Enrichment of Biscuits with Matcha Green Tea Powder: Its Impact on Consumer Acceptability and Acute Metabolic Response
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Shortbread Incorporated with MGTP
2.3. Defatting of Samples and Extraction of Catechins
2.4. HPLC Analysis of Catechins in Dough and Biscuit Samples
2.5. Sensory and Acceptability Studies
2.6. Human Intervention Study for Study of Metabolic Response
2.6.1. Reference and Test Meals
2.6.2. Glucose and Triglyceride Measurement
2.6.3. iAUC Calculation
2.7. Data Analysis
3. Results and Discussion
3.1. Stability of Catechins and Caffeine During the Baking Process
3.2. Sensory and Overall Acceptability of Biscuits
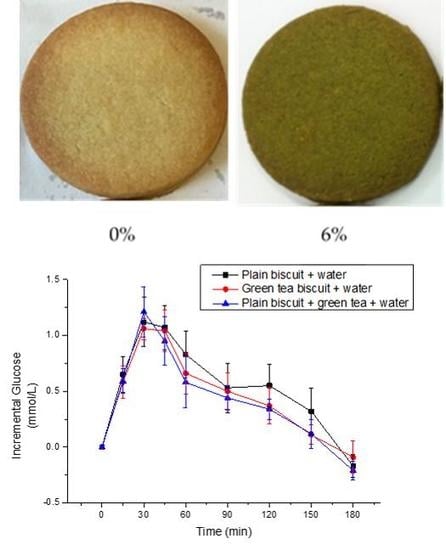
3.3. Metabolic Response to Matcha Green Tea Biscuits
3.3.1. Glucose Response
3.3.2. Triglyceride Response
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Wang, H.; Provan, G.; Helliwell, K.; Ransom, W. The functional benefits of flavonoids: The case of tea. In Phytochemical Functional Foods; Johnson, I., Williamson, G., Eds.; Woodhead Publishing: Sawston, Cambridge, UK, 2003; pp. 128–159. [Google Scholar]
- Wang, R.; Zhou, W.; Jiang, X. Reaction kinetics of degradation and epimerization of epigallocatechin gallate (EGCG) in aqueous system over a wide temperature range. J. Agric. Food Chem. 2008, 56, 694–2701. [Google Scholar] [CrossRef] [PubMed]
- Pelillo, M.; Biguzzi, B.; Bendini, A.; Toschi, T.G.; Vanzini, M.; Lercker, G. Preliminary investigation into development of HPLC with UV and MS-electrospray detection for the analysis of tea catechins. Food Chem. 2002, 78, 369–374. [Google Scholar] [CrossRef]
- Mennen, L.; Hirvonen, T.; Arnault, N.; Bertrais, S.; Galan, P.; Hercberg, S. Consumption of black, green and herbal tea and iron status in French adults. Eur. J. Clin. Nutr. 2007, 61, 1174–1179. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.-K.; Jombart, L.; Valentin, D.; Kim, K.O. A cross-cultural study using Napping®: Do Korean and French consumers perceive various green tea products differently? Food Res. Int. 2013, 53, 534–542. [Google Scholar] [CrossRef]
- Šebečić, B.; Vedrina Dragojević, I.; Vitali, D.; Hečimović, M.; Dragičević, I. Raw materials in fibre enriched biscuits production as source of total phenols. Agric. Conspec. Sci. 2007, 72, 265–270. [Google Scholar]
- Carrillo, E.; Varela, P.; Fiszman, S. Effects of food package information and sensory characteristics on the perception of healthiness and the acceptability of enriched biscuits. Food Res. Int. 2012, 48, 209–216. [Google Scholar] [CrossRef]
- Ahmad, M.; Baba, W.N.; Wani, T.A.; Gani, A.; Gani, A.; Shah, U.; Wani, S.M.; Masoodi, F.A. Effect of green tea powder on thermal, rheological & functional properties of wheat flour and physical, nutraceutical & sensory analysis of cookies. J. Food Sci. Technol. 2015, 52, 5799–5807. [Google Scholar] [PubMed]
- Lu, T.M.; Lee, C.C.; Mau, J.L.; Lin, S.D. Quality and antioxidant property of green tea sponge cake. Food Chem. 2010, 119, 1090–1095. [Google Scholar] [CrossRef]
- Li, M.; Zhang, J.H.; Zhu, K.X.; Peng, W.; Zhang, S.K.; Wang, B.; Zhu, Y.J.; Zhou, H.M. Effect of superfine green tea powder on the thermodynamic, rheological and fresh noodle making properties of wheat flour. LWT Food Sci. Technol. 2012, 46, 23–28. [Google Scholar] [CrossRef]
- Yang, J.; Mao, Q.X.; Xu, H.X.; Ma, X.; Zeng, C.Y. Tea consumption and risk of type 2 diabetes mellitus: A systematic review and meta-analysis update. BMJ Open 2014, 4, e005632. [Google Scholar] [CrossRef] [PubMed]
- Thielecke, F.; Boschmann, M. The potential role of green tea catechins in the prevention of the metabolic syndrome—A review. Phytochemistry 2009, 70, 11–24. [Google Scholar] [CrossRef] [PubMed]
- Zhong, L.; Furne, J.K.; Levitt, M.D. An extract of black, green, and mulberry teas causes malabsorption of carbohydrate but not of triacylglycerol in healthy volunteers. Am. J. Clin. Nutr. 2006, 84, 551–555. [Google Scholar] [PubMed]
- Koo, S.I.; Noh, S.K. Green tea as inhibitor of the intestinal absorption of lipids: Potential Mechanism for its Lipid-Lowering Effect. J. Nutr. Biochem. 2007, 18, 179–183. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, I.; Tsuda, K.; Suzuki, Y.; Kobayashi, M.; Unno, T.; Tomoyori, H.; Goto, H.; Kawata, Y.; Imaizumi, K.; Nozawa, A.; et al. Tea catechins with a galloyl moiety suppress postprandial hypertriacylglycerolemia by delaying lymphatic transport of dietary fat in rats. J. Nutr. 2005, 135, 155–159. [Google Scholar] [CrossRef] [PubMed]
- Unno, T.; Tago, M.; Suzuki, Y.; Nozawa, A.; Sagesaka, Y.M.; Kakuda, T.; Egawa, K.; Kondo, K. Effect of tea catechins on postprandial plasma lipid responses in human subjects. Br. J. Nutr. 2005, 93, 543–547. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Zhou, W. Stability of tea catechins in the breadmaking Process. J. Agric. Food Chem. 2004, 52, 8224–8229. [Google Scholar] [CrossRef] [PubMed]
- Food and Agriculture Organization. Carbohydrates in Human Nutrition. Report of a Joint FAO/WHO Expert Consultation; FAO Food Nutrition Paper; Food and Agriculture Organization of the United Nations: Rome, Itlay, 1998; Volume 66, pp. 1–140. [Google Scholar]
- Brouns, F.; Bjorck, I.; Frayn, K.N.; Gibbs, A.L.; Lang, V.; Slama, G.; Wolever, T.M.S. Glycaemic index methodology. Nutr. Res. Rev. 2005, 18, 145–171. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Zhou, W.; Jiang, X. Mathematical modeling of the stability of green tea catechin epigallocatechin gallate (EGCG) during bread baking. J. Food Eng. 2008, 87, 505–513. [Google Scholar] [CrossRef]
- Sharma, A.; Zhou, W. A stability study of green tea catechins during the biscuit making process. Food Chem. 2011, 126, 568–573. [Google Scholar] [CrossRef]
- Guo, Q.; Zhao, B.; Shen, S.; Hou, J.; Hu, J.; Xin, W. ESR study on the structure–antioxidant activity relationship of tea catechins and their epimers. Biochim. Biophys. Acta (BBA) Gen. Subj. 1999, 1427, 13–23. [Google Scholar] [CrossRef]
- Ananingsih, V.K.; Sharma, A.; Zhou, W. Green tea catechins during food processing and storage: A review on stability and detection. Food Res. Int. 2013, 50, 469–479. [Google Scholar] [CrossRef]
- Wang, L.-F.; So, S.; Baik, J.H.; Kim, H.J.; Moon, K.S.; Park, S.K. Aroma changes in green tea beverage during processing and storage. In Nutraceutical Beverages; American Chemical Society: Washington, DC, USA, 2003; pp. 162–188. [Google Scholar]
- Kim, E.S.; Liang, Y.R.; Jin, J.; Sun, Q.F.; Lu, J.L.; Du, Y.Y.; Lin, C. Impact of heating on chemical compositions of green tea liquor. Food Chem. 2007, 103, 1263–1267. [Google Scholar] [CrossRef]
- Wang, R.; Zhou, W.; Isabelle, M. Comparison study of the effect of green tea extract (GTE) on the quality of bread by instrumental analysis and sensory evaluation. Food Res. Int. 2007, 40, 470–479. [Google Scholar] [CrossRef]
- Green, B.G.; Lim, J.; Osterhoff, F.; Blacher, K.; Nachtigal, D. Taste mixture interactions: Suppression, additivity, and the predominance of sweetness. Physiol. Behave. 2010, 101, 731–737. [Google Scholar] [CrossRef] [PubMed]
- Forester, S.C.; Gu, Y.; Lambert, J.D. Inhibition of starch digestion by the green tea polyphenol, (−)-epigallocatechin-3-gallate. Mol. Nutr. Food Res. 2012, 56, 1647–1654. [Google Scholar] [CrossRef] [PubMed]
- Chen, N.; Bezzina, R.; Hinch, E.; Lewandowski, P.A.; Cameron-Smith, D.; Mathai, M.L.; Jois, M.; Sinclair, A.J.; Begg, D.P.; Wark, J.D.; et al. Green tea, black tea, and epigallocatechin modify body composition, improve glucose tolerance, and differentially alter metabolic gene expression in rats fed a high-fat diet. Nutr. Res. 2009, 29, 784–793. [Google Scholar] [CrossRef] [PubMed]
- Tsuneki, H.; Ishizuka, M.; Terasawa, M.; Wu, J.B.; Sasaoka, T.; Kimura, I. Effect of green tea on blood glucose levels and serum proteomic patterns in diabetic (db/db) mice and on glucose metabolism in healthy humans. BMC Pharmacol. 2004, 4, 18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lochocka, K.; Bajerska, J.; Glapa, A.; Fidler-Witon, E.; Nowak, J.K.; Szczapa, T.; Grebowiec, P.; Lisowska, A.; Walkowiak, J. Green tea extract decreases starch digestion and absorption from a test meal in humans: A randomized, placebo-controlled crossover study. Sci. Rep. 2015, 5, 12015. [Google Scholar] [CrossRef] [PubMed]
- Josic, J.; Olsson, A.T.; Wickeberg, J.; Lindstedt, S.; Hlebowicz, J. Does green tea affect postprandial glucose, insulin and satiety in healthy subjects: A randomized controlled trial. Nutr. J. 2010, 9, 63. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.Y.; Juan, C.C.; Ho, L.T.; Hsu, Y.P.; Hwang, L.S. Effect of green tea supplementation on insulin sensitivity in Sprague-Dawley rats. J. Agric. Food Chem. 2004, 52, 643–648. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.Y.; Juan, C.C.; Hwang, L.S.; Hsu, Y.P.; Ho, P.H.; Ho, L.T. Green tea supplementation ameliorates insulin resistance and increase glucose transporter IV content in a fructose-fed rat model. Eur. J. Nutr. 2004, 43, 116–124. [Google Scholar] [CrossRef] [PubMed]
- Park, J.H.; Jin, J.Y.; Baek, W.K.; Park, S.H.; Sung, H.Y.; Kim, Y.K.; Lee, J.; Song, D.K. Ambivalent role of gallated catechins in glucose tolerance in humans: A novel insight into non-absorbable gallated catechin-derived inhibitors of glucose absorption. J. Physiol. Pharmacol. 2009, 60, 101–109. [Google Scholar] [PubMed]
- Wolever, T.M.S. The Glycaemic Index: A Physiological Classification of Dietary Carbohydrate; CABI Pub.: Wallingford, Oxfordshire, UK; Cambridge, MA, USA, 2006; 227p. [Google Scholar]
- Suzuki, Y.; Unno, T.; Kobayashi, M.; Nozawa, A.; Sagesaka, Y.; Kakuda, T. Dose-dependent suppression of tea catechins with a galloyl moiety on postprandial hypertriglyceridemia in rats. Biosci. Biotechnol. Biochem. 2005, 69, 1288–1291. [Google Scholar] [CrossRef] [PubMed]
- Juhel, C.; Armand, M.; Pafumi, Y.; Rosier, C.; Vandermander, J.; Lairon, D. Green tea extract (AR25®) inhibits lipolysis of triglycerides in gastric and duodenal medium in vitro. J. Nutr. Biochem. 2000, 11, 45–51. [Google Scholar] [CrossRef]
- Kim, J.; Koo, S.I.; Noh, S.K. Green tea extract markedly lowers the lymphatic absorption and increases the biliary secretion of C-14-benzo a pyrene in rats. J. Nutr. Biochem. 2012, 23, 1007–1011. [Google Scholar] [CrossRef] [PubMed]
- Chan, C.Y.; Wei, L.; Castro-Muñozledo, F.; Koo, W.L. (−)-Epigallocatechin-3-gallate blocks 3T3-L1 adipose conversion by inhibition of cell proliferation and suppression of adipose phenotype expression. Life Sci. 2011, 89, 779–785. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.-S.; Kim, C.T.; Kim, I.H.; Kim, Y. Inhibitory Effects of Green Tea Catechin on the Lipid Accumulation in 3T3-L1 Adipocytes. Phytother. Res. 2009, 23, 1088–1091. [Google Scholar] [CrossRef] [PubMed]
- Walkowiak, J.; Bajerska, J.; Kargulewicz, A.; Lisowska, A.; Siedlerski, G.; Szczapa, T.; Kobelska-Dubiel, N.; Grzymisławski, M. Single dose of green tea extract decreases lipid digestion and absorption from a test meal in humans. Acta Biochim. Pol. 2013, 60, 481–483. [Google Scholar] [PubMed]
- Raederstorff, D.G.; Schlachter, M.F.; Elste, V.; Weber, P. Effect of EGCG on lipid absorption and plasma lipid levels in rats. J. Nutr. Biochem. 2003, 14, 326–332. [Google Scholar] [CrossRef]
- Liu, Z.-K.; Hu, M.; Baum, L.; Thomas, G.N.; Tomlinson, B. Associations of polymorphisms in the apolipoprotein A1/C3/A4/A5 gene cluster with familial combined hyperlipidaemia in Hong Kong Chinese. Atherosclerosis 2010, 208, 427–432. [Google Scholar] [CrossRef] [PubMed]
- Arjunan, S.P.; Bishop, N.; Reischak-Oliveira, A.; Stensel, D.J. Exercise and coronary heart disease risk markers in South Asian and European men. Med. Sci. Sports Exerc. 2013, 45, 1261–1268. [Google Scholar] [CrossRef] [PubMed]






| Compound | 2 g MGTP (%) | 4 g MGTP (%) | 6 g MGTP (%) |
|---|---|---|---|
| (−)-epigallocatechin (EGC) | 70.9 ± 9.5 | 81.5 ± 2.5 | 68.9 ± 1.6 |
| (−)-epicatechin (EC) | 72.6 ± 4.5 | 85.7 ± 3.5 | 80.2 ± 1.7 |
| (−)-epigallocatechin gallate (EGCG) | 84.9 ± 4.0 | 89.7 ± 2.1 | 81.8 ± 1.4 |
| (+)-gallocatechin gallate (GCG) | 133.0 ± 7.3 | 128.6 ± 11.0 | 140.3 ± 8.6 |
| (−)-epicatechin gallate (ECG) | 92.2 ± 3.9 | 94.3 ± 2.4 | 89.1 ± 1.2 |
| Total catechins | 82.4 ± 5.7 | 88.9 ± 2.5 | 81.1 ± 1.4 |
| Caffeine | 81.9 ± 5.0 | 76.3 ± 3.4 | 76.4 ± 1.1 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Phongnarisorn, B.; Orfila, C.; Holmes, M.; Marshall, L.J. Enrichment of Biscuits with Matcha Green Tea Powder: Its Impact on Consumer Acceptability and Acute Metabolic Response. Foods 2018, 7, 17. https://doi.org/10.3390/foods7020017
Phongnarisorn B, Orfila C, Holmes M, Marshall LJ. Enrichment of Biscuits with Matcha Green Tea Powder: Its Impact on Consumer Acceptability and Acute Metabolic Response. Foods. 2018; 7(2):17. https://doi.org/10.3390/foods7020017
Chicago/Turabian StylePhongnarisorn, Benjapor, Caroline Orfila, Melvin Holmes, and Lisa J. Marshall. 2018. "Enrichment of Biscuits with Matcha Green Tea Powder: Its Impact on Consumer Acceptability and Acute Metabolic Response" Foods 7, no. 2: 17. https://doi.org/10.3390/foods7020017
APA StylePhongnarisorn, B., Orfila, C., Holmes, M., & Marshall, L. J. (2018). Enrichment of Biscuits with Matcha Green Tea Powder: Its Impact on Consumer Acceptability and Acute Metabolic Response. Foods, 7(2), 17. https://doi.org/10.3390/foods7020017