Calabrian Goji vs. Chinese Goji: A Comparative Study on Biological Properties
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Instrumentation
2.3. Scavenging of DPPH Free Radical
2.4. ABTS Radical Scavenging Assay
2.5. Oxygen Radical Absorbance Capacity (ORAC) Assay
2.6. β-Carotene–Linoleic Acid Assay
2.7. Cholinesterase Inhibitory Assay
2.8. Nitrite Oxide Scavenging Activity
2.9. Total Carotenoids Content
2.10. Statistical analysis
3. Results
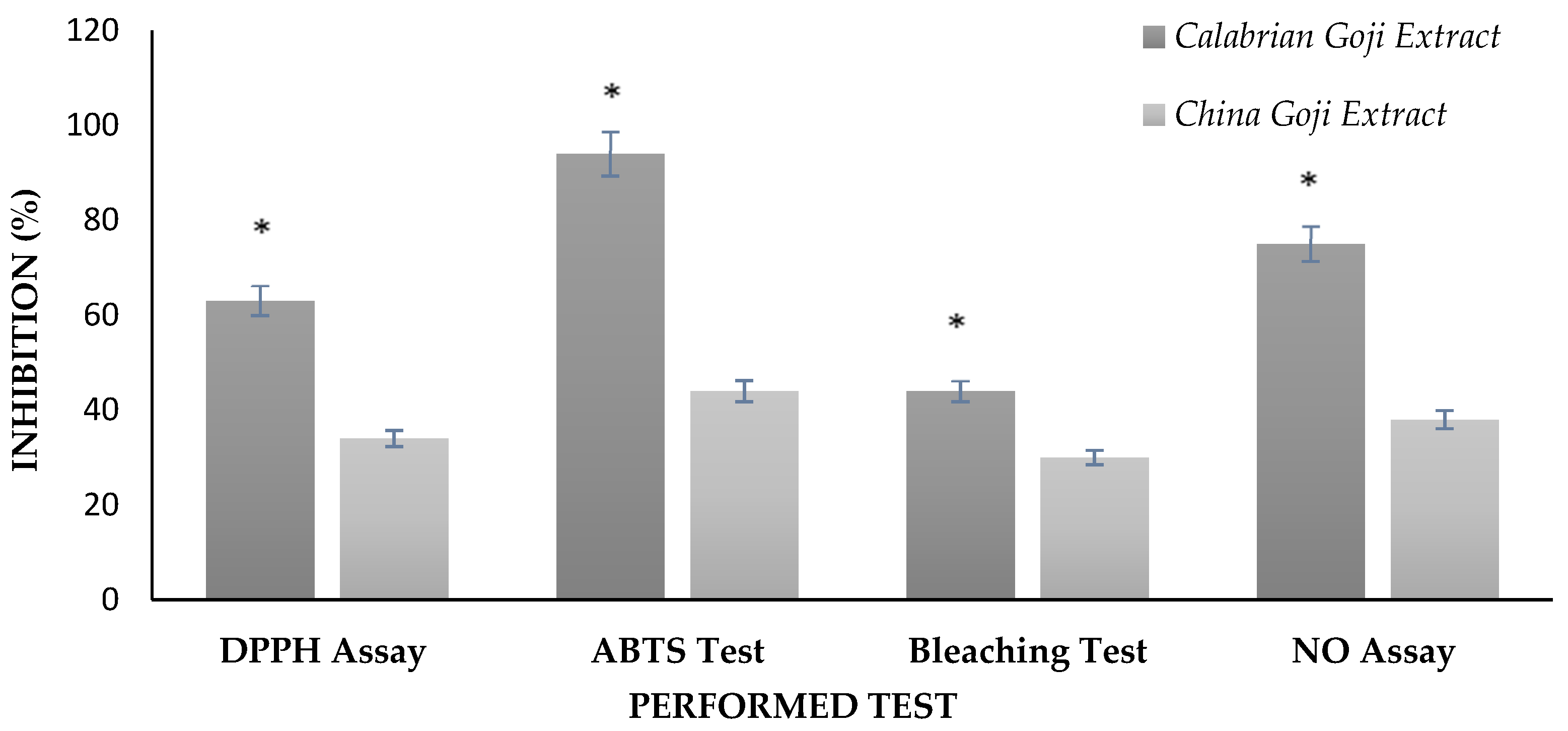
3.1. Scavenging of DPPH and ABTS Free Radicals
3.2. β-Carotene–Linoleic Acid Assay
3.3. Oxygen Radical Absorbance Capacity (ORAC) Assay
3.4. Nitric Oxide Radical Scavenging Assay
3.5. Cholinesterase Inhibitory Assay
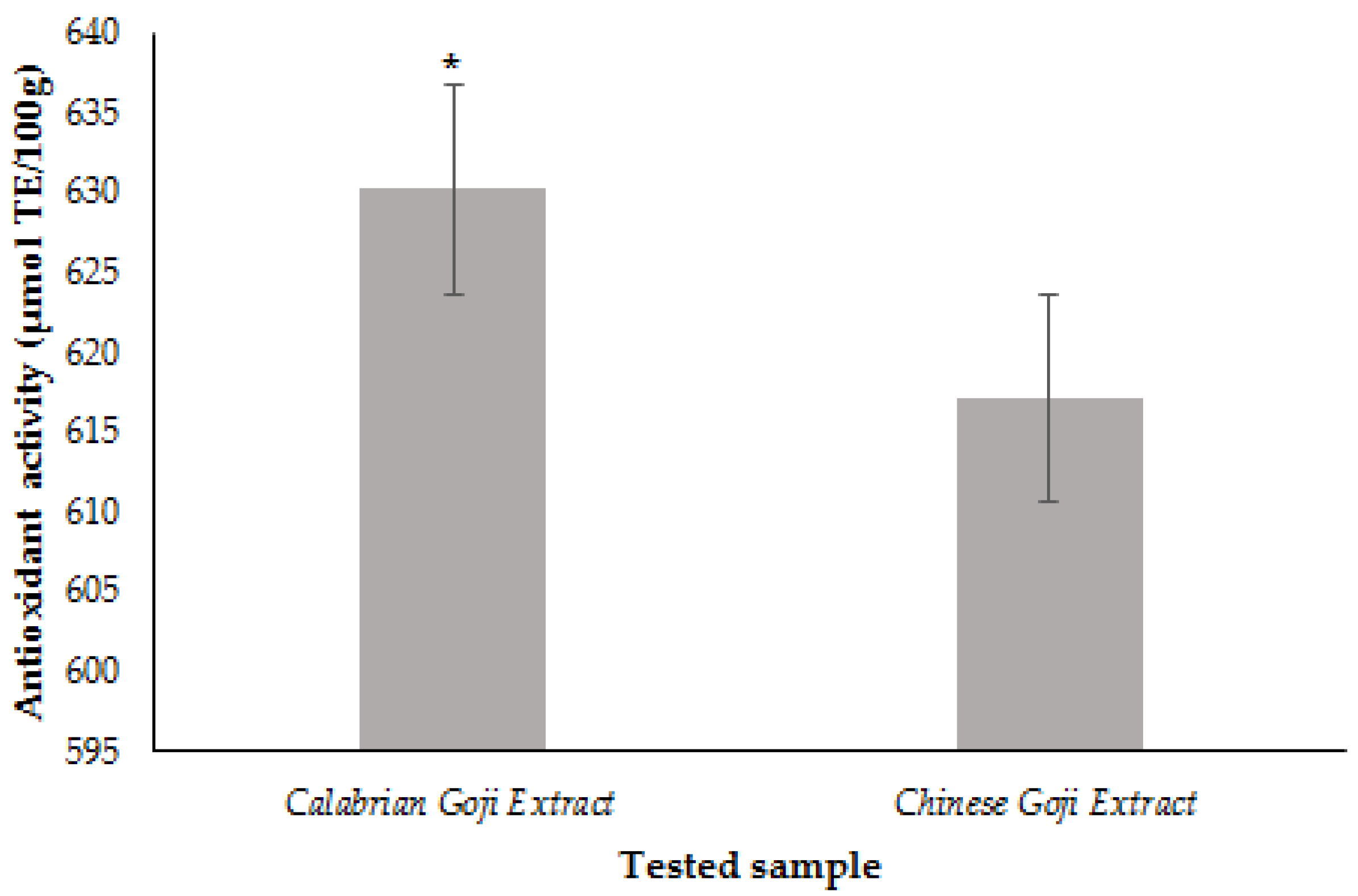
3.6. Total Carotenoids as Zeaxanthin Equivalents
4. Discussion
4.1. Antioxidant Properties
4.2. Cholinesterase Inhibitory Activity
4.3. Nitric Oxide Radical Scavenging Ability
4.4. Carotenoids Quantification in Zeaxanthin Equivalents
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Zhang, Q.; Chen, W.; Zhao, J.; Xi, W. Functional constituents and antioxidant activities of eight Chinese native Goji genotypes. Food Chem. 2016, 200, 230–236. [Google Scholar] [CrossRef] [PubMed]
- Le, K.; Chiu, F.; Ng, K. Identification and quantification of antioxidants in fructus lycii. Food Chem. 2007, 105, 353–363. [Google Scholar] [CrossRef]
- Amagase, H.; Sun, B.; Borek, C. Lycium barbarum (Goji) juice improves in vivo antioxidant biomarkers in serum of healthy adults. Nutr. Res. 2009, 29, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Kulczyński, B.; Gramza-Michałowska, A. Goji berry (Lycium barbarum): Composition and health effects—A review. Pol. J. Food Nutr. Sci. 2016, 66, 67–76. [Google Scholar]
- Feeney, J.; Finucane, C.; Savva, G.M.; Cronin, H.; Beatty, S.; Nolan, J.M.; Kenny, R.A. Low macular pigment optical density is associated with lower cognitive performance in a large, population-based sample of older adults. Neurobiol. Aging 2013, 34, 2449–2456. [Google Scholar] [CrossRef] [PubMed]
- Hempel, J.; Schädle, C.N.; Sprenger, J.; Heller, A.; Carle, R.; Schweiggert, R.M. Ultrastructural deposition forms and bioaccessibility of carotenoids and carotenoid esters from Goji berries (Lycium barbarum L.). Food Chem. 2017, 218, 525–533. [Google Scholar] [CrossRef] [PubMed]
- Johnson, E.J. Role of lutein and zeaxanthin in visual and cognitive function throughout the lifespan. Nutr. Rev. 2014, 72, 605–612. [Google Scholar] [CrossRef] [PubMed]
- Ong, S.Y.; Cheung, C.Y.; Li, X.; Lamoureux, E.L.; Ikram, M.K.; Ding, J.; Cheng, C.Y.; Haaland, B.A.; Saw, S.M.; Venketasubramanian, N. Visual impairment, age-related eye diseases, and cognitive function: The singapore malay eye study. Arch. Ophthalmol. 2012, 130, 895–900. [Google Scholar] [CrossRef] [PubMed]
- Johnson, E.J. A possible role for lutein and zeaxanthin in cognitive function in the elderly. Am. J. Clin. Nutr. 2012, 96, 1161S–1165S. [Google Scholar] [CrossRef] [PubMed]
- Teunissen, C.; van Boxtel, M.; Bosma, H.; Bosmans, E.; Delanghe, J.; de Bruijn, C.; Wauters, A.; Maes, M.; Jolles, J.; Steinbusch, H. Inflammation markers in relation to cognition in a healthy aging population. J. Neuroimmunol. 2003, 134, 142–150. [Google Scholar] [CrossRef]
- Spizzirri, U.; Altimari, I.; Puoci, F.; Parisi, O.; Iemma, F.; Picci, N. Innovative antioxidant thermo-responsive hydrogels by radical grafting of catechin on inulin chain. Carbohydr. Polym. 2011, 84, 517–523. [Google Scholar] [CrossRef]
- Zhang, M.; Wang, F.; Liu, R.; Tang, X.; Zhang, Q.; Zhang, Z. Effects of superfine grinding on physicochemical and antioxidant properties of Lycium barbarum polysaccharides. LWT-Food Sci. Technol. 2014, 58, 594–601. [Google Scholar] [CrossRef]
- Roy, M.K.; Koide, M.; Rao, T.P.; Okubo, T.; Ogasawara, Y.; Juneja, L.R. Orac and dpph assay comparison to assess antioxidant capacity of tea infusions: Relationship between total polyphenol and individual catechin content. Int. J. Food Sci. Nutr. 2010, 61, 109–124. [Google Scholar] [CrossRef] [PubMed]
- Cirillo, G.; Hampel, S.; Klingeler, R.; Puoci, F.; Iemma, F.; Curcio, M.; Parisi, O.I.; Spizzirri, U.G.; Picci, N.; Leonhardt, A. Antioxidant multi-walled carbon nanotubes by free radical grafting of gallic acid: New materials for biomedical applications. J. Pharm. Pharmacol. 2011, 63, 179–188. [Google Scholar] [CrossRef] [PubMed]
- Puoci, F.; Iemma, F.; Spizzirri, U.G.; Restuccia, D.; Pezzi, V.; Sirianni, R.; Manganaro, L.; Curcio, M.; Parisi, O.I.; Cirillo, G. Antioxidant activity of a mediterranean food product:“Fig syrup”. Nutrients 2011, 3, 317–329. [Google Scholar] [CrossRef] [PubMed]
- Parisi, O.I.; Aiello, D.; Casula, M.F.; Puoci, F.; Malivindi, R.; Scrivano, L.; Testa, F. Mesoporous nanocrystalline tio 2 loaded with ferulic acid for sunscreen and photo-protection: Safety and efficacy assessment. RSC Adv. 2016, 6, 83767–83775. [Google Scholar] [CrossRef]
- Menichini, F.; Tundis, R.; Bonesi, M.; Loizzo, M.R.; Conforti, F.; Statti, G.; de Cindio, B.; Houghton, P.J.; Menichini, F. The influence of fruit ripening on the phytochemical content and biological activity of capsicum chinense jacq. Cv habanero. Food Chem. 2009, 114, 553–560. [Google Scholar] [CrossRef]
- Trevithick-Sutton, C.C.; Foote, C.S.; Collins, M.; Trevithick, J.R. The retinal carotenoids zeaxanthin and lutein scavenge superoxide and hydroxyl radicals: A chemiluminescence and esr study. Mol. Vis. 2006, 12, 1127–1135. [Google Scholar] [PubMed]
- Wang, C.; Chang, S.; Inbaraj, B.S.; Chen, B. Isolation of carotenoids, flavonoids and polysaccharides from Lycium barbarum L. And evaluation of antioxidant activity. Food Chem. 2010, 120, 184–192. [Google Scholar] [CrossRef]
- Praticò, D. Alzheimer’s disease and oxygen radicals: New insights. Biochem. Pharmacol. 2002, 63, 563–567. [Google Scholar] [CrossRef]
- Pláteník, J.; Stopka, P.; Vejražka, M.; Štípek, S. Quinolinic acid-iron (II) complexes: Slow autoxidation, but enhanced hydroxyl radical production in the fenton reaction. Free Radic. Res. 2001, 34, 445–459. [Google Scholar] [CrossRef] [PubMed]
- Brunetti, L.; Michelotto, B.; Orlando, G.; Recinella, L.; di Nisio, C.; Ciabattoni, G.; Vacca, M. Aging increases amyloid β-peptide-induced 8-iso-prostaglandin F2α release from rat brain. Neurobiol. Aging 2004, 25, 125–129. [Google Scholar] [CrossRef]
- Brunetti, L.; Orlando, G.; Menghini, L.; Ferrante, C.; Chiavaroli, A.; Vacca, M. Ginkgo biloba leaf extract reverses amyloid β-peptide-induced isoprostane production in rat brain in vitro. Planta Medica 2006, 72, 1296–1299. [Google Scholar] [CrossRef] [PubMed]
- Brunetti, L.; Menghini, L.; Orlando, G.; Recinella, L.; Leone, S.; Epifano, F.; Lazzarin, F.; Chiavaroli, A.; Ferrante, C.; Vacca, M. Antioxidant effects of garlic in young and aged rat brain in vitro. J. Med. Food 2009, 12, 1166–1169. [Google Scholar] [CrossRef] [PubMed]
- Luo, Q.; Cai, Y.; Yan, J.; Sun, M.; Corke, H. Hypoglycemic and hypolipidemic effects and antioxidant activity of fruit extracts from Lycium barbarum. Life Sci. 2004, 76, 137–149. [Google Scholar] [CrossRef] [PubMed]
- Mottay, D.; Neergheen-Bhujun, V.S. Anticholinesterase and antioxidant effects of traditional herbal medicines used in the management of neurodegenerative diseases in mauritius. Arch. Med. Biomed. Res. 2015, 2, 114–130. [Google Scholar] [CrossRef]
- Gao, L.-B.; Yu, X.-F.; Chen, Q.; Zhou, D. Alzheimer’s disease therapeutics: Current and future therapies. Minerva Medica 2016, 107, 108–113. [Google Scholar] [PubMed]
- de Souza, L.G.; Rennó, M.N.; Figueroa-Villar, J.D. Coumarins as cholinesterase inhibitors: A review. Chem.-Biol. Interact. 2016, 254, 11–23. [Google Scholar] [CrossRef] [PubMed]
- Sharma, J.; Al-Omran, A.; Parvathy, S. Role of nitric oxide in inflammatory diseases. Inflammopharmacology 2007, 15, 252–259. [Google Scholar] [CrossRef] [PubMed]
- Saturnino, C.; Sinicropi, M.S.; Parisi, O.I.; Iacopetta, D.; Popolo, A.; Marzocco, S.; Caruso, A.; Cappello, A.R.; Longo, P.; Puoci, F. Acetylated hyaluronic acid: Enhanced bioavailability and biological studies. BioMed Res. Int. 2014. [Google Scholar] [CrossRef] [PubMed]
- Keller, J.; Schmitt, F.; Scheff, S.; Ding, Q.; Chen, Q.; Butterfield, D.; Markesbery, W. Evidence of increased oxidative damage in subjects with mild cognitive impairment. Neurology 2005, 64, 1152–1156. [Google Scholar] [CrossRef] [PubMed]
- Finkel, T.; Holbrook, N.J. Oxidants, oxidative stress and the biology of ageing. Nature 2000, 408, 239–247. [Google Scholar] [CrossRef] [PubMed]
- Krinsky, N.I. Possible biologic mechanisms for a protective role of xanthophylls. J. Nutr. 2002, 132, 540S–542S. [Google Scholar] [PubMed]
- Peng, Y.; Ma, C.; Li, Y.; Leung, K.S.-Y.; Jiang, Z.-H.; Zhao, Z. Quantification of zeaxanthin dipalmitate and total carotenoids in lycium fruits (fructus lycii). Plant Foods Hum. Nutr. 2005, 60, 161–164. [Google Scholar] [CrossRef] [PubMed]
- Ma, W.; Li, H.; Ye, L.; Chen, M.; Zhang, S. Changes of the main carotenoids pigment contents during the drying processes at different harvest stage in Lycium barbarum L. Fruits. Sci. Agric. Sin. 2007, 40, 1492–1497. [Google Scholar] [CrossRef]
| Samples | Total Carotenoids Content (mg eq Z/g) |
|---|---|
| Calabrian Goji extract | 7.8 ± 0.1 |
| Chinese Goji extract | 5.2 ± 0.3 1 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ruffo, M.; Parisi, O.I.; Amone, F.; Malivindi, R.; Gorgoglione, D.; De Biasio, F.; Scrivano, L.; Pezzi, V.; Puoci, F. Calabrian Goji vs. Chinese Goji: A Comparative Study on Biological Properties. Foods 2017, 6, 30. https://doi.org/10.3390/foods6040030
Ruffo M, Parisi OI, Amone F, Malivindi R, Gorgoglione D, De Biasio F, Scrivano L, Pezzi V, Puoci F. Calabrian Goji vs. Chinese Goji: A Comparative Study on Biological Properties. Foods. 2017; 6(4):30. https://doi.org/10.3390/foods6040030
Chicago/Turabian StyleRuffo, Mariarosa, Ortensia Ilaria Parisi, Fabio Amone, Rocco Malivindi, Domenico Gorgoglione, Filomena De Biasio, Luca Scrivano, Vincenzo Pezzi, and Francesco Puoci. 2017. "Calabrian Goji vs. Chinese Goji: A Comparative Study on Biological Properties" Foods 6, no. 4: 30. https://doi.org/10.3390/foods6040030