Unequivocal Identification of 1-Phenylethyl Acetate in Clove Buds (syzygium aromaticum (L.) Merr. & L.M.Perry) and Clove Essential Oil
Abstract
:1. Introduction
2. Materials and Methods
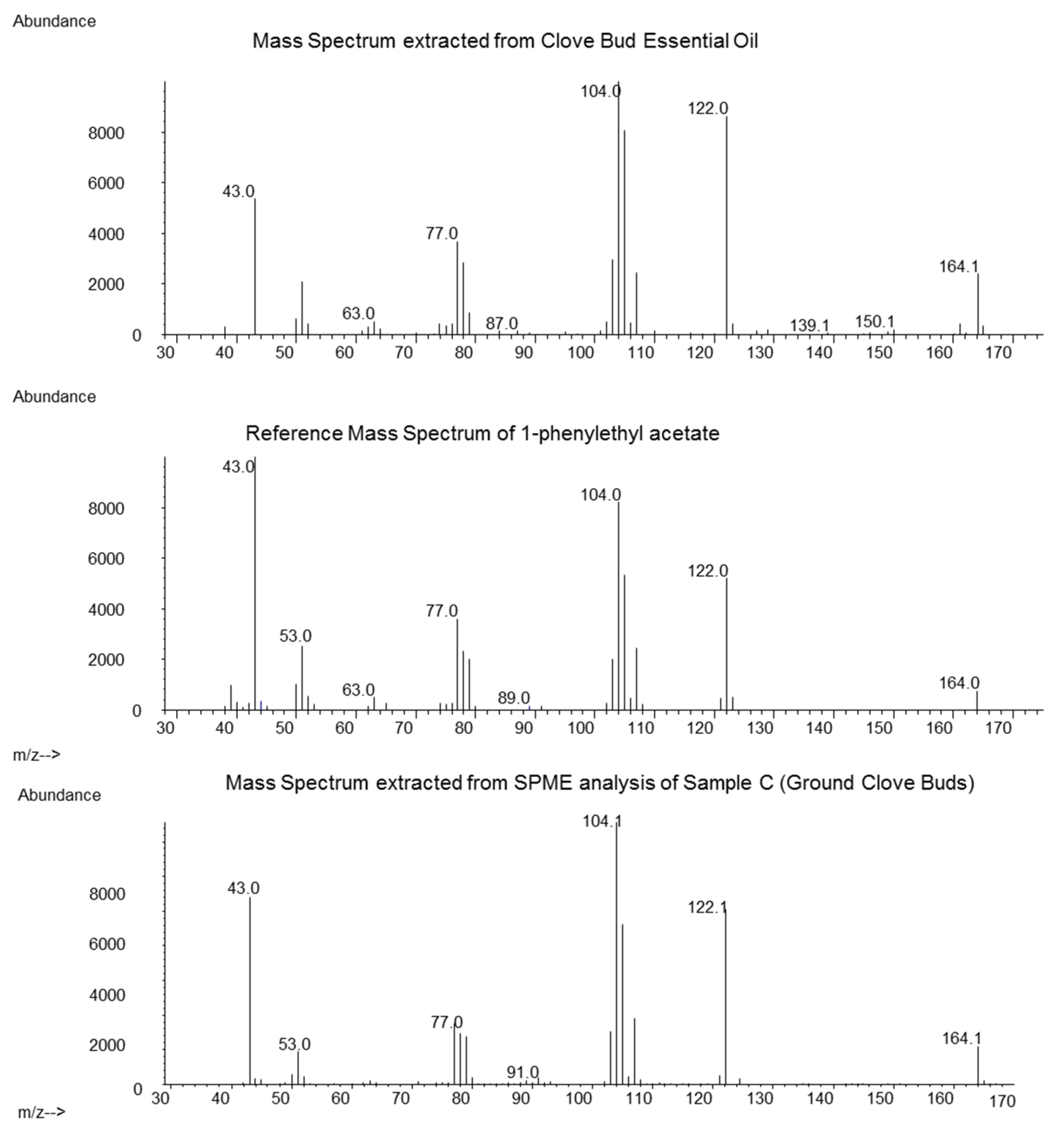
3. Results
4. Discussion and Conclusions
Author Contributions
Conflicts of Interest
References
- Mosciano, G. Organoleptic characteristics of flavor materials. Perfum. Flavorist 2006, 31, 46–49. [Google Scholar]
- Volatile Compounds in Food 16.3. Available online: http://www.vcf-online.nl/VcfHome.cfm (accessed on 8 March 2017).
- Yamaguchi, K.; Nishimura, O.; Toda, H.; Mihara, S.; Shibamoto, T. Chemical studies on tropical fruits. In Instrumental Analysis of Foods: Recent Progress; Charalambous, G., Inglett, G., Eds.; Academic Press: San Diego, CA, USA, 1983; pp. 93–117. [Google Scholar]
- Plutowska, B.; Chmiel, T.; Dymerski, T.; Wardencki, W. A headspace solid-phase microextraction method development and its application in the determination of volatiles in honeys by gas chromatography. Food Chem. 2011, 126, 1288–1298. [Google Scholar] [CrossRef]
- Chaparro-Torres, L.A.; Bueso, M.C.; Fernández-Trujillo, J.P. Aroma volatiles obtained at harvest by HS-SPME/GC-MS and INDEX/MS-E-nose fingerprint discriminate climacteric behaviour in melon fruit. J. Sci. Food Agric. 2016, 96, 2352–2365. [Google Scholar] [CrossRef] [PubMed]
- Pino, J.A.; Marbot, R.; Vázquez, C. Characterization of volatiles in strawberry guava (Psidium cattleianum Sabine) fruit. J. Agric. Food Chem. 2001, 49, 5883–5887. [Google Scholar] [CrossRef] [PubMed]
- Steingass, C.B.; Carle, C.; Schmarr, H.G. Ripening-dependent metabolic changes in the volatiles of pineapple (Ananas comosus (L.) Merr.) fruit: I. Characterization of pineapple aroma compounds by comprehensive two-dimensional gas chromatography-mass spectrometry. Anal. Bioanal. Chem. 2015, 407, 2591–2608. [Google Scholar] [CrossRef] [PubMed]
- Pino, J.A.; Febles, Y. Odour-active compounds in banana fruit cv. Giant Cavendish. Food Chem. 2013, 141, 795–801. [Google Scholar] [CrossRef] [PubMed]
- Brevard, H.; Cachet, T.; Chaintreau, A.; Demyttenaere, J.C.R.; Dijkhuizen, A.; Gassenmeier, K.; Joulain, D.; Koenig, T.; Leijs, H.; Liddle, P.; et al. Statement on the identification in nature of flavouring substances, made by the Working Group on Methods of Analysis of the International Organization of the Flavour Industry (IOFI). Flavour Fragr. J. 2006, 21, 185. [Google Scholar]
- Lawrence, B.M. Major tropical spices—Clove. In Essential Oils 1976–1977; Allured Publ Corp.: Carol Stream, IL, USA, 1979; pp. 84–145. [Google Scholar]
- Cheng, B.Q.; Yu, X.J.; Ding, J.K.; Xu, Y.; Sun, H.D.; Ma, X.X.; Yi, Y. Cinnamomum Plant Resources and Their Aromatic Constituents; Yunnan Science and Technology Press: Yunnan, China, 2001. [Google Scholar]
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gassenmeier, K.; Schwager, H.; Houben, E.; Clery, R. Unequivocal Identification of 1-Phenylethyl Acetate in Clove Buds (syzygium aromaticum (L.) Merr. & L.M.Perry) and Clove Essential Oil. Foods 2017, 6, 46. https://doi.org/10.3390/foods6070046
Gassenmeier K, Schwager H, Houben E, Clery R. Unequivocal Identification of 1-Phenylethyl Acetate in Clove Buds (syzygium aromaticum (L.) Merr. & L.M.Perry) and Clove Essential Oil. Foods. 2017; 6(7):46. https://doi.org/10.3390/foods6070046
Chicago/Turabian StyleGassenmeier, Klaus, Hugo Schwager, Eric Houben, and Robin Clery. 2017. "Unequivocal Identification of 1-Phenylethyl Acetate in Clove Buds (syzygium aromaticum (L.) Merr. & L.M.Perry) and Clove Essential Oil" Foods 6, no. 7: 46. https://doi.org/10.3390/foods6070046




