Honey Mitigates Radiation-Induced Oral Mucositis in Head and Neck Cancer Patients without Affecting the Tumor Response
Abstract
:1. Introduction
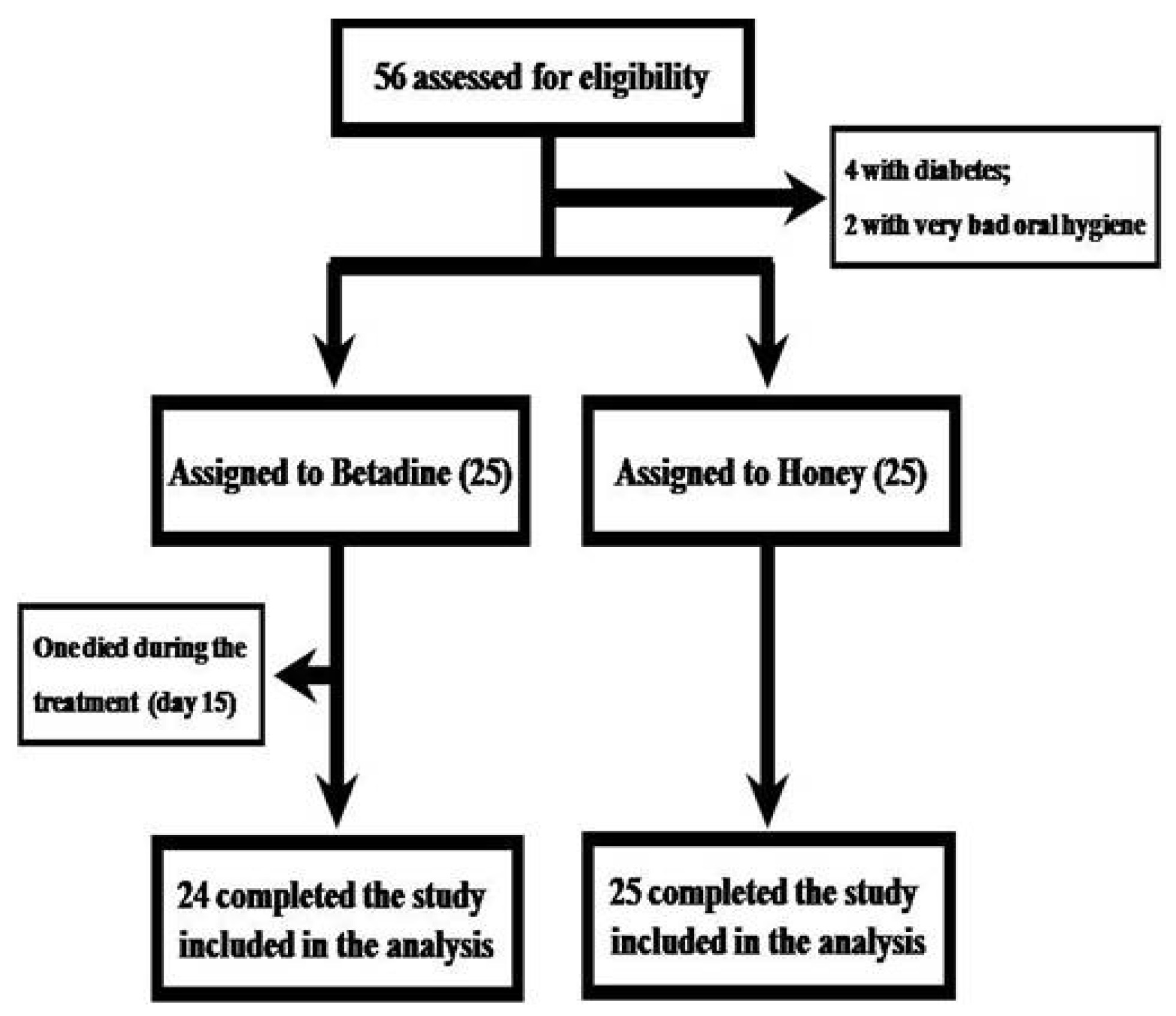
2. Patients and Methods
2.1. Patient Population
2.2. Study Design
2.3. Radiation Treatment
2.4. Study Mouthwashes
2.5. Outcome Measures
2.6. Treatment Response Evaluation after Treatment Completion
2.7. Statistical Analysis
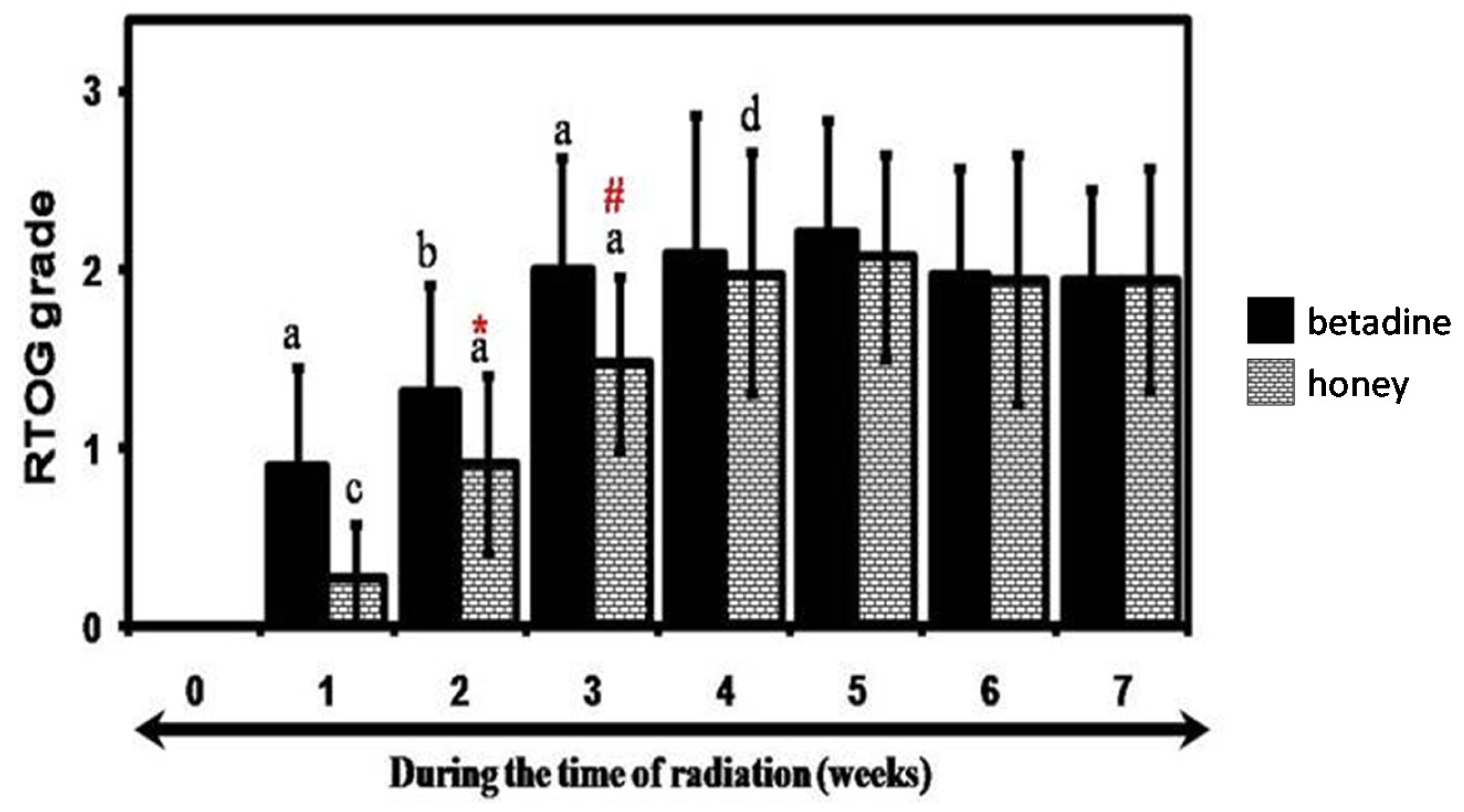
3. Results
Clinical Study
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Sonis, S.T. Oral mucositis in head and neck cancer: Risk, biology, and management. Am. Soc. Clin. Oncol. Educ. Book 2013, 33, e236. [Google Scholar] [CrossRef] [PubMed]
- Biswal, B.M.; Zakaria, A.; Ahmad, N.M. Topical application of honey in the management of radiation mucositis: A preliminary study. Support. Care Cancer 2003, 11, 242–248. [Google Scholar] [PubMed]
- Motallebnejad, M.; Akram, S.; Moghadamnia, A.; Moulana, Z.; Omidi, S. The effect of topical application of pure honey on radiation-induced mucositis: A randomized clinical trial. J. Contemp. Dent. Pract. 2008, 9, 40–47. [Google Scholar] [PubMed]
- Rashad, U.M.; Al-Gezawy, S.M.; El-Gezawy, E.; Azzaz, A.N. Honey as topical prophylaxis against radiochemotherapy-induced mucositis in head and neck cancer. J. Laryngol. Otol. 2009, 123, 223–228. [Google Scholar] [CrossRef] [PubMed]
- Khanal, B.; Baliga, M.; Uppal, N. Effect of topical honey on limitation of radiation-induced oral mucositis: An intervention study. Int. J. Oral Maxillofac. Surg. 2010, 39, 1181–1185. [Google Scholar] [CrossRef] [PubMed]
- Maiti, P.K.; Ray, A.; Mitra, T.N.; Jana, U.; Bhattacharya, J.; Ganguly, S. The effect of honey on mucositis induced by chemoradiation in head and neck cancer. J. Indian Med. Assoc. 2012, 110, 453–456. [Google Scholar] [PubMed]
- Jayachandran, S.; Balaji, N. Evaluating the effectiveness of topical application of natural honey and benzydamine hydrochloride in the management of radiation mucositis. Indian J. Palliat. Care 2012, 18, 190–195. [Google Scholar] [PubMed]
- Abdulrhman, M.; El Barbary, N.S.; Ahmed Amin, D.; SaeidEbrahim, R. Honey and a mixture of honey, beeswax, and olive oil-propolis extract in treatment of chemotherapy-induced oral mucositis: A randomized controlled pilot study. Pediatr. Hematol. Oncol. 2012, 29, 285–292. [Google Scholar] [CrossRef] [PubMed]
- Samdariya, S.; Lewis, S.; Kauser, H.; Ahmed, I.; Kumar, D. A randomized controlled trial evaluating the role of honey in reducing pain due to radiation induced mucositis in head and neck cancer patients. Indian J. Palliat. Care 2015, 21, 268–273. [Google Scholar] [CrossRef] [PubMed]
- KobyaBulut, H.; GüdücüTüfekci, F. Honey prevents oral mocositis in children undergoing chemotherapy: A quasi-experimental study with a control group. Complement. Ther. Med. 2016, 29, 132–140. [Google Scholar] [CrossRef] [PubMed]
- Jayalekshmi, J.L.; Lakshmi, R.; Mukerji, A. Honey on oral mucositis: A randomized controlled trial. Gulf J. Oncol. 2016, 1, 30–37. [Google Scholar]
- Al Jaouni, S.K.; Al Muhayawi, M.S.; Hussein, A.; Elfiki, I.; Al-Raddadi, R.; Al Muhayawi, S.M.; Almasaudi, S.; Kamal, M.A.; Harakeh, S. Effects of honey on oral mucositis among pediatric cancer patients undergoing chemo/radiotherapy treatment at King Abdulaziz University Hospital in Jeddah, Kingdom of Saudi Arabia. Evid.-Based Complement. Altern. Med. 2017, 2017, 5861024. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.L.; Xia, R.; Sun, Z.H.; Sun, L.; Min, X.; Liu, C.; Zhang, H.; Zhu, Y.M. Effects of honey use on the management of radio/chemotherapy-induced mucositis: A meta-analysis of randomized controlled trials. Int. J. Oral Maxillofac. Surg. 2016, 45, 1618–1625. [Google Scholar] [CrossRef] [PubMed]
- Co, J.L.; Mejia, M.B.; Que, J.C.; Dizon, J.M. Effectiveness of honey on radiation-induced oral mucositis, time to mucositis, weight loss, and treatment interruptions among patients with head and neck malignancies: A meta-analysis and systematic review of literature. Head Neck 2016, 38, 1119–1128. [Google Scholar] [CrossRef] [PubMed]
- Cho, H.K.; Jeong, Y.M.; Lee, H.S.; Lee, Y.J.; Hwang, S.H. Effects of honey on oral mucositis in patients with head and neck cancer: A meta-analysis. Laryngoscope 2015, 125, 2085–2092. [Google Scholar] [CrossRef] [PubMed]
- Elad, S.; Bowen, J.; Zadik, Y.; Lalla, R.V. Development of the MASCC/ISOO Clinical Practice Guidelines for Mucositis: Considerations underlying the process. Support. Care Cancer 2013, 21, 309–312. [Google Scholar] [CrossRef] [PubMed]
- Madan, P.D.; Sequeira, P.S.; Shenoy, K.; Shetty, J. The effect of three mouthwashes on radiation-induced oral mucositis in patients with head and neck malignancies: A randomized control trial. J. Cancer Res. Ther. 2008, 4, 3–8. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. WHO Handbook for Reporting Results of Cancer Treatment; WHO Offset Publication No. 48; World Health Organization: Geneva, Switzerland, 1979. [Google Scholar]
- Erejuwa, O.O.; Sulaiman, S.A.; Ab Wahab, M.S. Honey: A novel antioxidant. Molecules 2012, 17, 4400–4423. [Google Scholar] [CrossRef] [PubMed]
- Samarghandian, S.; Farkhondeh, T.; Samini, F. Honey and health: A review of recent clinical research. Pharmacogn. Res. 2017, 9, 121–127. [Google Scholar]
| Povidone-Iodine | Honey | |
|---|---|---|
| Age (years) | 55.8 ± 10.8 | 54.1 ± 11.3 |
| Gender | ||
| Male | 17 | 20 |
| Female | 8 | 5 |
| Site | ||
| Alveolus | 0 | 2 |
| Buccal mucosa | 4 | 3 |
| Floor of the Mouth | 0 | 2 |
| Gingivobuccal sulcus | 1 | 0 |
| Palate | 1 | 1 |
| Pyriform sinus | 0 | 2 |
| Pharynx (Oro and Hypo) | 0 | 1 |
| Retromolar trigone | 0 | 2 |
| Tongue | 17 | 12 |
| Tonsil | 2 | 0 |
| Tumor/Node/Metastasis (TNM) stage | ||
| Primary | ||
| T1 | 2 | 8 |
| T2 | 9 | 8 |
| T3 | 8 | 5 |
| T4 | 5 | 3 |
| TX | 1 | 1 |
| Regional nodes | ||
| N0 | 3 | 5 |
| N1 | 13 | 11 |
| N2 | 1 | 0 |
| N2a | 4 | 3 |
| N2b | 0 | 3 |
| N2c | 2 | 1 |
| N3 | 1 | 2 |
| NX | 1 | 0 |
| Metastasis | ||
| M0 | 0 | 0 |
| MX | 0 | 0 |
| Treatment Details | Povidone-Iodine | Honey |
|---|---|---|
| Radiation only | 2 | 1 |
| Chemoradiation | 23 | 24 |
| Dose of radiation | 67.0 ± 2.3 | 68.1 ± 2.3 |
| Death during the course of radiation treatment | 1 | 0 |
| Incidence of treatment breaks | 5/24 | 3/25 |
| Number of days | 7.4 ± 0.8 | 6.33 ± 0.95 |
| Appearance of mucositis | 7.24 ± 3.10 | 10.16 ± 2.35 * |
| Treatment response details | ||
| Complete response | 45.8% (11/24) | 52% (13/25) |
| Partial response | 25% (6/24) | 20% (5/25) |
| No response | 29.2% (7/24) | 28% (7/25) |
© 2017 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rao, S.; Hegde, S.K.; Rao, P.; Dinkar, C.; Thilakchand, K.R.; George, T.; Baliga-Rao, M.P.; Palatty, P.L.; Baliga, M.S. Honey Mitigates Radiation-Induced Oral Mucositis in Head and Neck Cancer Patients without Affecting the Tumor Response. Foods 2017, 6, 77. https://doi.org/10.3390/foods6090077
Rao S, Hegde SK, Rao P, Dinkar C, Thilakchand KR, George T, Baliga-Rao MP, Palatty PL, Baliga MS. Honey Mitigates Radiation-Induced Oral Mucositis in Head and Neck Cancer Patients without Affecting the Tumor Response. Foods. 2017; 6(9):77. https://doi.org/10.3390/foods6090077
Chicago/Turabian StyleRao, Suresh, Sanath K. Hegde, Pratima Rao, Chetana Dinkar, Karadka R. Thilakchand, Thomas George, Manjeshwar P. Baliga-Rao, Princy L. Palatty, and Manjeshwar S. Baliga. 2017. "Honey Mitigates Radiation-Induced Oral Mucositis in Head and Neck Cancer Patients without Affecting the Tumor Response" Foods 6, no. 9: 77. https://doi.org/10.3390/foods6090077




