Listeria monocytogenes in Fresh Produce: Outbreaks, Prevalence and Contamination Levels
Abstract
:1. Introduction
2. Foodborne Listeriosis
3. Fresh Produce—Associated Listeriosis Outbreaks
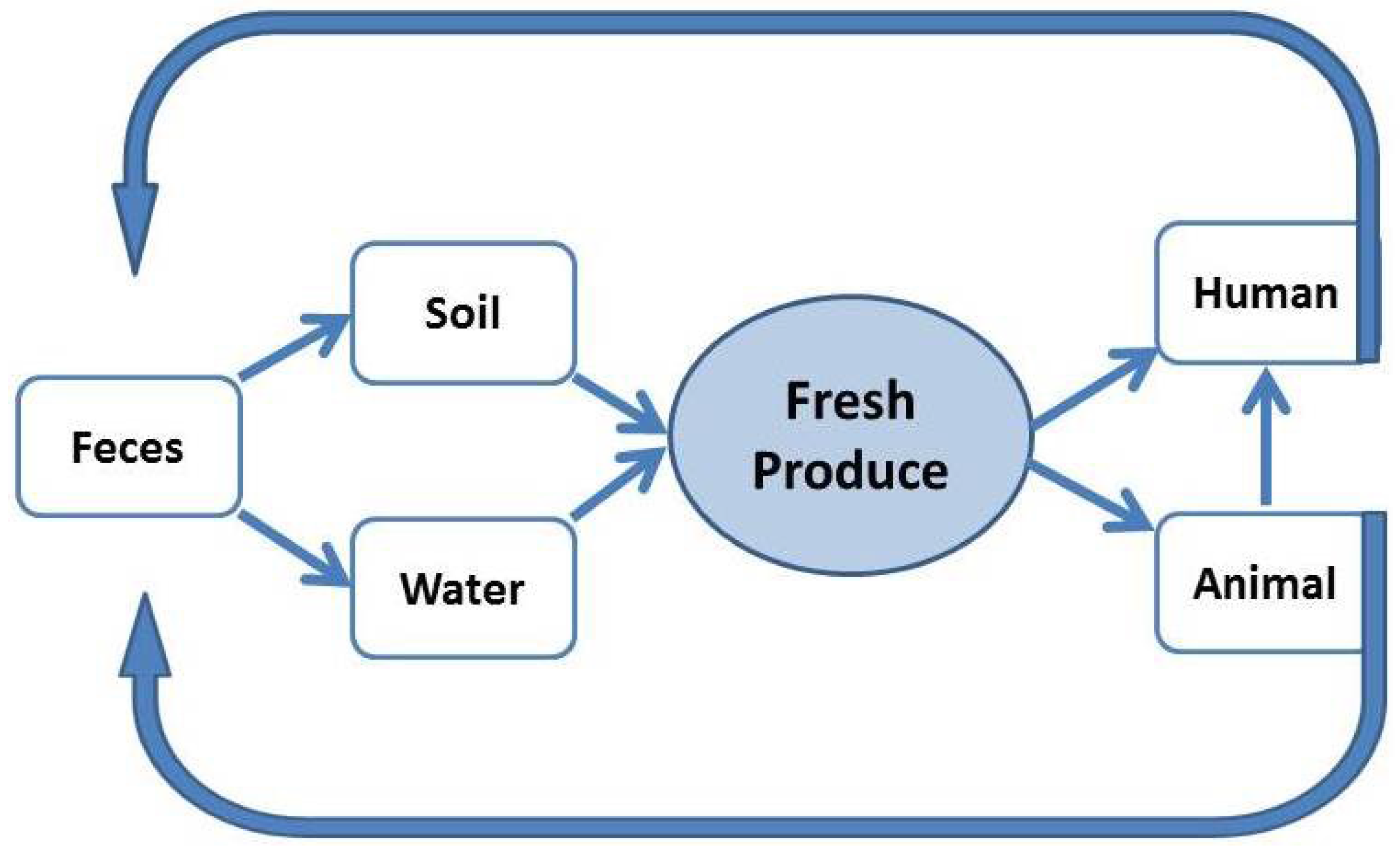
4. Prevalence and Survival of L. monocytogenes in Produce Growing Environments
5. L. monocytogenes Contamination Level of Fresh Produce
6. Prevention of Biofilm Formation to Reduce the Level of Contamination
7. Conclusions
Acknowledgments
Conflicts of Interest
References
- Jeyaletchumi, P.; Tunung, R.; Selina, P.M.; Chai, L.C.; Radu, S.; Farinazleen, M.G.; Cheah, Y.K.; Mitsuaki, N.; Yoshitsugu, N.; Kumar, M.P. Assessment of Listeria monocytogenes in salad vegetables through kitchen simulation study. J. Trop. Agric. Food Sci. 2012, 40, 55–62. [Google Scholar]
- Azizoglu, R.A.; Gorski, L.; Kathariou, S. Listeria and produce: A troublesome liaison! Available online: http://www.newfoodmagazine.com/advent-calendar/listeria-and-produce/ (accessed on 10 February 2017).
- Swaminathan, B.; Gerner-Smidt, P. The epidemiology of human listeriosis. Microb. Infect. 2007, 9, 1236–1243. [Google Scholar] [CrossRef] [PubMed]
- Okutani, A.; Okada, Y.; Yamamoto, S.; Igimi, S. Nationwide survey of human Listeria monocytogenes infection in Japan. Epidemiol. Infect. 2004, 132, 769–772. [Google Scholar] [CrossRef] [PubMed]
- CDC. Multistate Outbreak of Listeriosis Linked to Commercially Produced, Prepackaged Caramel Apples. 2015. Available online: http://www.cdc.gov/listeria/outbreaks/caramel-apples-12–14/index.html (accessed on 15 October 2015). [Google Scholar]
- Gillespie, I.A.; Mook, P.; Little, C.L.; Grant, K.; Adak, G.K. Listeria monocytogenes Infection in the Over-60s in England Between 2005 and 2008: A Retrospective Case–Control Study Utilizing Market Research Panel Data. Foodborne Pathog. Dis. 2010, 7, 1373–1379. [Google Scholar] [CrossRef] [PubMed]
- Buzby, J.C. Children and Microbial Foodborne Illness. Food Rev. 2001, 24, 32. [Google Scholar]
- Smith, B.; Kemp, M.; Ethelberg, S.; Schiellerup, P.; Bruun, B.G.; Gerner-Smidt, P.; Christensen, J.J. Listeria monocytogenes: Maternal-foetal infections in Denmark 1994–2005. Scand. J. Infect. Dis. 2009, 41, 21–25. [Google Scholar] [CrossRef] [PubMed]
- Dilber, E.; Aksoy, A.; Çakir, M.; Bahat, E.; Kamaşak, T.; Dilber, B. Listeria monocytogenes meningitis in two immunocompetent children. Ann. Trop. Paediatr. 2009, 29, 225–229. [Google Scholar] [CrossRef] [PubMed]
- FDA. Food Safety for Pregnant Women. 2011. Available online: http://www.fda.gov/food/foodborneillnesscontaminants/peopleatrisk/ucm312704.htm (accessed on 27 November 2015). [Google Scholar]
- Scallan, E.; Hoekstra, R.M.; Angulo, F.J.; Tauxe, R.V.; Widdowson, M.A.; Roy, S.L.; Jones, J.L.; Griffin, P.M. Foodborne illness acquired in the United States—Major pathogens. Emerg. Infect. Dis. 2011, 17, 7–15. [Google Scholar] [CrossRef] [PubMed]
- Ponniah, J.; Robin, T.; Paie, M.S.; Radu, S.; Ghazali, F.M.; Kqueen, C.Y.; Nishibuchi, M.; Nakaguchi, Y.; Malakar, P.K. Listeria monocytogenes in raw salad vegetables sold at retail level in Malaysia. Food Cont. 2010, 21, 774–778. [Google Scholar] [CrossRef]
- Aureli, P.; Fiorucci, G.C.; Caroli, D.; Marchiaro, G.; Novara, O.; Leone, L.; Salmaso, S. An Outbreak of Febrile Gastroenteritis Associated with Corn Contaminated by Listeria monocytogenes. N. Engl. J. Med. 2000, 342, 1236–1241. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Cruz, S.; Acedo-Félix, E.; Díaz-Cinco, M.; Islas-Osuna, M.A.; González-Aguilar, G.A. Efficacy of sanitizers in reducing Escherichia coli O157:H7, Salmonella spp. and Listeria monocytogenes populations on fresh-cut carrots. Food Cont. 2007, 18, 1383–1390. [Google Scholar] [CrossRef]
- Sy, K.V.; Murray, M.B.; Harrison, M.D.; Beuchat, L.R. Evaluation of Gaseous Chlorine Dioxide as a Sanitizer for Killing Salmonella, Escherichia coli O157:H7, Listeria monocytogenes, and Yeasts and Molds on Fresh and Fresh-Cut Produce. J. Food Prot. 2005, 68, 1176–1187. [Google Scholar] [CrossRef] [PubMed]
- Kamat, A.; Ghadge, N.; Ramamurthy, M.; Alur, M. Effect of low-dose irradiation on shelf life and microbiological safety of sliced carrot. J. Sci. Food Agric. 2005, 85, 2213–2219. [Google Scholar] [CrossRef]
- Thunberg, R.L.; Tran, T.T.; Bennett, R.W.; Matthews, R.N.; Belay, N. Microbial Evaluation of Selected Fresh Produce Obtained at Retail Markets. J. Food Prot. 2002, 65, 677–682. [Google Scholar] [CrossRef] [PubMed]
- Ding, T.; Iwahori, J.; Kasuga, F.; Wang, J.; Forghani, F.; Park, M.-S.; Oh, D.-H. Risk assessment for Listeria monocytogenes on lettuce from farm to table in Korea. Food Control 2013, 30, 190–199. [Google Scholar] [CrossRef]
- Althaus, D.; Hofer, E.; Corti, S.; Julmi, A.; Stephan, R. Bacteriological Survey of Ready-to-Eat Lettuce, Fresh-Cut Fruit, and Sprouts Collected from the Swiss Market. J. Food Prot. 2012, 75, 1338–1341. [Google Scholar] [CrossRef] [PubMed]
- Francis, G.A.; O’Beirne, D. Isolation and Pulsed-Field Gel Electrophoresis Typing of Listeria monocytogenes from Modified Atmosphere Packaged Fresh-Cut Vegetables Collected in Ireland. J. Food Prot. 2006, 69, 2524–2528. [Google Scholar] [CrossRef] [PubMed]
- Abadias, M.; Usall, J.; Anguera, M.; Solsona, C.; Viñas, I. Microbiological quality of fresh, minimally-processed fruit and vegetables, and sprouts from retail establishments. Int. J. Food Microbiol. 2008, 123, 121–129. [Google Scholar] [CrossRef] [PubMed]
- Soriano, J.M.; Rico, H.; Moltó, J.C.; Mañes, J. Listeria Species in Raw and Ready-to-Eat Foods from Restaurants. J. Food Prot. 2001, 64, 551–553. [Google Scholar] [CrossRef] [PubMed]
- Johannessen, G.S.; Loncarevic, S.; Kruse, H. Bacteriological analysis of fresh produce in Norway. Int. J. Food Microbiol. 2002, 77, 199–204. [Google Scholar] [CrossRef]
- Meldrum, R.J.; Little, C.L.; Sagoo, S.; Mithani, V.; McLauchlin, J.; de Pinna, E. Assessment of the microbiological safety of salad vegetables and sauces from kebab take-away restaurants in the United Kingdom. Food Microbiol. 2009, 26, 573–577. [Google Scholar] [CrossRef] [PubMed]
- Jamali, H.; Paydar, M.; Chung, C.Y.; Wong, W.F. Prevalence of Listeria species and Listeria monocytogenes serotypes in ready mayonnaise salads and salad vegetables in Iran. Afr. J. Microbiol. Res. 2013, 7, 1903–1906. [Google Scholar]
- Gómez-Govea, M.; Solís-Soto, L.; Heredia, N.; García, S.; Moreno, G.; Tovar, O.; Isunza, G. Analysis of microbial contamination levels of fruits and vegetables at retail in Monterrey, Mexico. J. Food Agric. Environ. 2012, 10, 152–156. [Google Scholar]
- Öktem, A.B.; Bayram, G.; Ceylan, A.E.; Yentür, G. Prevalence of Listeria monocytogenes in Some Turkish Foodstuffs. J. Food Qual. 2006, 29, 76–86. [Google Scholar] [CrossRef]
- Easa, S.M.H. Microorganisms found in fast and traditional fast food. J. Am. Sci. 2010, 6, 515–537. [Google Scholar]
- Skalina, L.; Nikolajeva, V. Growth potential of Listeria monocytogenes strains in mixed ready-to-eat salads. Int. J. Food Microbiol. 2010, 144, 317–321. [Google Scholar] [CrossRef] [PubMed]
- Gaul, L.K.; Farag, N.H.; Shim, T.; Kingsley, M.A.; Silk, B.J.; Hyytia-Trees, E. Hospital-Acquired Listeriosis Outbreak Caused by Contaminated Diced Celery—Texas, 2010. Clin. Infect. Dis. 2013, 56, 20–26. [Google Scholar] [CrossRef] [PubMed]
- CDC. Multistate Outbreak of Listeriosis Linked to Whole Cantaloupes from Jensen Farms, Colorado. 2011. Available online: http://www.cdc.gov/listeria/outbreaks/cantaloupes-jensen-farms/ (accessed on 15 January 2016). [Google Scholar]
- Liu, D. Handbook of Listeria monocytogenes; CRC Press: Boca Raton, FL, USA, 2008. [Google Scholar]
- Todd, E.C.D.; Notermans, S. Surveillance of listeriosis and its causative pathogen, Listeria monocytogenes. Food Cont. 2011, 22, 1484–1490. [Google Scholar] [CrossRef]
- Wing, E.J.; Gregory, S.H. Listeria monocytogenes: Clinical and Experimental Update. J. Infect. Dis. 2002, 185, S18–S24. [Google Scholar] [CrossRef] [PubMed]
- Aoshi, T.; Carrero, J.A.; Konjufca, V.; Koide, Y.; Unanue, E.R.; Miller, M.J. The cellular niche of Listeria monocytogenes infection changes rapidly in the spleen. Eur. J. Immunol. 2009, 39, 417–425. [Google Scholar] [CrossRef] [PubMed]
- Cone, L.A.; Leung, M.M.; Byrd, R.G.; Annunziata, G.M.; Lam, R.Y.; Herman, B.K. Multiple cerebral abscesses because of Listeria monocytogenes: Three case reports and a literature review of supratentorial listerial brain abscess(es). Surg. Neurol. 2003, 59, 320–328. [Google Scholar] [CrossRef]
- Bhat, S.A.; Willayat, M.M.; Roy, S.S.; Bhat, M.A.; Shah, S.N.; Ahmed, A.; Maqbool, S.; Ganayi, B.A. Isolation, molecular detection and antibiogram of Listeria monocytogenes from human clinical cases and fish of Kashmir, India. Comp. Clin. Pathol. 2012, 22, 661–665. [Google Scholar] [CrossRef]
- Salazar, J.K.; Wu, Z.; Yang, W.; Freitag, N.E.; Tortorello, M.L.; Wang, H.; Zhang, W. Roles of a Novel Crp/Fnr Family Transcription Factor Lmo0753 in Soil Survival, Biofilm Production and Surface Attachment to Fresh Produce of Listeria monocytogenes. PLoS ONE 2013, 8, e75736. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Trallero, E.; Zigorraga, C.; Artieda, J.; Alkorta, M.; Marimón, J.M. Two Outbreaks of Listeria monocytogenes Infection, Northern Spain. Emerg. Infect. Dis. 2014, 20, 2155–2157. [Google Scholar] [CrossRef] [PubMed]
- Khoury, N.T.; Hossain, M.M.; Wootton, S.H.; Salazar, L.; Hasbun, R. Meningitis With a Negative Cerebrospinal Fluid Gram Stain in Adults: Risk Classification for an Adverse Clinical Outcome. Mayo Clin. Proc. 2012, 87, 1181–1188. [Google Scholar] [CrossRef] [PubMed]
- Brouwer, M.C.; van de Beek, D.; Heckenberg, S.G.B.; Spanjaard, L.; de Gans, J. Community-Acquired Listeria monocytogenes Meningitis in Adults. Clin. Infect. Dis. 2006, 43, 1233–1238. [Google Scholar] [CrossRef] [PubMed]
- Okike, I.O.; Lamont, R.F.; Heath, P.T. Do We Really Need to Worry About Listeria in Newborn Infants? Pediatr. Infect. Dis. J. 2013, 32, 405–406. [Google Scholar] [CrossRef] [PubMed]
- Gaschignard, J.; Levy, C.; Romain, O.; Cohen, R.; Bingen, E.; Aujard, Y.; Boileau, P. Neonatal Bacterial Meningitis: 444 Cases in 7 Years. Pediatr. Infect. Dis. J. 2011, 30, 212–217. [Google Scholar] [CrossRef] [PubMed]
- Camacho-Gonzalez, A.; Spearman, P.W.; Stoll, B.J. Neonatal Infectious Diseases: Evaluation of Neonatal Sepsis. Pediatr. Clin. North Am. 2013, 60, 367–389. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.-M.; Takeuchi, K.; Zhang, L.; Dohm, C.B.; Meyer, J.D.; Hall, P.A.; Doyle, M.P. Cross-Contamination between Processing Equipment and Deli Meats by Listeria monocytogenes. J. Food Prot. 2006, 69, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Gottlieb, S.L.; Newbern, E.C.; Griffin, P.M.; Graves, L.M.; Hoekstra, R.M.; Baker, N.L.; Hunter, S.B.; Holt, K.G.; Ramsey, F.; Head, M.; et al. Multistate Outbreak of Listeriosis Linked to Turkey Deli Meat and Subsequent Changes in US Regulatory Policy. Clin. Infect. Dis. 2006, 42, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Proctor, M.E.; Brosch, R.; Mellen, J.W.; Garrett, L.A.; Kaspar, C.W.; Luchansky, J.B. Use of pulsed-field gel electrophoresis to link sporadic cases of invasive listeriosis with recalled chocolate milk. Appl. Environ. Microbiol. 1995, 61, 3177–3179. [Google Scholar] [PubMed]
- CDC. Multistate Outbreak of Listeriosis Linked to Roos Foods Dairy Products. Available online: http://www.cdc.gov/listeria/outbreaks/cheese-02–14/index.html (accessed on 4 February 2015).
- CDC. Listeriosis Infections Linked to Marte Brand Frescolina Ricotta Salata Cheese. Available online: http://www.cdc.gov/listeria/outbreaks/cheese-09–12/index.html (accessed on 4 February 2015).
- CDC. Multistate Outbreak of Listeriosis Linked to Packaged Salads Produced at Springfield, Ohio Dole Processing Facility (Final Update). Available online: http://www.cdc.gov/listeria/outbreaks/bagged-salads-01–16/ (accessed on 15 August 2016).
- Tham, W.; Ericsson, H.; Loncarevic, S.; Unnerstad, H.; Danielsson-Tham, M.-L. Lessons from an outbreak of listeriosis related to vacuum-packed gravad and cold-smoked fish. Int. J. Food Microbiol. 2000, 62, 173–175. [Google Scholar] [CrossRef]
- Rørvik, L.M. Listeria monocytogenes in the smoked salmon industry. Int. J. Food Microbiol. 2000, 62, 183–190. [Google Scholar] [CrossRef]
- Mukherjee, A.; Speh, D.; Jones, A.T.; Buesing, K.M.; Diez-Gonzalez, F. Longitudinal microbiological survey of fresh produce grown by farmers in the upper Midwest. J. Food Prot. 2006, 69, 1928–1936. [Google Scholar] [CrossRef] [PubMed]
- CDC. Listeria outbreaks. Available online: https://www.cdc.gov/listeria/outbreaks/ (accessed on 25 September 2016).
- Shrivastava, S. Listeria Outbreak—Bacteria Found in Romaine Lettuce: FDA. Available online: http://www.ibtimes.com/listeria-outbreak-bacteria-found-romaine-lettuce-fda-320544 (accessed on 14 March 2016).
- Ho, J.L.; Shands, K.N.; Friedland, G.; Eckind, P.; Fraser, D.W. An outbreak of type 4b Listeria monocytogenes infection involving patients from eight boston hospitals. Arch. Intern. Med. 1986, 146, 520–524. [Google Scholar] [CrossRef] [PubMed]
- Schlech, W.F.; Lavigne, P.M.; Bortolussi, R.A.; Allen, A.C.; Haldane, E.V.; Wort, A.J.; Hightower, A.W.; Johnson, S.E.; King, S.H.; Nicholls, E.S.; et al. Epidemic Listeriosis — Evidence for Transmission by Food. N. Engl. J. Med. 1983, 308, 203–206. [Google Scholar] [CrossRef] [PubMed]
- Garner, D.; Kathariou, S. Fresh produce-associated listeriosis outbreaks, sources of concern, teachable moments, and insights. J. Food. Prot. 2016, 79, 337–344. [Google Scholar] [CrossRef] [PubMed]
- Vela, A.I.; Fernandez-Garayzabal, J.F.; Vazquez, J.A.; Latre, M.V.; Blanco, M.M.; Moreno, M.A.; de la Fuente, L.; Marco, J.; Franco, C.; Cepeda, A.; et al. Molecular Typing by Pulsed-Field Gel Electrophoresis of Spanish Animal and Human Listeria monocytogenes Isolates. Appl. Environ. Microbiol. 2001, 67, 5840–5843. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, V.; Nam, H.M.; Nguyen, L.T.; Tamilselvam, B.; Murinda, S.E.; Oliver, S.P. Prevalence of Antimicrobial Resistance Genes in Listeria monocytogenes Isolated from Dairy Farms. Foodborne Pathog. Dis. 2005, 2, 201–211. [Google Scholar] [CrossRef] [PubMed]
- Olier, M.; Pierre, F.; Lemaître, J.-P.; Divies, C.; Rousset, A.; Guzzo, J. Assessment of the pathogenic potential of two Listeria monocytogenes human faecal carriage isolates. Microbiology 2002, 148, 1855–1862. [Google Scholar] [CrossRef] [PubMed]
- Nightingale, K.K.; Schukken, Y.H.; Nightingale, C.R.; Fortes, E.D.; Ho, A.J.; Her, Z.; Grohn, Y.T.; McDonough, P.L.; Wiedmann, M. Ecology and Transmission of Listeria monocytogenes Infecting Ruminants and in the Farm Environment. Appl. Environ. Microbiol. 2004, 70, 4458–4467. [Google Scholar] [CrossRef] [PubMed]
- Esteban, J.I.; Oporto, B.; Aduriz, G.; Juste, R.A.; Hurtado, A. Faecal shedding and strain diversity of Listeria monocytogenes in healthy ruminants and swine in Northern Spain. BMC Vet. Res. 2009, 5, 2. [Google Scholar] [CrossRef] [PubMed]
- Food Safety Authority of Ireland. The Control and Management of Listeria monocytogenes Contamination of Food; Food Safety Authority of Ireland: Dublin, Ireland, 2005. [Google Scholar]
- Freitag, N.E.; Port, G.C.; Miner, M.D. Listeria monocytogenes—From saprophyte to intracellular pathogen. Nat. Rev. Microbiol. 2009, 7, 623–628. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, M.; Viñas, I.; Colàs, P.; Anguera, M.; Usall, J.; Abadias, M. Effectiveness of a bacteriophage in reducing Listeria monocytogenes on fresh-cut fruits and fruit juices. Food Microbiol. 2014, 38, 137–142. [Google Scholar] [CrossRef] [PubMed]
- Vandamm, J.P.; Li, D.; Harris, L.J.; Schaffner, D.W.; Danyluk, M.D. Fate of Escherichia coli O157:H7, Listeria monocytogenes, and Salmonella on fresh-cut celery. Food Microbiol. 2013, 34, 151–157. [Google Scholar] [CrossRef] [PubMed]
- Junttila, J.R.; Niemelä, S.I.; Hirn, J. Minimum growth temperature of Listeria monocytogenes and non-haemolytic listeria. J. Appl. Bacteriol. 1988, 65, 321–327. [Google Scholar] [CrossRef] [PubMed]
- Te Giffel, M.C.; Zwietering, M.H. Validation of predictive models describing the growth of Listeria monocytogenes. Int. J. Food Microbiol. 1999, 46, 135–149. [Google Scholar] [CrossRef]
- Strawn, L.K.; Fortes, E.D.; Bihn, E.A.; Nightingale, K.K.; Gröhn, Y.T.; Worobo, R.W.; Wiedmann, M.; Bergholz, P.W. Landscape and Meteorological Factors Affecting Prevalence of Three Food-Borne Pathogens in Fruit and Vegetable Farms. Appl. Environ. Microbiol. 2013, 79, 588–600. [Google Scholar] [CrossRef] [PubMed]
- Strawn, L.K.; Gröhn, Y.T.; Warchocki, S.; Worobo, R.W.; Bihn, E.A.; Wiedmann, M. Risk Factors Associated with Salmonella and Listeria monocytogenes Contamination of Produce Fields. Appl. Environ. Microbiol. 2013, 79, 7618–7627. [Google Scholar] [CrossRef] [PubMed]
- Szymczak, B.; Szymczak, M.; Sawicki, W.; Dabrowski, W. Anthropogenic impact on the presence of L. monocytogenes in soil, fruits, and vegetables. Folia Microbiol Praha 2014, 59, 23–29. [Google Scholar] [CrossRef] [PubMed]
- McLaughlin, H.P.; Casey, P.G.; Cotter, J.; Gahan, C.G.M.; Hill, C. Factors affecting survival of Listeria monocytogenes and Listeria innocua in soil samples. Arch. Microbiol. 2011, 193, 775–785. [Google Scholar] [CrossRef] [PubMed]
- Locatelli, A.; Spor, A.; Jolivet, C.; Piveteau, P.; Hartmann, A. Biotic and Abiotic Soil Properties Influence Survival of Listeria monocytogenes in Soil. PLoS ONE 2013, 8, e75969. [Google Scholar] [CrossRef] [PubMed]
- Nastou, A.; Rhoades, J.; Smirniotis, P.; Makri, I.; Kontominas, M.; Likotrafiti, E. Efficacy of household washing treatments for the control of Listeria monocytogenes on salad vegetables. Int. J. Food Microbiol. 2012, 159, 247–253. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; W, Q.; Zhang, J.; Chen, M.; Yan, Z.; Hu, H. Listeria monocytogenes prevalence and characteristics in retails raw foods in China. PLoS ONE 2015, 10, e0136682. [Google Scholar] [CrossRef] [PubMed]
- Aparecida, O.M.; Abeid Ribeiro, E.G.; Morato Bergamini, A.M.; Pereira De Martinis, E.C. Quantification of Listeria monocytogenes in minimally processed leafy vegetables using a combined method based on enrichment and 16S rRNA real-time PCR. Food Microbiol. 2010, 27, 19–23. [Google Scholar] [CrossRef] [PubMed]
- Sant’Ana, A.S.; Igarashi, M.C.; Landgraf, M.; Destro, M.T.; Franco, B.D.G.M. Prevalence, populations and pheno- and genotypic characteristics of Listeria monocytogenes isolated from ready-to-eat vegetables marketed in São Paulo, Brazil. Int. J. Food Microbiol. 2012, 155, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Uzeh, R.; Adepoju, A. Incidence and survival of Escherichia coli O157: H7 and Listeria monocytogenes on salad vegetables. Int. Food Res. J. 2013, 20, 1921–1925. [Google Scholar]
- Zhu, Q.; Hussain, M.A. Prevalence of Listeria species in fresh salad vegetables and ready-to-eat foods containing fresh produce marketed in canterbury, New Zealand. Adv. Food Technol. Nutr. Sci. Open J. 2014, 1, 5–9. [Google Scholar] [CrossRef]
- Seo, Y.-H.; Jang, J.-H.; Moon, K.-D. Microbial evaluation of minimally processed vegetables and sprouts produced in Seoul, Korea. Food Sci. Biotechnol. 2010, 19, 1283–1288. [Google Scholar] [CrossRef]
- de Oliveira, M.M.M.; Brugnera, D.F.; Alves, E.; Piccoli, R.H. Biofilm formation by Listeria monocytogenes on stainless steel surface and biotransfer potential. Braz. J. Microbiol. 2010, 41, 97–106. [Google Scholar] [CrossRef] [PubMed]
- Bonsaglia, E.C.R.; Silva, N.C.C.; Fernades Júnior, A.; Araújo Júnior, J.P.; Tsunemi, M.H.; Rall, V.L.M. Production of biofilm by Listeria monocytogenes in different materials and temperatures. Food Cont. 2014, 35, 386–391. [Google Scholar] [CrossRef]
- Botticella, G.; Russo, R.; Capozzi, V.; Amado, M.L.; Massa, S.; Spano, G.; Beneduce, L. Listeria monocytogenes, biofilm formation and fresh cut produce. 2013. Available online: http://www.formatex.info/microbiology4/vol1/114-123.pdf (accessed on 5 February 2017).
- Srey, S.; Park, S.Y.; Jahid, I.K.; Ha, S.-D. Reduction effect of the selected chemical and physical treatments to reduce L. monocytogenes biofilms formed on lettuce and cabbage. Food Res. Int. 2014, 62, 484–491. [Google Scholar] [CrossRef]
- Bae, Y.-M.; Choi, N.-Y.; Heu, S.; Kang, D.-H.; Lee, S.-Y. Inhibitory effects of organic acids combined with modified atmosphere packaging on foodborne pathogens on cabbage. J. Korean Soc. Appl. Biol. Chem. 2011, 54, 993–997. [Google Scholar] [CrossRef]
| Outbreak Location/Year | Deaths/Cases (% Mortality) | Food Vehicle | References |
|---|---|---|---|
| Boston, USA, 1979 | 3/20 (15) | Raw vegetables | Ho et al. [56] |
| Nova Scotia, Canada, 1981 | 17/41 (41) | Vegetable mix for coleslaw | Schlech et al. [57] |
| Moncalieri and Giaveno, Italy, 1997 | 0/2930 (0) | Corn | Aureli et al. [13] |
| Texas, USA, 2010 | 5/10 (50) | Chopped celery | Gaul et al. [30] |
| Colorado, USA, 2011 | 33/147 (22) | Whole cantaloupes | CDC [54] |
| Colorado, USA, 2011 | 15/99 (15) | Lettuce | Shrivastava et al. [55] |
| Illinois and Michigan, USA, 2014 | 2/5 (40) | Mung bean sprouts | Garner and Kathariou [58] |
| California, USA, 2014 | 1/32 (3) | Caramel apples | CDC [5] |
| Ohio, USA, 2016 | 1/19 (5) | Packaged salads | CDC [50] |
| Country | Environment (Total Number of Samples) | Frequency a Number of Positive Samples (%) | References |
|---|---|---|---|
| USA | Soil (178) | 16 (9%) | Strawn et al. [70] |
| Drag swab (175) | 15 (9%) | ||
| Fecal (61) | 9 (15%) | ||
| Water (174) | 48 (28%) | ||
| Engineered (28) | 0 (0%) | ||
| Surface (146) | 48 (33%) | ||
| USA | Field | 263 (17.5%) | Strawn et al. [71] |
| Water | 74 (30%) | ||
| Poland | Soil (1000) | 55 (5.5%) | Szymczak et al. [72] |
| Ireland | Soil | McLaughlin et al. [73] | |
| French | soil | Locatelli et al. [74] |
| Produce | Country | Prevalence a | References |
|---|---|---|---|
| Vegetables | China | 140 (8, 5.7%) | Wu et al. [76] |
| Parsley | Poland | 30 (3, 10.0%) | Szymczak et al. [72] |
| Malaysia | 16 (4, 25.0%) | Ponniah et al. [11] | |
| Brazil | 22 (1, 4.5%) | Aparecida de Oliveira et al. [77] | |
| Greece | Nastou et al. [75] | ||
| Collard greens | Brazil | 30 (1, 3.3%) | Aparecida de Oliveira et al. [77] |
| Brazil | 24 (1, 4.2%) | Sant’Ana et al. [78] | |
| Lettuce | Korea | Ding et al. [18] | |
| Brazil | 152 (3, 2.0%) | Sant’Ana et al. [78] | |
| Nigeria | Uzeh et al. [79] | ||
| Greece | Nastou et al. [75] | ||
| Cabbage | Malaysia | 32 (7, 21.9%) | Ponniah et al. [11] |
| Brazil | 11 (2, 18.2%) | Sant’Ana et al. [78] | |
| Nigeria New Zealand | Uzeh et al. [79] Zhu et al. [80] | ||
| Spinach | Brazil | 11 (1, 9.1%) | Sant’Ana et al. [78] |
| Carrot | Malaysia | 33 (8, 24.2%) | Ponniah et al. [11] |
| Tomato | Malaysia | 32 (7, 21.9%) | Ponniah et al. [11] |
| Cucumber | Malaysia | 32 (7, 21.9%) | Ponniah et al. [11] |
| Greece | Nastou et al. [75] | ||
| Sprouts | Korean | 112 (1, 0.9%) | Seo et al. [81] |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, Q.; Gooneratne, R.; Hussain, M.A. Listeria monocytogenes in Fresh Produce: Outbreaks, Prevalence and Contamination Levels. Foods 2017, 6, 21. https://doi.org/10.3390/foods6030021
Zhu Q, Gooneratne R, Hussain MA. Listeria monocytogenes in Fresh Produce: Outbreaks, Prevalence and Contamination Levels. Foods. 2017; 6(3):21. https://doi.org/10.3390/foods6030021
Chicago/Turabian StyleZhu, Qi, Ravi Gooneratne, and Malik Altaf Hussain. 2017. "Listeria monocytogenes in Fresh Produce: Outbreaks, Prevalence and Contamination Levels" Foods 6, no. 3: 21. https://doi.org/10.3390/foods6030021






