analysis of AOAC International; AOAC International: Gaithersburg, MD, USA, 1995.
- Hinds, M.J.; Chinnan, M.S.; Beuchat, L.R. Unhydrogenated palm oil as a stabilizer for peanut butter. J. Food Sci. 1994, 59, 816–820. [Google Scholar] [CrossRef]
- Ahmed, E.M.; Ali, T. Textural Quality of Peanut Butter as Influenced by Peanut Seed and Oil Contents 1. Peanut Sci. 1986, 13, 18–20. [Google Scholar] [CrossRef]
- Tarrago-Trani, M.T.; Phillips, K.M.; Cotty, M. Matrix-specific method validation for quantitative analysis of vitamin C in diverse foods. J. Food Compos. Anal. 2012, 26, 12–25. [Google Scholar] [CrossRef]
- De Pee, S.; Bloem, M.W. Current and potential role of specially formulated foods and food supplements for preventing malnutrition among 6-to 23-month-old children and for treating moderate malnutrition among 6-to 59-montn-old children. Food Nutr. Bull. 2009, 30, 434–463. [Google Scholar] [CrossRef] [PubMed]
- Franklin, K.K. Reduced Fat Peanut Butter and Method of Making Same. U.S. Patent 5,302,409, 12 April 1994. [Google Scholar]
- Singh, S.K.; Castell-Perez, M.E.; Moreira, R.G. Viscosity and Textural Attributes of Reduced-fat Peanut Pastes. J. Food Sci. 2000, 65, 849–853. [Google Scholar] [CrossRef]
- Hobbs, L. Sweeteners from Starch. In Starch Chemistry and Technology; BeMiller, J., Whistler, R., Eds.; Elsevier: Amsterdam, The Netherlands, 2009; pp. 797–832. [Google Scholar]
- Richards, M. Lipids: Functional Properties. In Food Chemistry: Principles and Applications; Hui, Y.H., Ed.; Science Technology System: West Sacramento, CA, USA, 2007. [Google Scholar]
- Institute of Shortening and Edible Oils, Inc. Food Fats and Oils, 10th ed.; Institute of Shortening and Edible Oils, Inc.: Washington, DC, USA, 2016. [Google Scholar]
- Addaquay, J. The Shea Butter Value Chain: Refining in West Africa. USAID: Washington, DC, USA, 2004. [Google Scholar]
- Firestone, D. (Ed.) Physical and Chemical Characteristics of Oils, Fats, and Waxes, 3rd ed.; AOCS Press: Urbana, IL, USA, 2013. [Google Scholar]
- United States Department of Agriculture. Agricultural Marketing Service Commercial Item Description: Ready-to-Use Therapeutic Food (RUTF). Available online: https://www.ams.usda.gov/sites/default/files/media/CID%20Therapeutic%20Food%2C%20Ready-To-Use.pdf (accessed on 19 September 2016).
- Nguyen, V.H.; Bouzambou, H. Technical Specifications for Lipid-Based Nutrient Supplement—Large Quantity—LNS-LQ. World Food Program: Rome, Italy, 2014. [Google Scholar]
- Jelensperger, C. Technical Specifications for Lipid-Based Nutrient Supplement—Medium Quantity—LNS MQ. World Food Program: Rome, Italy, 2016. [Google Scholar]
- Gaur, S.; Sloffer, E.M.; Ojha, A.; Patra, F.; Shukla, D.; Engeseth, N.J.; Patel, P.R.; Andrade, J.E. Omega-3-Fortified Lipid-Based Nutrient Supplement: Development, Characterization, and Consumer Acceptability. Food Nutr. Bull. 2017, 38, 158–171. [Google Scholar] [CrossRef] [PubMed]

| A-20 | SB | SB + F | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Ingredient | Wt (g) | Calories (kcal) | % kcal | Wt (g) | Calories (kcal) | % kcal | Wt (g) | Calories (kcal) | % kcal |
| Maltodextrin M 100 | 25.9 | 103.5 | 20% | 0.0 | 0.0 | 0% | 0.0 | 0.0 | 0% |
| Sugar, confectioners/powdered | 18.0 | 70.2 | 14% | 26.9 | 104.7 | 20% | 26.9 | 104.7 | 20% |
| Milk, nonfat/skim, dry | 11.0 | 42.1 | 8% | 11.0 | 42.1 | 8% | 11.0 | 42.1 | 8% |
| Protein, whey, concentrate | 6.0 | 24.0 | 5% | 6.0 | 24.0 | 5% | 6.0 | 24.0 | 5% |
| Dried Cream Powder | 5.5 | 39.6 | 8% | 5.5 | 39.6 | 8% | 5.5 | 39.6 | 8% |
| Pea Protein | 2.2 | 8.0 | 2% | 2.2 | 8.0 | 2% | 2.2 | 8.0 | 2% |
| Oil, soybean, refined | 24.0 | 212.2 | 41% | 0.0 | 0.0 | 0% | 0.0 | 0.0 | 0% |
| Oil, Shea nut | 0.0 | 0.0 | 0% | 5.0 | 44.2 | 8% | 5.0 | 44.2 | 8% |
| Oil, canola | 0.0 | 0.0 | 0% | 25.0 | 221.0 | 42% | 20.0 | 176.8 | 34% |
| Oil, flaxseed | 0.0 | 0.0 | 0% | 0.0 | 0.0 | 0% | 5.0 | 46.4 | 9% |
| Oil, soybean lecithin | 2.5 | 19.1 | 4% | 2.5 | 19.1 | 4% | 2.5 | 19.1 | 4% |
| Cocoa, powder, unsweetened | 0.0 | 0.0 | 0% | 10.0 | 22.8 | 4% | 10.0 | 22.8 | 4% |
| Vitamin mineral mix | 3.8 | 0.0 | 0% | 3.8 | 0.0 | 0% | 3.8 | 0.0 | 0% |
| Total | 100.0 | 518.6 | 100% | 100.0 | 525.5 | 100% | 100.0 | 527.7 | 100% |
| Lipid | 30.8 | 270.3 | 52% | 38.2 | 335.7 | 64% | 38.2 | 336.5 | 64% |
| Carbohydrate | 50.8 | 203.4 | 39% | 40.6 | 162.4 | 31% | 40.6 | 162.4 | 31% |
| Protein | 11.5 | 45.8 | 9% | 13.4 | 53.6 | 10% | 13.4 | 53.6 | 10% |
| Fatty Acid 1 | A-20 | SB | SB + F |
|---|---|---|---|
| 16:0 | 2.7 | 1.2 | 1.3 |
| 16:1 | 0.0 | 0.1 | 0.1 |
| 9c-16:1 | 0.0 | 0.4 | 0.3 |
| 18:0 | 1.1 | 2.5 | 2.9 |
| 18:1 Total | 5.5 | 13.3 | 12.1 |
| Undefined 18:2 | 12.9 | 4.7 | 4.6 |
| Undefined 18:3 | 2.0 | 5.0 | 6.6 |
| 20:0 | 0.1 | 0.4 | 0.4 |
| 20:1 Total | 0.0 | 2.3 | 1.8 |
| Total Saturated fat | 3.9 | 4.1 | 4.6 |
| Total Monounsaturated fat | 5.6 | 16.0 | 14.2 |
| Total Polyunsaturated fat | 14.9 | 9.8 | 11.2 |
| ω-3/ω-6 ratio | 0.2 | 1.1 | 1.4 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sloffer, E.M.; Gaur, S.; Engeseth, N.J.; Andrade, J.E. Development and Physico-Chemical Characterization of a Shea Butter-Containing Lipid Nutrition Supplement for Sub-Saharan Africa. Foods 2017, 6, 97. https://doi.org/10.3390/foods6110097
Sloffer EM, Gaur S, Engeseth NJ, Andrade JE. Development and Physico-Chemical Characterization of a Shea Butter-Containing Lipid Nutrition Supplement for Sub-Saharan Africa. Foods. 2017; 6(11):97. https://doi.org/10.3390/foods6110097
Chicago/Turabian StyleSloffer, Elizabeth M., Shashank Gaur, Nicki J. Engeseth, and Juan E. Andrade. 2017. "Development and Physico-Chemical Characterization of a Shea Butter-Containing Lipid Nutrition Supplement for Sub-Saharan Africa" Foods 6, no. 11: 97. https://doi.org/10.3390/foods6110097





