Dairy Fats and Cardiovascular Disease: Do We Really Need to Be Concerned?
Abstract
:1. Introduction
2. Dietary Guidelines and Dairy Product Consumption
3. Saturated Fat, Cholesterol and Dairy Products
3.1. Saturated Fat
3.2. Dietary Cholesterol
3.3. Low-Fat Dairy products
3.4. Limitations to Dairy Research: The Dairy Matrix Effect
4. Dairy Products and Cardiometabolic Health
4.1. Dairy Products and Hypertension
4.2. Dairy Products and Diabetes
4.3. Dairy Products and Obesity
5. Anti-Inflammatory Properties of Dairy Products
6. Trans Fatty Acids
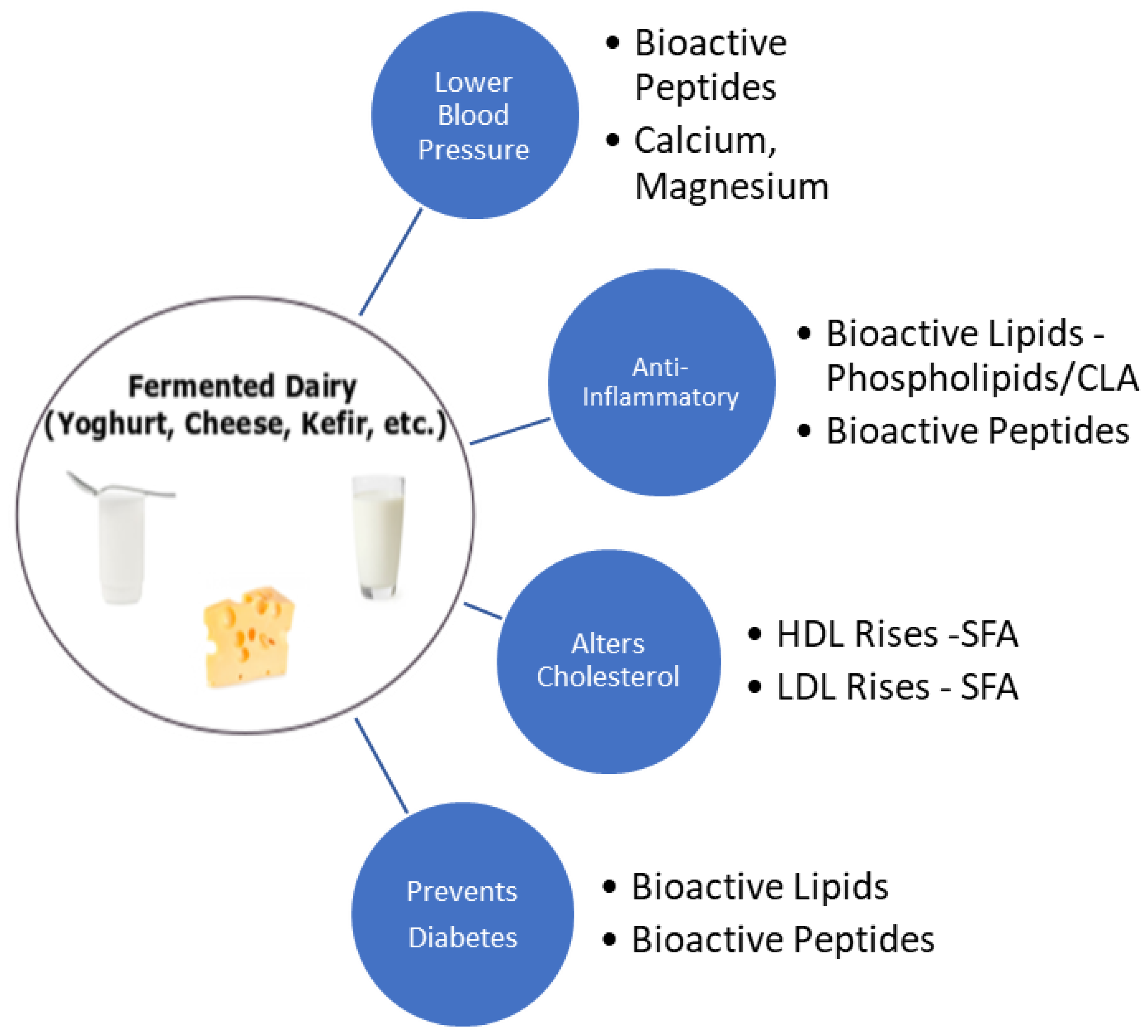
7. Fermented Dairy Products and Cardiovascular Health
8. Functional Alternative Dairy Foods and Consumer Trends
8.1. Cholesterol-Lowering Dairy products
8.2. Plant Based Milk Alternatives
8.3. Alternatives to Bovine Milk: Caprine and Ovine Milk
8.4. Functional Foods—Kefir
9. Conclusion: Dairy Fats and Cardiovascular Diseases, Do We Really Need to Be Concerned?
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Wilkins, E.; Wilson, L.; Wickramasinghe, K.; Bhatnagar, P.; Leal, J.; Luengo-Fernandez, R.; Burns, R.; Rayner, M.; Townsend, N. European Cardiovascular Disease Statistics 2017; European Heart Network: Brussels, Belgium, 2017. [Google Scholar]
- Benjamin, E.J.; Blaha, M.J.; Chiuve, S.E.; Cushman, M.; Das, S.R.; Deo, R.; de Ferranti, S.D.; Floyd, J.; Fornage, M.; Gillespie, C.; et al. Heart disease and stroke statistics—2017 update: A report from the american heart association. Circulation 2017, 135, e146–e603. [Google Scholar] [CrossRef] [PubMed]
- Health Service Executive Ireland. Coronary Heart Disease. Available online: http://www.hse.ie/eng/health/az/C/Coronary-heart-disease/ (accessed on 20 January 2017).
- Lordan, R.; Zabetakis, I. Invited review: The anti-inflammatory properties of dairy lipids. J. Dairy Sci. 2017, 100, 4197–4212. [Google Scholar] [CrossRef] [PubMed]
- O’Keefe, J.H.; Gheewala, N.M.; O’Keefe, J.O. Dietary strategies for improving post-prandial glucose, lipids, inflammation, and cardiovascular health. J. Am. Coll. Cardiol. 2008, 51, 249–255. [Google Scholar] [CrossRef] [PubMed]
- Mozaffarian, D.; Appel, L.J.; Van Horn, L. Components of a cardioprotective diet new insights. Circulation 2011, 123, 2870–2891. [Google Scholar] [CrossRef] [PubMed]
- Kapaj, A.; Deci, E. Chapter 7—World milk production and socio-economic factors effecting its consumption a2—Watson, ronald ross. In Dairy in Human Health and Disease Across the Lifespan; Collier, R.J., Preedy, V.R., Eds.; Academic Press: Cambridge, MA, USA, 2017; pp. 107–115. [Google Scholar]
- Artaud-Wild, S.M.; Connor, S.; Sexton, G.; Connor, W.E. Differences in coronary mortality can be explained by differences in cholesterol and saturated fat intakes in 40 countries but not in France and Finland. A paradox. Circulation 1993, 88, 2771–2779. [Google Scholar] [CrossRef] [PubMed]
- Turpeinen, O. Effect of cholesterol-lowering diet on mortality from coronary heart disease and other causes. Circulation 1979, 59, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Huth, P.J.; Park, K.M. Influence of dairy product and milk fat consumption on cardiovascular disease risk: A review of the evidence. Adv. Nutr. 2012, 3, 266–285. [Google Scholar] [CrossRef] [PubMed]
- Grosso, G. Chapter 30—Milk and chronic-degenerative diseases: Main components and potential mechanisms a2—Watson, ronald ross. In Dairy in Human Health and Disease Across the Lifespan; Collier, R.J., Preedy, V.R., Eds.; Academic Press: Cambridge, MA, USA, 2017; pp. 385–393. [Google Scholar]
- Guo, J.; Astrup, A.; Lovegrove, J.A.; Gijsbers, L.; Givens, D.I.; Soedamah-Muthu, S.S. Milk and dairy consumption and risk of cardiovascular diseases and all-cause mortality: Dose-response meta-analysis of prospective cohort studies. Eur. J. Epidemiol. 2017, 32, 269–287. [Google Scholar] [CrossRef] [PubMed]
- Goldbohm, R.A.; Chorus, A.M.J.; Garre, F.G.; Schouten, L.J.; van den Brandt, P.A. Dairy consumption and 10-y total and cardiovascular mortality: A prospective cohort study in The Netherlands. Am. J. Clin. Nutr. 2011, 93, 615–627. [Google Scholar] [CrossRef] [PubMed]
- Lamarche, B.; Givens, D.I.; Soedamah-Muthu, S.; Krauss, R.M.; Jakobsen, M.U.; Bischoff-Ferrari, H.A.; Pan, A.; Després, J.-P. Does milk consumption contribute to cardiometabolic health and overall diet quality? Can. J. Cardiol. 2016, 32, 1026–1032. [Google Scholar] [CrossRef] [PubMed]
- Dumas, A.-A.; Lapointe, A.; Dugrenier, M.; Provencher, V.; Lamarche, B.; Desroches, S. A systematic review of the effect of yogurt consumption on chronic diseases risk markers in adults. Eur. J. Clin. Nutr. 2017, 56, 1375–1392. [Google Scholar] [CrossRef] [PubMed]
- Alexander, D.D.; Bylsma, L.C.; Vargas, A.J.; Cohen, S.S.; Doucette, A.; Mohamed, M.; Irvin, S.R.; Miller, P.E.; Watson, H.; Fryzek, J.P. Dairy consumption and CVD: A systematic review and meta-analysis. Br. J. Nutr. 2016, 115, 737–750. [Google Scholar] [CrossRef] [PubMed]
- Qin, L.-Q.; Xu, J.-Y.; Han, S.-F.; Zhang, Z.-L.; Zhao, Y.-Y.; Szeto, I.M. Dairy consumption and risk of cardiovascular disease: An updated meta-analysis of prospective cohort studies. Asia Pac. J. Clin. Nutr. 2015, 24, 90–100. [Google Scholar] [PubMed]
- Crichton, G.E.; Elias, M.F. Dairy food intake and cardiovascular health: The maine-syracuse study. Adv. Dairy Res. 2014, 2, 1–8. [Google Scholar] [CrossRef]
- Crichton, G.E.; Alkerwi, A. Dairy food intake is positively associated with cardiovascular health: Findings from observation of cardiovascular risk factors in Luxembourg study. Nutr. Res. 2014, 34, 1036–1044. [Google Scholar] [CrossRef] [PubMed]
- Gijsbers, L.; Ding, E.L.; Malik, V.S.; de Goede, J.; Geleijnse, J.M.; Soedamah-Muthu, S.S. Consumption of dairy foods and diabetes incidence: A dose-response meta-analysis of observational studies. Am. J. Clin. Nutr. 2016, 103, 1111–1124. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.; Xun, P.; Wan, Y.; He, K.; Cai, W. Long-term association between dairy consumption and risk of childhood obesity: A systematic review and meta-analysis of prospective cohort studies. Eur. J. Clin. Nutr. 2016, 70, 414–423. [Google Scholar] [CrossRef] [PubMed]
- Crichton, G.E.; Alkerwi, A. Whole-fat dairy food intake is inversely associated with obesity prevalence: Findings from the observation of cardiovascular risk factors in Luxembourg study. Nutr. Res. 2014, 34, 936–943. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.; Cho, W. The consumption of dairy products is associated with reduced risks of obesity and metabolic syndrome in Korean women but not in men. Nutrients 2017, 9, 630. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.C.; Szeto, I.; Chen, L.; Han, S.; Li, Y.; van Hekezen, R.; Qin, L. Dairy products consumption and metabolic syndrome in adults: Systematic review and meta-analysis of observational studies. Sci. Rep. 2015, 5, 14606. [Google Scholar] [CrossRef] [PubMed]
- Liu, A.G.; Ford, N.A.; Hu, F.B.; Zelman, K.M.; Mozaffarian, D.; Kris-Etherton, P.M. A healthy approach to dietary fats: Understanding the science and taking action to reduce consumer confusion. Nutr. J. 2017, 16, 53. [Google Scholar] [CrossRef] [PubMed]
- Rousseau, S. The celebrity quick-fix. Food Cult. Soc. 2015, 18, 265–287. [Google Scholar] [CrossRef]
- United States Department of Health and Human Services. Dietary Guidelines for Americans 2015–2020; Skyhorse Publishing Inc.: New York, NY, USA, 2017.
- Health Service Executive Ireland. Healthy Food for Life Guidelines. Available online: http://www.hse.ie/eng/about/Who/healthwellbeing/Our-Priority-Programmes/HEAL/Healthy-Eating-Guidelines/ (accessed on 17 January 2018).
- Weaver, C.M. How sound is the science behind the dietary recommendations for dairy? Am. J. Clin. Nutr. 2014, 99, 1217S–1222S. [Google Scholar] [CrossRef] [PubMed]
- Bantle, J.P.; Wylie-Rosett, J.; Albright, A.L.; Apovian, C.M.; Clark, N.G.; Franz, M.J.; Hoogwerf, B.J.; Lichtenstein, A.H.; Mayer-Davis, E.; Mooradian, A.D. Nutrition recommendations and interventions for diabetes: A position statement of the American Diabetes Association. Diabetes Care 2008, 31, S61–S78. [Google Scholar] [PubMed]
- Benatar, J.R. Chapter 20—Does dairy food have effects on cardiovascular disease and cardiometabolic risk? A2—Watson, ronald ross. In Dairy in Human Health and Disease Across the Lifespan; Collier, R.J., Preedy, V.R., Eds.; Academic Press: Cambridge, MA, USA, 2017; pp. 263–271. [Google Scholar]
- Harvard School of Public Health. Healthy Eating Plate and Healthy Eating Pyramid. Available online: https://www.hsph.harvard.edu/nutritionsource/healthy-eating-plate/ (accessed on 21 January 2018).
- National Health and Medical Research Council. Australian Dietary Guidelines Ageing; Department of Health and Ageing, Ed.; National Health and Medical Research Council Canberra: Canberra, Australia, 2013.
- Markey, O.; Vasilopoulou, D.; Givens, D.I.; Lovegrove, J.A. Dairy and cardiovascular health: Friend or foe? Nutr. Bull. 2014, 39, 161–171. [Google Scholar] [CrossRef] [PubMed]
- Wade, A.T.; Davis, C.R.; Dyer, K.A.; Hodgson, J.M.; Woodman, R.J.; Keage, H.A.; Murphy, K.J. A mediterranean diet to improve cardiovascular and cognitive health: Protocol for a randomised controlled intervention study. Nutrients 2017, 9, 145. [Google Scholar] [CrossRef] [PubMed]
- Van Ballegooijen, A.J.; Beulens, J.W. The role of vitamin K status in cardiovascular health: Evidence from observational and clinical studies. Curr. Nutr. Rep. 2017, 6, 197–205. [Google Scholar] [CrossRef] [PubMed]
- Heaney, R.P. Calcium, dairy products and osteoporosis. J. Am. Coll. Nutr. 2000, 19, 83S–99S. [Google Scholar] [CrossRef] [PubMed]
- Edem, D.O. Palm oil: Biochemical, physiological, nutritional, hematological and toxicological aspects: A review. Plant Foods Hum. Nutr. 2002, 57, 319–341. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, S.; Mitra, A. Health effects of palm oil. J. Hum. Ecol. 2009, 26, 197–203. [Google Scholar] [CrossRef]
- Pehowich, D.J.; Gomes, A.V.; Barnes, J.A. Fatty acid composition and possible health effects of coconut constituents. West Indian Med. J. 2000, 49, 128–133. [Google Scholar] [PubMed]
- Visioli, F.; Strata, A. Milk, dairy products, and their functional effects in humans: A narrative review of recent evidence. Adv. Nutr. 2014, 5, 131–143. [Google Scholar] [CrossRef] [PubMed]
- Hooper, L.; Summerbell, C.D.; Thompson, R.; Sills, D.; Roberts, F.G.; Moore, H.; Smith, G.D. Reduced or modified dietary fat for preventing cardiovascular disease. Cochrane Database Syst. Rev. 2011. [Google Scholar] [CrossRef] [Green Version]
- Micha, R.; Mozaffarian, D. Saturated fat and cardiometabolic risk factors, coronary heart disease, stroke, and diabetes: A fresh look at the evidence. Lipids 2010, 45, 893–905. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jakobsen, M.U.; O’Reilly, E.J.; Heitmann, B.L.; Pereira, M.A.; Bälter, K.; Fraser, G.E.; Goldbourt, U.; Hallmans, G.; Knekt, P.; Liu, S. Major types of dietary fat and risk of coronary heart disease: A pooled analysis of 11 cohort studies. Am. J. Clin. Nutr. 2009, 89, 1425–1432. [Google Scholar] [CrossRef] [PubMed]
- Nettleton, J.A.; Brouwer, I.A.; Geleijnse, J.M.; Hornstra, G. Saturated fat consumption and risk of coronary heart disease and ischemic stroke: A science update. Ann. Nutr. Metab. 2017, 70, 26–33. [Google Scholar] [CrossRef] [PubMed]
- Association, A.H. Dietary Fat and Its Relation to Heart Attacks and Strokes; Report of the Committee for Medical and Community Program of the American Heart Association; American Heart Association: Dallas, TX, USA, 1961; pp. 133–136. [Google Scholar]
- Kannel, W.B.; Dawber, T.R.; Kagan, A.; Revotskie, N.; Stokes, J. Factors of risk in the development of coronary heart disease—Six-year follow-up experiencethe Framingham study. Ann. Intern. Med. 1961, 55, 33–50. [Google Scholar] [CrossRef] [PubMed]
- La Berge, A.F. How the ideology of low fat conquered America. J. Hist. Med. Allied Sci. 2008, 63, 139–177. [Google Scholar] [CrossRef] [PubMed]
- Siri-Tarino, P.W.; Sun, Q.; Hu, F.B.; Krauss, R.M. Meta-analysis of prospective cohort studies evaluating the association of saturated fat with cardiovascular disease. Am. J. Clin. Nutr. 2010, 91, 535–546. [Google Scholar] [CrossRef] [PubMed]
- Legrand, P.; Rioux, V. The complex and important cellular and metabolic functions of saturated fatty acids. Lipids 2010, 45, 941–946. [Google Scholar] [CrossRef] [PubMed]
- Kubow, S. The influence of positional distribution of fatty acids in native, interesterified and structure-specific lipids on lipoprotein metabolism and atherogenesis. J. Nutr. Biochem. 1996, 7, 530–541. [Google Scholar] [CrossRef]
- Astrup, A.; Dyerberg, J.; Elwood, P.; Hermansen, K.; Hu, F.B.; Jakobsen, M.U.; Kok, F.J.; Krauss, R.M.; Lecerf, J.M.; LeGrand, P. The role of reducing intakes of saturated fat in the prevention of cardiovascular disease: Where does the evidence stand in 2010? Am. J. Clin. Nutr. 2011, 93, 684–688. [Google Scholar] [CrossRef] [PubMed]
- Lordan, R.; Tsoupras, A.; Zabetakis, I. Phospholipids of animal and marine origin: Structure, function, and anti-inflammatory properties. Molecules 2017, 22, 1964. [Google Scholar] [CrossRef] [PubMed]
- Walz, C.P.; Barry, A.R.; Koshman, S.L. Omega-3 polyunsaturated fatty acid supplementation in the prevention of cardiovascular disease. Can. Pharm. J. 2016, 149, 166–173. [Google Scholar] [CrossRef] [PubMed]
- Marventano, S.; Kolacz, P.; Castellano, S.; Galvano, F.; Buscemi, S.; Mistretta, A.; Grosso, G. A review of recent evidence in human studies of n-3 and n-6 PUFA intake on cardiovascular disease, cancer, and depressive disorders: Does the ratio really matter? Int. J. Food Sci. Nutr. 2015, 66, 611–622. [Google Scholar] [CrossRef] [PubMed]
- Nettleton, J.A.; Legrand, P.; Mensink, R.P. Issfal 2014 debate: It is time to update saturated fat recommendations. Ann. Nutr. Metab. 2015, 66, 104–108. [Google Scholar] [CrossRef] [PubMed]
- Livingstone, K.M.; Lovegrove, J.A.; Givens, D.I. The impact of substituting SFA in dairy products with MUFA or PUFA on CVD risk: Evidence from human intervention studies. Nutr. Res. Rev. 2012, 25, 193–206. [Google Scholar] [CrossRef] [PubMed]
- Markey, O.; Souroullas, K.; Fagan, C.C.; Kliem, K.E.; Vasilopoulou, D.; Jackson, K.G.; Humphries, D.J.; Grandison, A.S.; Givens, D.I.; Lovegrove, J.A.; et al. Consumer acceptance of dairy products with a saturated fatty acid–reduced, monounsaturated fatty acid–enriched content. J. Dairy Sci. 2017, 100, 7953–7966. [Google Scholar] [CrossRef] [PubMed]
- Bayat, A.R.; Tapio, I.; Vilkki, J.; Shingfield, K.J.; Leskinen, H. Plant oil supplements reduce methane emissions and improve milk fatty acid composition in dairy cows fed grass silage-based diets without affecting milk yield. J. Dairy Sci. 2018, 101, 1136–1151. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, M.L.; West, K.L. Mechanisms by which dietary fatty acids modulate plasma lipids. J. Nutr. 2005, 135, 2075–2078. [Google Scholar] [CrossRef] [PubMed]
- Mensink, R.P.; Zock, P.L.; Kester, A.D.; Katan, M.B. Effects of dietary fatty acids and carbohydrates on the ratio of serum total to HDL cholesterol and on serum lipids and apolipoproteins: A meta-analysis of 60 controlled trials. Am. J. Clin. Nutr. 2003, 77, 1146–1155. [Google Scholar] [CrossRef] [PubMed]
- German, J.B.; Dillard, C.J. Saturated fats: What dietary intake? Am. J. Clin. Nutr. 2004, 80, 550–559. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, G.D. Dietary fats and health: Dietary recommendations in the context of scientific evidence. Adv. Nutr. 2013, 4, 294–302. [Google Scholar] [CrossRef] [PubMed]
- Skeaff, C.M.; Miller, J. Dietary fat and coronary heart disease: Summary of evidence from prospective cohort and randomised controlled trials. Ann. Nutr. Metab. 2009, 55, 173–201. [Google Scholar] [CrossRef] [PubMed]
- Dehghan, M.; Mente, A.; Zhang, X.; Swaminathan, S.; Li, W.; Mohan, V.; Iqbal, R.; Kumar, R.; Wentzel-Viljoen, E.; Rosengren, A.; et al. Associations of fats and carbohydrate intake with cardiovascular disease and mortality in 18 countries from five continents (pure): A prospective cohort study. Lancet 2017, 390, 2050–2062. [Google Scholar] [CrossRef]
- Fernandez, M.L.; Calle, M. Revisiting dietary cholesterol recommendations: Does the evidence support a limit of 300 mg/d? Curr. Atheroscler. Rep. 2010, 12, 377–383. [Google Scholar] [CrossRef] [PubMed]
- Rong, Y.; Chen, L.; Zhu, T.; Song, Y.; Yu, M.; Shan, Z.; Sands, A.; Hu, F.B.; Liu, L. Egg consumption and risk of coronary heart disease and stroke: Dose-response meta-analysis of prospective cohort studies. BMJ 2013, 346, e8539. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krauss, R.M.; Eckel, R.H.; Howard, B.; Appel, L.J.; Daniels, S.R.; Deckelbaum, R.J.; Erdman, J.W.; Kris-Etherton, P.; Goldberg, I.J.; Kotchen, T.A.; et al. Aha dietary guidelines. Revision 2000: A Statement for Healthcare professionals From the Nutrition Committee of the American Heart Association. Circulation 2000, 102, 2284–2299. [Google Scholar] [CrossRef] [PubMed]
- Genest, J.; McPherson, R.; Frohlich, J.; Anderson, T.; Campbell, N.; Carpentier, A.; Couture, P.; Dufour, R.; Fodor, G.; Francis, G.A.; et al. 2009 canadian cardiovascular society/canadian guidelines for the diagnosis and treatment of dyslipidemia and prevention of cardiovascular disease in the adult—2009 recommendations. Can. J. Cardiol. 2009, 25, 567–579. [Google Scholar] [CrossRef]
- Public Health England. The Eatwell Guide—Government Dietary Recommendations: Government Recommendations for Energy and Nutrients for Males and Females Aged 1–18 Years and 19+ Years; Public Health England: London, UK, 2016.
- Jang, Y.-A.; Lee, H.-S.; Kim, B.-H.; Lee, Y.-N.; Lee, H.-J.; Moon, J.-J.; Kim, C.-I. Revised dietary guidelines for Koreans. Asia Pac. J. Clin. Nutr. 2008, 17, 55–58. [Google Scholar] [PubMed]
- Ministry of Health. Food and Nutrition Guidelines for Healthy Adults: A Background Paper; Ministry of Health: Wellington, New Zealand, 2003.
- McNamara, D.J. Cholesterol intake and plasma cholesterol: An update. J. Am. Coll. Nutr. 1997, 16, 530–534. [Google Scholar]
- Hu, F.B.; Stampfer, M.J.; Rimm, E.B.; Manson, J.E.; Ascherio, A.; Colditz, G.A.; Rosner, B.A.; Spiegelman, D.; Speizer, F.E.; Sacks, F.M.; et al. A prospective study of egg consumption and risk of cardiovascular disease in men and women. JAMA 1999, 281, 1387–1394. [Google Scholar] [CrossRef] [PubMed]
- Esrey, K.L.; Joseph, L.; Grover, S.A. Relationship between dietary intake and coronary heart disease mortality: Lipid research clinics prevalence follow-up study. J. Clin. Epidemiol. 1996, 49, 211–216. [Google Scholar] [CrossRef]
- Herron, K.L.; Vega-Lopez, S.; Conde, K.; Ramjiganesh, T.; Roy, S.; Shachter, N.S.; Fernandez, M.L. Pre-menopausal women, classified as hypo- or hyper-responders, do not alter their LDL/HDL ratio following a high dietary cholesterol challenge. J. Am. Coll. Nutr. 2002, 21, 250–258. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, M.L.; Webb, D. The LDL to HDL cholesterol ratio as a valuable tool to evaluate coronary heart disease risk. J. Am. Coll. Nutr. 2008, 27, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Blesso, C. Egg phospholipids and cardiovascular health. Nutrients 2015, 7, 2731. [Google Scholar] [CrossRef] [PubMed]
- Mann, G.V.; Spoerry, A. Studies of a surfactant and cholesteremia in the Maasai. Am. J. Clin. Nutr. 1974, 27, 464–469. [Google Scholar] [CrossRef] [PubMed]
- Steinmetz, K.A.; Childs, M.T.; Stimson, C.; Kushi, L.H.; McGovern, P.G.; Potter, J.D.; Yamanaka, W.K. Effect of consumption of whole milk and skim milk on blood lipid profiles in healthy men. Am. J. Clin. Nutr. 1994, 59, 612–618. [Google Scholar] [CrossRef] [PubMed]
- Sharpe, S.J.; Gamble, G.D.; Sharpe, D.N. Cholesterol-lowering and blood pressure effects of immune milk. Am. J. Clin. Nutr. 1994, 59, 929–934. [Google Scholar] [CrossRef] [PubMed]
- Buonopane, G.J.; Kilara, A.; Smith, J.S.; McCarthy, R.D. Effect of skim milk supplementation on blood cholesterol concentration, blood pressure, and triglycerides in a free-living human population. J. Am. Coll. Nutr. 1992, 11, 56–67. [Google Scholar] [CrossRef] [PubMed]
- St-Onge, M.-P.; Farnworth, E.R.; Jones, P.J.H. Consumption of fermented and nonfermented dairy products: Effects on cholesterol concentrations and metabolism. Am. J. Clin. Nutr. 2000, 71, 674–681. [Google Scholar] [CrossRef] [PubMed]
- Tholstrup, T.; Høy, C.-E.; Andersen, L.N.; Christensen, R.D.K.; Sandström, B. Does fat in milk, butter and cheese affect blood lipids and cholesterol differently? J. Am. Coll. Nutr. 2004, 23, 169–176. [Google Scholar] [CrossRef] [PubMed]
- Biong, A.S.; Muller, H.; Seljeflot, I.; Veierod, M.B.; Pedersen, J.I. A comparison of the effects of cheese and butter on serum lipids, haemostatic variables and homocysteine. Br. J. Nutr. 2004, 92, 791–797. [Google Scholar] [CrossRef] [PubMed]
- Nestel, P.J.; Chronopulos, A.; Cehun, M. Dairy fat in cheese raises LDL cholesterol less than that in butter in mildly hypercholesterolaemic subjects. Eur. J. Clin. Nutr. 2005, 59, 1059–1063. [Google Scholar] [CrossRef] [PubMed]
- Hjerpsted, J.; Leedo, E.; Tholstrup, T. Cheese intake in large amounts lowers LDL-cholesterol concentrations compared with butter intake of equal fat content. Am. J. Clin. Nutr. 2011, 94, 1479–1484. [Google Scholar] [CrossRef] [PubMed]
- Brassard, D.; Tessier-Grenier, M.; Allaire, J.; Rajendiran, E.; She, Y.; Ramprasath, V.; Gigleux, I.; Talbot, D.; Levy, E.; Tremblay, A. Comparison of the impact of SFAs from cheese and butter on cardiometabolic risk factors: A randomized controlled trial. Am. J. Clin. Nutr. 2017, 105, 800–809. [Google Scholar] [CrossRef] [PubMed]
- Lorenzen, J.K.; Astrup, A. Dairy calcium intake modifies responsiveness of fat metabolism and blood lipids to a high-fat diet. Br. J. Nutr. 2011, 105, 1823–1831. [Google Scholar] [CrossRef] [PubMed]
- Givens, D.I. Saturated fats, dairy foods and health: A curious paradox? Nutr. Bull. 2017, 42, 274–282. [Google Scholar] [CrossRef]
- Hjerpsted, J.B.; Dragsted, L.O.; Tholstrup, T. Cheese intake lowers plasma cholesterol concentrations without increasing bile acid excretion. J. Nutr. Intermed. Metab. 2016, 3, 12–17. [Google Scholar] [CrossRef]
- Walsh, B. The truth about fat. Time Magazine, 12 June 2014; 28–35. [Google Scholar]
- Matthan, N.R.; Welty, F.K.; Barrett, P.H.R.; Harausz, C.; Dolnikowski, G.G.; Parks, J.S.; Eckel, R.H.; Schaefer, E.J.; Lichtenstein, A.H. Dietary hydrogenated fat increases high-density lipoprotein apoA-I catabolism and decreases low-density lipoprotein apoB-100 catabolism in hypercholesterolemic women. Arterioscler. Thromb. Vasc. Biol. 2004, 24, 1092–1097. [Google Scholar] [CrossRef] [PubMed]
- Zock, P.L.; Katan, M.B. Butter, margarine and serum lipoproteins. Atherosclerosis 1997, 131, 7–16. [Google Scholar] [CrossRef]
- Pimpin, L.; Wu, J.H.; Haskelberg, H.; Del Gobbo, L.; Mozaffarian, D. Is butter back? A systematic review and meta-analysis of butter consumption and risk of cardiovascular disease, diabetes, and total mortality. PLoS ONE 2016, 11, e0158118. [Google Scholar] [CrossRef] [PubMed]
- United States Senate Select Committee on Nutrition and Human Needs. Dietary Goals for the United States, Supplemental Views; US Goverment Printing Office: Washington, DC, USA, 1977.
- Reedy, J. How the US Low-Fat Diet Recommendations of 1977 Contributed to the Declining Health of Americans. Ph.D. Thesis, University of Connecticut, Storrs, CT, USA, 2016. [Google Scholar]
- Ludwig, D.S. Lowering the bar on the low-fat diet. JAMA 2016, 316, 2087–2088. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Fox, C.S.; Troy, L.M.; Mckeown, N.M.; Jacques, P.F. Longitudinal association of dairy consumption with the changes in blood pressure and the risk of incident hypertension: The framingham heart study. Br. J. Nutr. 2015, 114, 1887–1899. [Google Scholar] [CrossRef] [PubMed]
- Ralston, R.A.; Lee, J.H.; Truby, H.; Palermo, C.E.; Walker, K.Z. A systematic review and meta-analysis of elevated blood pressure and consumption of dairy foods. J. Hum. Hypertens. 2012, 26, 3–13. [Google Scholar] [CrossRef] [PubMed]
- Soedamah-Muthu, S.S.; Verberne, L.D.M.; Ding, E.L.; Engberink, M.F.; Geleijnse, J.M. Dairy consumption and incidence of hypertension: A dose-response meta-analysis of prospective cohort studies. Hypertension 2012, 71. [Google Scholar] [CrossRef] [PubMed]
- Drouin-Chartier, J.-P.; Brassard, D.; Tessier-Grenier, M.; Côté, J.A.; Labonté, M.-È.; Desroches, S.; Couture, P.; Lamarche, B. Systematic review of the association between dairy product consumption and risk of cardiovascular-related clinical outcomes. Adv. Nutr. 2016, 7, 1026–1040. [Google Scholar] [CrossRef] [PubMed]
- Appel, L.J.; Brands, M.W.; Daniels, S.R.; Karanja, N.; Elmer, P.J.; Sacks, F.M. Dietary approaches to prevent and treat hypertension: A Scientific Statement From the American Heart Association. Hypertension 2006, 47, 296–308. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, M.S.; Rudkowska, I. Dairy products on metabolic health: Current research and clinical implications. Maturitas 2014, 77, 221–228. [Google Scholar] [CrossRef] [PubMed]
- Alonso, A.; Zozaya, C.; Vázquez, Z.; Alfredo Martínez, J.; Martínez-González, M.A. The effect of low-fat versus whole-fat dairy product intake on blood pressure and weight in young normotensive adults. J. Hum. Nutr. Diet. 2009, 22, 336–342. [Google Scholar] [CrossRef] [PubMed]
- Spence, L.A.; Cifelli, C.J.; Miller, G.D. The role of dairy products in healthy weight and body composition in children and adolescents. Curr. Nutr. Food Sci. 2011, 7, 40–49. [Google Scholar] [CrossRef] [PubMed]
- Vanderhout, S.M.; Birken, C.S.; Parkin, P.C.; Lebovic, G.; Chen, Y.; O’Connor, D.L.; Maguire, J.L.; Collaboration, T.T.K. Relation between milk-fat percentage, vitamin D, and BMI z score in early childhood. Am. J. Clin. Nutr. 2016, 104, 1657–1664. [Google Scholar] [CrossRef] [PubMed]
- García Yu, I.A.-L.; Sánchez-Aguadero, N.; Recio-Rodríguez, J.I. Chapter 25—Effect of the fat component of dairy products in cardiovascular health, vascular structure and function a2—Watson, ronald ross. In Nutrients in Dairy and Their Implications on Health and Disease; Collier, R.J., Preedy, V.R., Eds.; Academic Press: Cambridge, MA, USA, 2017; pp. 325–332. [Google Scholar]
- Drehmer, M.; Pereira, M.A.; Schmidt, M.I.; Alvim, S.; Lotufo, P.A.; Luft, V.C.; Duncan, B.B. Total and full-fat, but not low-fat, dairy product intakes are inversely associated with metabolic syndrome in adults. J. Nutr. 2015, 146, 81–89. [Google Scholar] [CrossRef] [PubMed]
- Eussen, S.J.P.M.; van Dongen, M.C.J.M.; Wijckmans, N.; den Biggelaar, L.; Oude Elferink, S.J.W.H.; Singh-Povel, C.M.; Schram, M.T.; Sep, S.J.S.; van der Kallen, C.J.; Koster, A.; et al. Consumption of dairy foods in relation to impaired glucose metabolism and type 2 diabetes mellitus: The Maastricht study. Br. J. Nutr. 2016, 115, 1453–1461. [Google Scholar] [CrossRef] [PubMed]
- Soedamah-Muthu, S.S.; Ding, E.L.; Al-Delaimy, W.K.; Hu, F.B.; Engberink, M.F.; Willett, W.C.; Geleijnse, J.M. Milk and dairy consumption and incidence of cardiovascular diseases and all-cause mortality: Dose-response meta-analysis of prospective cohort studies. Am. J. Clin. Nutr. 2011, 93, 158–171. [Google Scholar] [CrossRef] [PubMed]
- Tremblay, A.; Gilbert, J.-A. Milk products, insulin resistance syndrome and type 2 diabetes. J. Am. Coll. Nutr. 2009, 28, 91S–102S. [Google Scholar] [CrossRef] [PubMed]
- Thorning, T.K.; Bertram, H.C.; Bonjour, J.-P.; De Groot, L.; Dupont, D.; Feeney, E.; Ipsen, R.; Lecerf, J.M.; Mackie, A.; McKinley, M.C. Whole dairy matrix or single nutrients in assessment of health effects: Current evidence and knowledge gaps. Am. J. Clin. Nutr. 2017, 105, 1033–1045. [Google Scholar] [CrossRef] [PubMed]
- Guarner, V.; Rubio-Ruiz, M.E. Low-grade systemic inflammation connects aging, metabolic syndrome and cardiovascular disease. In Aging and Health—A Systems Biology Perspective; Karger Publishers: Basel, Switzerland, 2014; Volume 40, pp. 99–106. [Google Scholar]
- Vishnu, A.; Gurka, M.J.; DeBoer, M.D. The severity of the metabolic syndrome increases over time within individuals, independent of baseline metabolic syndrome status and medication use: The atherosclerosis risk in communities study. Atherosclerosis 2015, 243, 278–285. [Google Scholar] [CrossRef] [PubMed]
- Givens, D.I.; Livingstone, K.M.; Pickering, J.E.; Fekete, Á.A.; Dougkas, A.; Elwood, P.C. Milk: White elixir or white poison? An examination of the associations between dairy consumption and disease in human subjects. Anim. Front. 2014, 4, 8–15. [Google Scholar] [CrossRef]
- Laurent, S.; Boutouyrie, P.; Asmar, R.; Gautier, I.; Laloux, B.; Guize, L.; Ducimetiere, P.; Benetos, A. Aortic stiffness is an independent predictor of all-cause and cardiovascular mortality in hypertensive patients. Hypertension 2001, 37, 1236–1241. [Google Scholar] [CrossRef] [PubMed]
- Livingstone, K.M.; Lovegrove, J.A.; Cockcroft, J.R.; Elwood, P.C.; Pickering, J.E.; Givens, D.I. Does dairy food intake predict arterial stiffness and blood pressure in men? Evidence from the caerphilly prospective study. Hypertension 2013, 61, 42–47. [Google Scholar] [CrossRef] [PubMed]
- Appel, L.J.; Moore, T.J.; Obarzanek, E.; Vollmer, W.M.; Svetkey, L.P.; Sacks, F.M.; Bray, G.A.; Vogt, T.M.; Cutler, J.A.; Windhauser, M.M.; et al. A clinical trial of the effects of dietary patterns on blood pressure. Dash collaborative research group. N. Engl. J. Med. 1997, 336, 1117–1124. [Google Scholar] [CrossRef] [PubMed]
- Van Meijl, L.E.; Mensink, R.P. Low-fat dairy consumption reduces systolic blood pressure, but does not improve other metabolic risk parameters in overweight and obese subjects. Nutr. Metab. Cardiovasc. Dis. 2011, 21, 355–361. [Google Scholar] [CrossRef] [PubMed]
- Buendia, J.R.; Li, Y.; Hu, F.B.; Cabral, H.J.; Bradlee, M.L.; Quatromoni, P.A.; Singer, M.R.; Curhan, G.C.; Moore, L.L. Regular yogurt intake and risk of cardiovascular disease among hypertensive adults. Am. J. Hypertens. 2018. [Google Scholar] [CrossRef] [PubMed]
- Gholami, F.; Khoramdad, M.; Esmailnasab, N.; Moradi, G.; Nouri, B.; Safiri, S.; Alimohamadi, Y. The effect of dairy consumption on the prevention of cardiovascular diseases: A meta-analysis of prospective studies. J. Cardiovasc. Thorac. Res. 2017, 9, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Beltrán-Barrientos, L.M.; Hernández-Mendoza, A.; Torres-Llanez, M.J.; González-Córdova, A.F.; Vallejo-Córdoba, B. Invited review: Fermented milk as antihypertensive functional food. J. Dairy Sci. 2016, 99, 4099–4110. [Google Scholar] [CrossRef] [PubMed]
- O’Keeffe, M.B.; FitzGerald, R.J. Whey protein hydrolysate induced modulation of endothelial cell gene expression. J. Funct. Foods 2018, 40, 102–109. [Google Scholar] [CrossRef]
- Morio, B.; Fardet, A.; Legrand, P.; Lecerf, J.-M. Involvement of dietary saturated fats, from all sources or of dairy origin only, in insulin resistance and type 2 diabetes. Nutr. Rev. 2016, 74, 33–47. [Google Scholar] [CrossRef] [PubMed]
- Elwood, P.C.; Pickering, J.E.; Fehily, A.M. Milk and dairy consumption, diabetes and the metabolic syndrome: The caerphilly prospective study. J. Epidemiol. Community Health 2007, 61, 695–698. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.K.; Willett, W.C.; Stampfer, M.J.; Rimm, E.; Hu, F.B. Dairy consumption and risk of type 2 diabetes mellitus in men: A prospective study. Arch. Intern. Med. 2005, 165, 997–1003. [Google Scholar] [CrossRef] [PubMed]
- Crichton, G.E.; Bryan, J.; Buckley, J.; Murphy, K.J. Dairy consumption and metabolic syndrome: A systematic review of findings and methodological issues. Obes. Rev. 2011, 12, e190–e201. [Google Scholar] [CrossRef] [PubMed]
- Fumeron, F.; Lamri, A.; Abi Khalil, C.; Jaziri, R.; Porchay-Baldérelli, I.; Lantieri, O.; Vol, S.; Balkau, B.; Marre, M.; The Data from the Epidemiological Study on the Insulin Resistance Syndrome Study Group. Dairy consumption and the incidence of hyperglycemia and the metabolic syndrome: Results from a french prospective study, data from the epidemiological study on the insulin resistance syndrome (DESIR). Diabetes Care 2011, 34, 813–817. [Google Scholar] [CrossRef] [PubMed]
- Benatar, J.R.; Sidhu, K.; Stewart, R.A.H. Effects of high and low fat dairy food on cardio-metabolic risk factors: A meta-analysis of randomized studies. PLoS ONE 2013, 8, e76480. [Google Scholar] [CrossRef] [PubMed]
- Tong, X.; Dong, J.Y.; Wu, Z.W.; Li, W.; Qin, L.Q. Dairy consumption and risk of type 2 diabetes mellitus: A meta-analysis of cohort studies. Eur. J. Clin. Nutr. 2011, 65, 1027–1031. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Sun, Q.; Giovannucci, E.; Mozaffarian, D.; Manson, J.E.; Willett, W.C.; Hu, F.B. Dairy consumption and risk of type 2 diabetes: 3 cohorts of us adults and an updated meta-analysis. BMC Med. 2014, 12, 215. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, D.; Ning, N.; Wang, C.; Wang, Y.; Li, Q.; Meng, Z.; Liu, Y.; Li, Q. Dairy products consumption and risk of type 2 diabetes: Systematic review and dose-response meta-analysis. PLoS ONE 2013, 8, e73965. [Google Scholar] [CrossRef] [PubMed]
- Aune, D.; Norat, T.; Romundstad, P.; Vatten, L.J. Dairy products and the risk of type 2 diabetes: A systematic review and dose-response meta-analysis of cohort studies. Am. J. Clin. Nutr. 2013, 98, 1066–1083. [Google Scholar] [CrossRef] [PubMed]
- Bergholdt, H.K.M.; Nordestgaard, B.G.; Ellervik, C. Milk intake is not associated with low risk of diabetes or overweight-obesity: A mendelian randomization study in 97,811 Danish individuals. Am. J. Clin. Nutr. 2015, 102, 487–496. [Google Scholar] [CrossRef] [PubMed]
- Sluijs, I.; Forouhi, N.G.; Beulens, J.W.J.; van der Schouw, Y.T.; Agnoli, C.; Arriola, L.; Balkau, B.; Barricarte, A.; Boeing, H.; Bueno-de-Mesquita, H.B. The amount and type of dairy product intake and incident type 2 diabetes: Results from the epic-interact study. Am. J. Clin. Nutr. 2012, 96, 382–390. [Google Scholar] [PubMed]
- Clifton, P. Chapter 32—The influence of dairy consumption on the risk of type 2 diabetes, metabolic syndrome, and impaired glucose tolerance or insulin resistance: A review of cohort and intervention studies a2—Watson, ronald ross. In Dairy in Human Health and Disease Across the Lifespan; Collier, R.J., Preedy, V.R., Eds.; Academic Press: Cambridge, MA, USA, 2017; pp. 411–422. [Google Scholar]
- Parodi, P.W. Cooperative action of bioactive components in milk fat with ppars may explain its anti-diabetogenic properties. Med. Hypotheses 2016, 89, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Keast, D.; Hill Gallant, K.; Albertson, A.; Gugger, C.; Holschuh, N. Associations between yogurt, dairy, calcium, and vitamin D intake and obesity among U.S. Children aged 8–18 years: NHANES, 2005–2008. Nutrients 2015, 7, 1577. [Google Scholar] [CrossRef] [PubMed]
- Walsh, B.; Cullinan, J. Decomposing socioeconomic inequalities in childhood obesity: Evidence from Ireland. Econ. Hum. Biol. 2015, 16, 60–72. [Google Scholar] [CrossRef] [PubMed]
- Moore, L.L.; Singer, M.R.; Qureshi, M.M.; Bradlee, M.L. Dairy intake and anthropometric measures of body fat among children and adolescents in NHANES. J. Am. Coll. Nutr. 2008, 27, 702–710. [Google Scholar] [CrossRef] [PubMed]
- Bradlee, M.L.; Singer, M.R.; Qureshi, M.M.; Moore, L.L. Food group intake and central obesity among children and adolescents in the third national health and nutrition examination survey (NHANES III). Public Health Nutr. 2009, 13, 797–805. [Google Scholar] [CrossRef] [PubMed]
- Wiley, A.S. Dairy and milk consumption and child growth: Is BMI involved? An analysis of NHANES 1999–2004. Am. J. Hum. Biol. 2010, 22, 517–525. [Google Scholar] [CrossRef] [PubMed]
- Sayon-Orea, C.; Martínez-González, M.A.; Ruiz-Canela, M.; Bes-Rastrollo, M. Associations between yogurt consumption and weight gain and risk of obesity and metabolic syndrome: A systematic review. Adv. Nutr. 2017, 8, 146S–154S. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, M.S.; Rudkowska, I. Dairy nutrients and their effect on inflammatory profile in molecular studies. Mol. Nutr. Food Res. 2015, 59, 1249–1263. [Google Scholar] [CrossRef] [PubMed]
- Palur Ramakrishnan, A.V.K.; Varghese, T.P.; Vanapalli, S.; Nair, N.K.; Mingate, M.D. Platelet activating factor: A potential biomarker in acute coronary syndrome? Cardiovasc. Ther. 2017, 35, 64–70. [Google Scholar] [CrossRef] [PubMed]
- Castro Faria Neto, H.C.; Stafforini, D.M.; Prescott, S.M.; Zimmerman, G.A. Regulating inflammation through the anti-inflammatory enzyme platelet-activating factor-acetylhydrolase. Mem. Inst. Oswaldo Cruz 2005, 100, 83–91. [Google Scholar] [CrossRef] [PubMed]
- Lordan, R.; Zabetakis, I. Ovine and caprine lipids promoting cardiovascular health in milk and its derivatives. Adv. Dairy Res 2017, 5. [Google Scholar] [CrossRef]
- Antonopoulou, S.; Semidalas, C.E.; Koussissis, S.; Demopoulos, C.A. Platelet-activating factor (PAF) antagonists in foods: A study of lipids with PAF or anti-PAF-like activity in cow’s milk and yogurt. J. Agric. Food Chem. 1996, 44, 3047–3051. [Google Scholar] [CrossRef]
- Poutzalis, S.; Anastasiadou, A.; Nasopoulou, C.; Megalemou, K.; Sioriki, E.; Zabetakis, I. Evaluation of the in vitro anti-atherogenic activities of goat milk and goat dairy products. Dairy Sci. Technol. 2016, 96, 317–327. [Google Scholar] [CrossRef]
- Tsorotioti, S.E.; Nasopoulou, C.; Detopoulou, M.; Sioriki, E.; Demopoulos, C.A.; Zabetakis, I. In vitro anti-atherogenic properties of traditional Greek cheese lipid fractions. Dairy Sci. Technol. 2014, 94, 269–281. [Google Scholar] [CrossRef]
- Megalemou, K.; Sioriki, E.; Lordan, R.; Dermiki, M.; Nasopoulou, C.; Zabetakis, I. Evaluation of sensory and in vitro anti-thrombotic properties of traditional Greek yogurts derived from different types of milk. Heliyon 2017, 3, e00227. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, M.S.; Rudkowska, I. Chapter 22—Macro components in dairy and their effects on inflammation parameters: Preclinical studies a2—Watson, ronald ross. In Nutrients in Dairy and Their Implications on Health and Disease; Collier, R.J., Preedy, V.R., Eds.; Academic Press: Cambridge, MA, USA, 2017; pp. 287–302. [Google Scholar]
- Stancliffe, R.A.; Thorpe, T.; Zemel, M.B. Dairy attentuates oxidative and inflammatory stress in metabolic syndrome. Am. J. Clin. Nutr. 2011, 94, 422–430. [Google Scholar] [CrossRef] [PubMed]
- Thompson, W.G.; Holdman, N.R.; Janzow, D.J.; Slezak, J.M.; Morris, K.L.; Zemel, M.B. Effect of energy-reduced diets high in dairy products and fiber on weight loss in obese adults. Obes. Res. 2005, 13, 1344–1353. [Google Scholar] [CrossRef] [PubMed]
- Sofi, F.; Buccioni, A.; Cesari, F.; Gori, A.M.; Minieri, S.; Mannini, L.; Casini, A.; Gensini, G.F.; Abbate, R.; Antongiovanni, M. Effects of a dairy product (pecorino cheese) naturally rich in cis-9, trans-11 conjugated linoleic acid on lipid, inflammatory and haemorheological variables: A dietary intervention study. Nutr. Metab. Cardiovasc. Dis. 2010, 20, 117–124. [Google Scholar] [CrossRef] [PubMed]
- Rosado, J.L.; Garcia, O.P.; Ronquillo, D.; Hervert-Hernández, D.; Caamaño, M.D.C.; Martínez, G.; Gutiérrez, J.; García, S. Intake of milk with added micronutrients increases the effectiveness of an energy-restricted diet to reduce body weight: A randomized controlled clinical trial in Mexican women. J. Am. Diet. Assoc. 2011, 111, 1507–1516. [Google Scholar] [CrossRef] [PubMed]
- Nestel, P.J.; Pally, S.; MacIntosh, G.L.; Greeve, M.A.; Middleton, S.; Jowett, J.; Meikle, P.J. Circulating inflammatory and atherogenic biomarkers are not increased following single meals of dairy foods. Eur. J. Clin. Nutr. 2012, 66, 25–31. [Google Scholar] [CrossRef] [PubMed]
- Esmaillzadeh, A.; Azadbakht, L. Dairy consumption and circulating levels of inflammatory markers among Iranian women. Public Health Nutr. 2010, 13, 1395–1402. [Google Scholar] [CrossRef] [PubMed]
- Panagiotakos, D.B.; Pitsavos, C.H.; Zampelas, A.D.; Chrysohoou, C.A.; Stefanadis, C.I. Dairy products consumption is associated with decreased levels of inflammatory markers related to cardiovascular disease in apparently healthy adults: The ATTICA study. J. Am. Coll. Nutr. 2010, 29, 357–364. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Steffen, L.M.; Vessby, B.; Basu, S.; Steinberger, J.; Moran, A.; Jacobs, D.R.; Hong, C.-P.; Sinaiko, A.R. Obesity modifies the relations between serum markers of dairy fats and inflammation and oxidative stress among adolescents. Obesity 2011, 19, 2404–2410. [Google Scholar] [CrossRef] [PubMed]
- Gadotti, T.N.; Norde, M.M.; Rogero, M.M.; Fisberg, M.; Fisberg, R.M.; Oki, E.; Martini, L.A. Dairy consumption and inflammatory profile: A cross-sectional population-based study, São Paulo, Brazil. Nutrition 2017, 48, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Van Meijl, L.E.C.; Mensink, R.P. Effects of low-fat dairy consumption on markers of low-grade systemic inflammation and endothelial function in overweight and obese subjects: An intervention study. Br. J. Nutr. 2010, 104, 1523–1527. [Google Scholar] [CrossRef] [PubMed]
- Zemel, M.B.; Sun, X.; Sobhani, T.; Wilson, B. Effects of dairy compared with soy on oxidative and inflammatory stress in overweight and obese subjects. Am. J. Clin. Nutr. 2010, 91, 16–22. [Google Scholar] [CrossRef] [PubMed]
- Nestel, P.J.; Mellett, N.; Pally, S.; Wong, G.; Barlow, C.K.; Croft, K.; Mori, T.A.; Meikle, P.J. Effects of low-fat or full-fat fermented and non-fermented dairy foods on selected cardiovascular biomarkers in overweight adults. Br. J. Nutr. 2013, 110, 2242–2249. [Google Scholar] [CrossRef] [PubMed]
- Labonté, M.-È.; Cyr, A.; Abdullah, M.M.; Lépine, M.-C.; Vohl, M.-C.; Jones, P.; Couture, P.; Lamarche, B. Dairy product consumption has no impact on biomarkers of inflammation among men and women with low-grade systemic inflammation. J. Nutr. 2014, 144, 1760–1767. [Google Scholar] [CrossRef] [PubMed]
- Dugan, C.E.; Aguilar, D.; Park, Y.K.; Lee, J.Y.; Fernandez, M.L. Dairy consumption lowers systemic inflammation and liver enzymes in typically low-dairy consumers with clinical characteristics of metabolic syndrome. J. Am. Coll. Nutr. 2016, 35, 255–261. [Google Scholar] [CrossRef] [PubMed]
- Zemel, M.B.; Sun, X. Dietary calcium and dairy products modulate oxidative and inflammatory stress in mice and humans. J. Nutr. 2008, 138, 1047–1052. [Google Scholar] [CrossRef] [PubMed]
- De Aguilar-Nascimento, J.E.; Prado Silveira, B.R.; Dock-Nascimento, D.B. Early enteral nutrition with whey protein or casein in elderly patients with acute ischemic stroke: A double-blind randomized trial. Nutrition 2011, 27, 440–444. [Google Scholar] [CrossRef] [PubMed]
- Jones, K.W.; Eller, L.K.; Parnell, J.A.; Doyle-Baker, P.K.; Edwards, A.L.; Reimer, R.A. Effect of a dairy-and calcium-rich diet on weight loss and appetite during energy restriction in overweight and obese adults: A randomized trial. Eur. J. Clin. Nutr. 2013, 67, 371–376. [Google Scholar] [CrossRef] [PubMed]
- Pei, R.; DiMarco, D.M.; Putt, K.K.; Martin, D.A.; Gu, Q.; Chitchumroonchokchai, C.; White, H.M.; Scarlett, C.O.; Bruno, R.S.; Bolling, B.W. Low-fat yogurt consumption reduces biomarkers of chronic inflammation and inhibits markers of endotoxin exposure in healthy premenopausal women: A randomised controlled trial. Br. J. Nutr. 2017, 118, 1043–1051. [Google Scholar] [CrossRef] [PubMed]
- Wannamethee, S.G.; Jefferis, B.J.; Lennon, L.; Papacosta, O.; Whincup, P.H.; Hingorani, A.D. Serum conjugated linoleic acid and risk of incident heart failure in older men: The British regional heart study. JAMA 2018, 7, e006653. [Google Scholar] [CrossRef] [PubMed]
- De Souza, R.J.; Mente, A.; Maroleanu, A.; Cozma, A.I.; Ha, V.; Kishibe, T.; Uleryk, E.; Budylowski, P.; Schünemann, H.; Beyene, J.; et al. Intake of saturated and trans unsaturated fatty acids and risk of all cause mortality, cardiovascular disease, and type 2 diabetes: Systematic review and meta-analysis of observational studies. BMJ 2015, 351, h3978. [Google Scholar] [CrossRef] [PubMed]
- Poudyal, H.; Brown, L. Should the pharmacological actions of dietary fatty acids in cardiometabolic disorders be classified based on biological or chemical function? Prog. Lipid Res. 2015, 59, 172–200. [Google Scholar] [CrossRef] [PubMed]
- Micha, R.; Mozaffarian, D. Trans fatty acids: Effects on cardiometabolic health and implications for policy. Prostaglandins Leukot. Essent. Fat. Acids 2008, 79, 147–152. [Google Scholar] [CrossRef] [PubMed]
- Ganguly, R.; Pierce, G.N. Trans fat involvement in cardiovascular disease. Mol. Nutr. Food Res. 2012, 56, 1090–1096. [Google Scholar] [CrossRef] [PubMed]
- Stone, N.J.; Robinson, J.; Lichtenstein, A.H.; Merz, C.N.B.; Blum, C.B.; Eckel, R.H.; Goldberg, A.C.; Gordon, D.; Levy, D.; Lloyd-Jones, D.M.; et al. 2013 ACC/AHA Guideline on the Treatment of Blood Cholesterol to Reduce Atherosclerotic Cardiovascular Risk in Adults; A Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines; American College of Cardiology: Washington, DC, USA, 2013. [Google Scholar]
- Lichtenstein, A.H.; Appel, L.J.; Brands, M.; Carnethon, M.; Daniels, S.; Franch, H.A.; Franklin, B.; Kris-Etherton, P.; Harris, W.S.; Howard, B. Diet and lifestyle recommendations revision 2006. Circulation 2006, 114, 82–96. [Google Scholar] [CrossRef] [PubMed]
- L’Abbé, M.R.; Stender, S.; Skeaff, C.M.; Ghafoorunissa; Tavella, M. Approaches to removing trans fats from the food supply in industrialized and developing countries. Eur. J. Clin. Nutr. 2009, 63, S50–S67. [Google Scholar] [CrossRef]
- Dawczynski, C.; Lorkowski, S. Trans-fatty acids and cardiovascular risk: Does origin matter? Expert Rev. Cardiovasc. Ther. 2016, 14, 1001–1005. [Google Scholar] [CrossRef] [PubMed]
- Mozaffarian, D.; Katan, M.B.; Ascherio, A.; Stampfer, M.J.; Willett, W.C. Trans fatty acids and cardiovascular disease. N. Engl. J. Med. 2006, 354, 1601–1613. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Jacome-Sosa, M.M.; Proctor, S.D. The role of ruminant trans fat as a potential nutraceutical in the prevention of cardiovascular disease. Food Res. Int. 2012, 46, 460–468. [Google Scholar] [CrossRef]
- Lichtenstein, A.H. Dietary trans fatty acids and cardiovascular disease risk: Past and present. Curr. Atheroscler. Rep. 2014, 16, 433. [Google Scholar] [CrossRef] [PubMed]
- Ascherio, A.; Hennekens, C.H.; Buring, J.E.; Master, C.; Stampfer, M.J.; Willett, W.C. Trans-fatty acids intake and risk of myocardial infarction. Circulation 1994, 89, 94–101. [Google Scholar] [CrossRef] [PubMed]
- Gebauer, S.K.; Chardigny, J.-M.; Jakobsen, M.U.; Lamarche, B.; Lock, A.L.; Proctor, S.D.; Baer, D.J. Effects of ruminant trans fatty acids on cardiovascular disease and cancer: A comprehensive review of epidemiological, clinical, and mechanistic studies. Adv. Nutr. 2011, 2, 332–354. [Google Scholar] [CrossRef] [PubMed]
- Bassett, C.M.C.; Edel, A.L.; Patenaude, A.F.; McCullough, R.S.; Blackwood, D.P.; Chouinard, P.Y.; Paquin, P.; Lamarche, B.T.; Pierce, G.N. Dietary vaccenic acid has antiatherogenic effects in LDLr−/− mice. J. Nutr. 2010, 140, 18–24. [Google Scholar] [CrossRef] [PubMed]
- Van de Vijver, L.; Kardinaal, A.; Couet, C.; Aro, A. Association between trans fatty acid intake and cardiovascular risk factors in Europe: The transfair study. Eur. J. Clin. Nutr. 2000, 54, 126–135. [Google Scholar] [CrossRef] [PubMed]
- Martínez-González, M.A.; Salas-Salvadó, J.; Estruch, R.; Corella, D.; Fitó, M.; Ros, E. Benefits of the mediterranean diet: Insights from the predimed study. Prog. Cardiovasc. Dis. 2015, 58, 50–60. [Google Scholar] [CrossRef] [PubMed]
- Smit, L.A.; Baylin, A.; Campos, H. Conjugated linoleic acid in adipose tissue and risk of myocardial infarction. Am. J. Clin. Nutr. 2010, 92, 34–40. [Google Scholar] [CrossRef] [PubMed]
- Moloney, F.; Toomey, S.; Noone, E.; Nugent, A.; Allan, B.; Loscher, C.E.; Roche, H.M. Antidiabetic effects of cis-9, trans-11–conjugated linoleic acid may be mediated via anti-inflammatory effects in white adipose tissue. Diabetes 2007, 56, 574–582. [Google Scholar] [CrossRef] [PubMed]
- Ahn, I.-S.; Choi, B.-H.; Ha, J.-H.; Byun, J.-M.; Shin, H.-G.; Park, K.-Y.; Do, M.-S. Isomer-specific effect of conjugated linoleic acid on inflammatory adipokines associated with fat accumulation in 3T3-L1 adipocytes. J. Med. Food 2006, 9, 307–312. [Google Scholar] [CrossRef] [PubMed]
- Mozaffarian, D.; Cao, H.; King, I.B.; Lemaitre, R.N.; Song, X.; Siscovick, D.S.; Hotamisligil, G.K.S. Trans-palmitoleic acid, metabolic risk factors, and new-onset diabetes in us adults. Ann. Intern. Med. 2010, 153, 790–799. [Google Scholar] [CrossRef] [PubMed]
- Bolton-Smith, C.; Woodward, M.; Fenton, S.; Brown, C. Does dietary trans fatty acid intake relate to the prevalence of coronary heart disease in Scotland? Eur. Heart J. 1996, 17, 837–845. [Google Scholar] [CrossRef] [PubMed]
- Olmedilla-Alonso, B.; Nova-Rebato, E.; García-González, N.; Martín-Diana, A.-B.; Fontecha, J.; Delgado, D.; Gredilla, A.-E.; Bueno, F.; Asensio-Vegas, C. Effect of ewe’s (semi-skimmed and whole) and cow’s milk yogurt consumption on the lipid profile of control subjects: A crossover study. Food Nutr. Res. 2017, 61, 1391669. [Google Scholar] [CrossRef] [PubMed]
- Zoumpopoulou, G.; Pot, B.; Tsakalidou, E.; Papadimitriou, K. Dairy probiotics: Beyond the role of promoting gut and immune health. Int. Dairy J. 2017, 67, 46–60. [Google Scholar] [CrossRef]
- Elwood, P.C.; Pickering, J.E.; Givens, D.I.; Gallacher, J.E. The consumption of milk and dairy foods and the incidence of vascular disease and diabetes: An overview of the evidence. Lipids 2010, 45, 925–939. [Google Scholar] [CrossRef] [PubMed]
- Darmon, N.; Drewnowski, A. Does social class predict diet quality? Am. J. Clin. Nutr. 2008, 87, 1107–1117. [Google Scholar] [CrossRef] [PubMed]
- Hobbs, D.A.; Givens, D.I.; Lovegrove, J.A. Yogurt consumption is associated with higher nutrient intake, diet quality and favourable metabolic profle in children: A cross-sectional analysis using data from years 1–4 of the national diet and nutrition survey, UK. Eur. J. Nutr. 2018, 1–14. [Google Scholar] [CrossRef]
- Tognon, G.; Nilsson, L.M.; Shungin, D.; Lissner, L.; Jansson, J.-H.; Renström, F.; Wennberg, M.; Winkvist, A.; Johansson, I. Nonfermented milk and other dairy products: Associations with all-cause mortality. Am. J. Clin. Nutr. 2017, 105, 1502–1511. [Google Scholar] [CrossRef] [PubMed]
- Tong, X.; Chen, G.-C.; Zhang, Z.; Wei, Y.-L.; Xu, J.-Y.; Qin, L.-Q. Cheese consumption and risk of all-cause mortality: A meta-analysis of prospective studies. Nutrients 2017, 9, 63. [Google Scholar] [CrossRef] [PubMed]
- Kießling, G.; Schneider, J.; Jahreis, G. Long-term consumption of fermented dairy products over 6 months increases HDL cholesterol. Eur. J. Clin. Nutr. 2002, 56, 843–849. [Google Scholar] [CrossRef] [PubMed]
- Turner, K.M.; Keogh, J.B.; Clifton, P.M. Dairy consumption and insulin sensitivity: A systematic review of short- and long-term intervention studies. Nutr. Metab. Cardiovasc. Dis. 2015, 25, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Hove, K.D.; Brøns, C.; Færch, K.; Lund, S.S.; Rossing, P.; Vaag, A. Effects of 12 weeks of treatment with fermented milk on blood pressure, glucose metabolism and markers of cardiovascular risk in patients with type 2 diabetes: A randomised double-blind placebo-controlled study. Eur. J. Endocrinol. 2015, 172, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Houston, D.K.; Driver, K.E.; Bush, A.J.; Kritchevsky, S.B. The association between cheese consumption and cardiovascular risk factors among adults. J. Hum. Nutr. Diet. 2008, 21, 129–140. [Google Scholar] [CrossRef] [PubMed]
- Tholstrup, T. Dairy products and cardiovascular disease. Curr. Opin. Lipidol. 2006, 17, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Tavani, A.; Gallus, S.; Negri, E.; La Vecchia, C. Milk, dairy products, and coronary heart disease. J. Epidemiol. Community Health 2002, 56, 471–472. [Google Scholar] [CrossRef] [PubMed]
- Hjerpsted, J.; Tholstrup, T. Cheese and cardiovascular disease risk: A review of the evidence and discussion of possible mechanisms. Crit. Rev. Food Sci. Nutr. 2016, 56, 1389–1403. [Google Scholar] [CrossRef] [PubMed]
- Praagman, J.; Dalmeijer, G.W.; van der Schouw, Y.T.; Soedamah-Muthu, S.S.; Monique Verschuren, W.M.; Bas Bueno-de-Mesquita, H.; Geleijnse, J.M.; Beulens, J.W.J. The relationship between fermented food intake and mortality risk in the European prospective investigation into cancer and nutrition-netherlands cohort. Br. J. Nutr. 2015, 113, 498–506. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Sun, D. Consumption of yogurt and the incident risk of cardiovascular disease: A meta-analysis of nine cohort studies. Nutrients 2017, 9, 315. [Google Scholar] [CrossRef] [PubMed]
- Sayón-Orea, C.; Bes-Rastrollo, M.; Martí, A.; Pimenta, A.M.; Martín-Calvo, N.; Martínez-González, M.A. Association between yogurt consumption and the risk of metabolic syndrome over 6 years in the sun study. BMC Public Health 2015, 15, 170. [Google Scholar] [CrossRef] [PubMed]
- Renaud, S.; de Lorgeril, M. Wine, alcohol, platelets, and the french paradox for coronary heart disease. Lancet 1992, 339, 1523–1526. [Google Scholar] [CrossRef]
- Lallès, J.-P. Dairy products and the french paradox: Could alkaline phosphatases play a role? Med. Hypotheses 2016, 92, 7–11. [Google Scholar] [CrossRef] [PubMed]
- Petyaev, I.M.; Bashmakov, Y.K. Could cheese be the missing piece in the French paradox puzzle? Med. Hypotheses 2012, 79, 746–749. [Google Scholar] [CrossRef] [PubMed]
- Parvez, S.; Malik, K.A.; Ah Kang, S.; Kim, H.Y. Probiotics and their fermented food products are beneficial for health. J. Appl. Microbiol. 2006, 100, 1171–1185. [Google Scholar] [CrossRef] [PubMed]
- Booth, S.L. Roles for vitamin K beyond coagulation. Annu. Rev. Nutr. 2009, 29, 89–110. [Google Scholar] [CrossRef] [PubMed]
- O’Connor, E.M.; Durack, E. Osteocalcin: The extra-skeletal role of a vitamin K-dependent protein in glucose metabolism. J. Nutr. Intermed. Metab. 2017, 7, 8–13. [Google Scholar] [CrossRef]
- Walther, B.; Karl, J.P.; Booth, S.L.; Boyaval, P. Menaquinones, bacteria, and the food supply: The relevance of dairy and fermented food products to vitamin K requirements. Adv. Nutr. 2013, 4, 463–473. [Google Scholar] [CrossRef] [PubMed]
- Booth, S.L.; Rajabi, A.A. Determinants of vitamin K status in humans. Vitam. Horm. 2008, 78, 1–22. [Google Scholar] [PubMed]
- Fu, X.; Harshman, S.G.; Shen, X.; Haytowitz, D.B.; Karl, J.P.; Wolfe, B.E.; Booth, S.L. Multiple vitamin K forms exist in dairy foods. Curr. Dev. Nutr. 2017, 1, e000638. [Google Scholar] [CrossRef]
- Shea, M.K.; Holden, R.M. Vitamin K status and vascular calcification: Evidence from observational and clinical studies. Adv. Nutr. 2012, 3, 158–165. [Google Scholar] [CrossRef] [PubMed]
- Harshman, S.G.; Shea, M.K. The role of vitamin K in chronic aging diseases: Inflammation, cardiovascular disease, and osteoarthritis. Curr. Nutr. Rep. 2016, 5, 90–98. [Google Scholar] [CrossRef] [PubMed]
- Gast, G.C.M.; de Roos, N.M.; Sluijs, I.; Bots, M.L.; Beulens, J.W.J.; Geleijnse, J.M.; Witteman, J.C.; Grobbee, D.E.; Peeters, P.H.M.; van der Schouw, Y.T. A high menaquinone intake reduces the incidence of coronary heart disease. Nutr. Metab. Cardiovasc. Dis. 2009, 19, 504–510. [Google Scholar] [CrossRef] [PubMed]
- Nagata, C.; Wada, K.; Tamura, T.; Konishi, K.; Goto, Y.; Koda, S.; Kawachi, T.; Tsuji, M.; Nakamura, K. Dietary soy and natto intake and cardiovascular disease mortality in Japanese adults: The Takayama study. Am. J. Clin. Nutr. 2017, 105, 426–431. [Google Scholar] [CrossRef] [PubMed]
- Keyzer, C.A.; Vermeer, C.; Joosten, M.M.; Knapen, M.H.; Drummen, N.E.; Navis, G.; Bakker, S.J.; de Borst, M.H. Vitamin K status and mortality after kidney transplantation: A cohort study. Am. J. Kidney Dis. 2015, 65, 474–483. [Google Scholar] [CrossRef] [PubMed]
- Rosa, D.D.; Dias, M.M.S.; Grześkowiak, Ł.M.; Reis, S.A.; Conceição, L.L.; Peluzio, M.D.C.G. Milk kefir: Nutritional, microbiological and health benefits. Nutr. Res. Rev. 2017, 30, 82–96. [Google Scholar] [CrossRef] [PubMed]
- Nestel, P. Chapter 16—Fermented Dairy Foods and Cardiovascular Risk a2—Watson, Ronald Ross. In Dairy in Human Health and Disease Across the Lifespan; Collier, R.J., Preedy, V.R., Eds.; Academic Press: Cambridge, MA, USA, 2017; pp. 225–229. [Google Scholar]
- Rosqvist, F.; Smedman, A.; Lindmark-Månsson, H.; Paulsson, M.; Petrus, P.; Straniero, S.; Rudling, M.; Dahlman, I.; Risérus, U. Potential role of milk fat globule membrane in modulating plasma lipoproteins, gene expression, and cholesterol metabolism in humans: A randomized study. Am. J. Clin. Nutr. 2015, 102, 20–30. [Google Scholar] [CrossRef] [PubMed]
- Granato, D.; Branco, G.F.; Cruz, A.G.; Faria, J.D.A.F.; Shah, N.P. Probiotic dairy products as functional foods. Compr. Rev. Food Sci. Food Saf. 2010, 9, 455–470. [Google Scholar] [CrossRef]
- Shortt, C.; O’Brien, J. Handbook of Functional Dairy Products; CRC Press: Boca Raton, FL, USA, 2016. [Google Scholar]
- Vásquez-Trespalacios, E.M.; Romero-Palacio, J. Efficacy of yogurt drink with added plant stanol esters (Benecol®, Colanta) in reducing total and LDL cholesterol in subjects with moderate hypercholesterolemia: A randomized placebo-controlled crossover trial NCT01461798. Lipids Health Dis. 2014, 13, 125. [Google Scholar] [CrossRef] [PubMed]
- Law, M. Plant sterol and stanol margarines and health. BMJ 2000, 320, 861–864. [Google Scholar] [CrossRef] [PubMed]
- Harland, J.I. Food combinations for cholesterol lowering. Nutr. Res. Rev. 2012, 25, 249–266. [Google Scholar] [CrossRef] [PubMed]
- Özer, B.H.; Kirmaci, H.A. Functional milks and dairy beverages. Int. J. Dairy Technol. 2010, 63, 1–15. [Google Scholar] [CrossRef]
- Rocha, M.; Banuls, C.; Bellod, L.; Jover, A.; M Victor, V.; Hernandez-Mijares, A. A review on the role of phytosterols: New insights into cardiovascular risk. Curr. Pharm. Des. 2011, 17, 4061–4075. [Google Scholar] [CrossRef] [PubMed]
- Yildiz, F. Development and Manufacture of Yogurt and Other Functional Dairy Products; CRC Press Taylor & Francis Group: Boca Raton, FL, USA, 2016; p. 435. [Google Scholar]
- Jones, P.J.; Ntanios, F.Y.; Raeini-Sarjaz, M.; Vanstone, C.A. Cholesterol-lowering efficacy of a sitostanol-containing phytosterol mixture with a prudent diet in hyperlipidemic men. Am. J. Clin. Nutr. 1999, 69, 1144–1150. [Google Scholar] [CrossRef] [PubMed]
- Hansel, B.; Nicolle, C.; Lalanne, F.; Tondu, F.; Lassel, T.; Donazzolo, Y.; Ferrières, J.; Krempf, M.; Schlienger, J.-L.; Verges, B.; et al. Effect of low-fat, fermented milk enriched with plant sterols on serum lipid profile and oxidative stress in moderate hypercholesterolemia. Am. J. Clin. Nutr. 2007, 86, 790–796. [Google Scholar] [CrossRef] [PubMed]
- Mäkinen, O.E.; Wanhalinna, V.; Zannini, E.; Arendt, E.K. Foods for special dietary needs: Non-dairy plant-based milk substitutes and fermented dairy-type products. Crit. Rev. Food Sci. Nutr. 2016, 56, 339–349. [Google Scholar] [CrossRef] [PubMed]
- Stall, S.; Adams, G. Can almond milk be called milk? J. Renal Nutr. 2017, 27, e15–e17. [Google Scholar] [CrossRef]
- Jeske, S.; Zannini, E.; Arendt, E.K. Evaluation of physicochemical and glycaemic properties of commercial plant-based milk substitutes. Plant Foods Hum. Nutr. 2017, 72, 26–33. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, D.J.; Mirrahimi, A.; Srichaikul, K.; Berryman, C.E.; Wang, L.; Carleton, A.; Abdulnour, S.; Sievenpiper, J.L.; Kendall, C.W.; Kris-Etherton, P.M. Soy protein reduces serum cholesterol by both intrinsic and food displacement mechanisms. J. Nutr. 2010, 140, 2302S–2311S. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.X.; Li, S.H.; Chen, J.Z.; Sun, K.; Wang, X.J.; Wang, X.G.; Hui, R.T. Effect of soy isoflavones on blood pressure: A meta-analysis of randomized controlled trials. Nutr. Metab. Cardiovasc. Dis. 2012, 22, 463–470. [Google Scholar] [CrossRef] [PubMed]
- Messina, M. Soy and health update: Evaluation of the clinical and epidemiologic literature. Nutrients 2016, 8, 754. [Google Scholar] [CrossRef] [PubMed]
- Lecomte, M.; Couëdelo, L.; Meugnier, E.; Plaisancié, P.; Létisse, M.; Benoit, B.; Gabert, L.; Penhoat, A.; Durand, A.; Pineau, G.; et al. Dietary emulsifiers from milk and soybean differently impact adiposity and inflammation in association with modulation of colonic goblet cells in high-fat fed mice. Mol. Nutr. Food Res. 2016, 60, 609–620. [Google Scholar] [CrossRef] [PubMed]
- O’Connor, A. Got Almond Milk? Dairy Farms Protest Milk Label on Nondairy Drinks. Available online: https://www.nytimes.com/2017/02/13/well/eat/got-almond-milk-dairy-farms-protest-milk-label-on-nondairy-drinks.html?_r=0 (accessed on 17 January 2018).
- Balthazar, C.; Junior, C.C.; Moraes, J.; Costa, M.; Raices, R.; Franco, R.; Cruz, A.; Silva, A. Physicochemical evaluation of sheep milk yogurts containing different levels of inulin. J. Dairy Sci. 2016, 99, 4160–4168. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.W. Impact of goat milk and milk products on human nutrition. Perspect. Agric. Vet. Sci. Nutr. Nat. Resour. 2007, 2. [Google Scholar] [CrossRef]
- Costa, M.P.; Monteiro, M.L.G.; Frasao, B.S.; Silva, V.L.M.; Rodrigues, B.L.; Chiappini, C.C.J.; Conte-Junior, C.A. Consumer perception, health information, and instrumental parameters of cupuassu (Theobroma grandiflorum) goat milk yogurts. J. Dairy Sci. 2017, 100, 157–168. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.W.; Juárez, M.; Ramos, M.; Haenlein, G.F.W. Physico-chemical characteristics of goat and sheep milk. Small Rumin. Res. 2007, 68, 88–113. [Google Scholar] [CrossRef]
- Haenlein, G.F.W. Goat milk in human nutrition. Small Rumin. Res. 2004, 51, 155–163. [Google Scholar] [CrossRef]
- Pereira, P.C. Milk nutritional composition and its role in human health. Nutrition 2014, 30, 619–627. [Google Scholar] [CrossRef] [PubMed]
- Contarini, G.; Pelizzola, V.; Povolo, M. Content of conjugated linoleic acid in neutral and polar lipid fractions of milk of different ruminant species. Int. Dairy J. 2009, 19, 342–344. [Google Scholar] [CrossRef]
- Pintus, S.; Murru, E.; Carta, G.; Cordeddu, L.; Batetta, B.; Accossu, S.; Pistis, D.; Uda, S.; Elena Ghiani, M.; Mele, M.; et al. Sheep cheese naturally enriched in α-linolenic, conjugated linoleic and vaccenic acids improves the lipid profile and reduces anandamide in the plasma of hypercholesterolaemic subjects. Br. J. Nutr. 2013, 109, 1453–1462. [Google Scholar] [CrossRef] [PubMed]
- Barłowska, J.; Szwajkowska, M.; Litwińczuk, Z.; Król, J. Nutritional value and technological suitability of milk from various animal species used for dairy production. Compr. Rev. Food Sci. Food Saf. 2011, 10, 291–302. [Google Scholar] [CrossRef]
- Haenlein, G.F.W.; Wendorff, W.L. Sheep milk. In Handbook of Milk of Non-Bovine Mammals; Blackwell Publishing Professional: Hoboken, NJ, USA, 2008; pp. 137–194. [Google Scholar]
- Balthazar, C.F.; Pimentel, T.C.; Ferrão, L.L.; Almada, C.N.; Santillo, A.; Albenzio, M.; Mollakhalili, N.; Mortazavian, A.M.; Nascimento, J.S.; Silva, M.C.; et al. Sheep milk: Physicochemical characteristics and relevance for functional food development. Compr. Rev. Food Sci. Food Saf. 2017, 16, 247–262. [Google Scholar] [CrossRef]
- Molkentin, J. Occurrence and biochemical characteristics of natural bioactive substances in bovine milk lipids. Br. J. Nutr. 2000, 84, 47–53. [Google Scholar] [CrossRef]
- Balthazar, C.F.; Cruz, A.G. Sheep milk: An unexplored food matrix to develop functional foods. Inform 2017, 28, 32–33. [Google Scholar] [CrossRef]
- Corrêa, A.P.F.; Daroit, D.J.; Coelho, J.; Meira, S.M.M.; Lopes, F.C.; Segalin, J.; Risso, P.H.; Brandelli, A. Antioxidant, antihypertensive and antimicrobial properties of ovine milk caseinate hydrolyzed with a microbial protease. J. Sci. Food Agric. 2011, 91, 2247–2254. [Google Scholar] [CrossRef] [PubMed]
- Marsh, A.J.; O’Sullivan, O.; Hill, C.; Ross, R.P.; Cotter, P.D. Sequencing-based analysis of the bacterial and fungal composition of kefir grains and milks from multiple sources. PLoS ONE 2013, 8, e69371. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Farnworth, E.R. Kefir—A complex probiotic. Food Sci. Technol. Bull. 2006, 2, 1–17. [Google Scholar] [CrossRef]
- Vieira, C.; Álvares, T.; Gomes, L.; Torres, A.; Paschoalin, V.; Conte-Junior, C. Kefir grains change fatty acid profile of milk during fermentation and storage. PLoS ONE 2015, 10, e0139910. [Google Scholar] [CrossRef] [PubMed]
- Bourrie, B.C.; Willing, B.P.; Cotter, P.D. The microbiota and health promoting characteristics of the fermented beverage kefir. Front. Microbiol. 2016, 7, 647. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maeda, H.; Zhu, X.; Suzuki, S.; Suzuki, K.; Kitamura, S. Structural characterization and biological activities of an exopolysaccharide kefiran produced by Lactobacillus kefiranofaciens Wt-2BT. J. Agric. Food Chem. 2004, 52, 5533–5538. [Google Scholar] [CrossRef] [PubMed]
- Urdaneta, E.; Barrenetxe, J.; Aranguren, P.; Irigoyen, A.; Marzo, F.; Ibáñez, F.C. Intestinal beneficial effects of kefir-supplemented diet in rats. Nutr. Res. 2007, 27, 653–658. [Google Scholar] [CrossRef]
- St-Onge, M.-P.; Farnworth, E.R.; Savard, T.; Chabot, D.; Mafu, A.; Jones, P.J. Kefir consumption does not alter plasma lipid levels or cholesterol fractional synthesis rates relative to milk in hyperlipidemic men: A randomized controlled trial (ISRCTN10820810). BMC Complement. Altern. Med. 2002, 2, 1. [Google Scholar] [CrossRef] [Green Version]
- Ostadrahimi, A.; Taghizadeh, A.; Mobasseri, M.; Farrin, N.; Payahoo, L.; Beyramalipoor Gheshlaghi, Z.; Vahedjabbari, M. Effect of probiotic fermented milk (kefir) on glycemic control and lipid profile in type 2 diabetic patients: A randomized double-blind placebo-controlled clinical trial. Iran. J. Public Health 2015, 44, 228–237. [Google Scholar] [PubMed]
- Givens, D.I. Dairy products: Good or bad for cardiometabolic disease? Am. J. Clin. Nutr. 2015, 101, 695–696. [Google Scholar] [CrossRef] [PubMed]
- Astrup, A. Yogurt and dairy product consumption to prevent cardiometabolic diseases: Epidemiologic and experimental studies. Am. J. Clin. Nutr. 2014, 99, 1235S–1242S. [Google Scholar] [CrossRef] [PubMed]
| Author, Year | Country | Study Design | Study Focus | Outcome | Conclusion |
|---|---|---|---|---|---|
| Thompson, 2005 [155] | USA | Dietary intervention | The effects of high-dairy and high-fibre consumption on weight loss in 90 obese subjects was assessed | CRP was reduced by 0.8 mg/L from baseline (p < 0.0001), however there was no significant difference between the dairy diet and the others tested | An insignificant reduction of CRP was observed following dairy consumption in obese participants |
| Sofi, 2010 [156] | Italy | Dietary intervention | Effect of pecorino cheese naturally enriched with cis-9, trans-11 CLA on inflammatory markers in 10 healthy participants | Reduction in arachidonic acid-induced platelet aggregation (pre: 87.8 ± 1.76% vs. post: 77.7 ± 3.56%; p = 0.04), improvement of erythrocyte filtration rate and a reduction of TNF-α (40.1%), IL-6 (43.2%) and IL-8 (36.5%) | Dietary short-term intake of pecorino cheese rich in cis-9, trans-11 CLA caused favourable biochemical changes of inflammatory and atherosclerotic markers |
| Rosado, 2011 [157] | Mexico | Dietary intervention | Effect of adding low-fat milk on anthropometrics, body composition, CRP etc. in energy restricted diets in 139 women | Change in CRP after low-fat milk was 0.2 mg/L (95% CI 1.1–1.6) | Dairy intake had no significant effect on CRP concentrations |
| Stancliffe, 2011 [154] | USA | Dietary intervention | Effects of an adequate full-fat dairy diet versus low-dairy (both mainly milk and yoghurt) intake on inflammatory markers in 40 overweight individuals with metabolic syndrome over a 12-week period versus a low-fat control | After 7 days, the adequate full-fat dairy diet decreased plasma malondialdehyde and oxidised LDL (35% and 11% respectively, p < 0.01), TNF-α decreased by 35% (p < 0.05), which further decreased by week 12. By week 12, decreases in IL-6 (21%, p < 0.02) and MCP-1 (24%, p < 0.05) were observed. Low-dairy intake exerted no effects on oxidative or inflammatory markers | An increase in dairy intake attenuates oxidative and inflammatory stress in metabolic syndrome |
| Nestel, 2012 [158] | Australia | Dietary intervention | Assessing the effects of low-fat or fermented dairy product intake on inflammation and atherogenesis on 13 overweight participants, using 5-single meal tests | No significant changes in the levels of inflammatory biomarkers (CRP, IL-6, IL-13, TNF-α, VCAM-1 and others) were observed | Authors could not confirm the reported increments in inflammation after high fat meals |
| Esmaillzadeh, 2010 [159] | Iran | Cross-sectional | Assessing the effect of dairy products on inflammatory markers in 486 women | Low-fat dairy was inversely associated with CRP (β = −0.04), IL-6 (β = −0.02) and VCAM-1 (β = −0.06); high fat dairy was positively associated with log-transformed values of serum amyloid A (β = 0.08) and VCAM-1 (β = 0.05) | Evidence suggests there is an independent relationship between dairy consumption and some markers of inflammation and endothelial dysfunction |
| Panagiotakis, 2010 [160] | Greece | Cross-sectional | The evaluation of effects of dairy product consumption on levels of inflammatory markers in blood samples from fasting adults with no evidence of previous chronic inflammatory disease | Levels of inflammatory markers such as CRP, IL-6 and TNF-α were 29, 9 and 20% lower, respectively (p = 0.01), in people who consumed more than 14 servings of dairy per week compared with those who had fewer than 8 servings per week (p = 0.05) | This inverse association between dairy consumption and levels of inflammatory markers in healthy adults indicates that dairy products may be protective against chronic inflammatory diseases |
| Wang, 2011 [161] | USA | Cross-sectional | 305 adolescents were tested for serum phospholipid fatty acid markers of dairy intake (C15:0 & C17:0), which were linked to biomarkers of inflammation by generalised linear regression analyses adjusted for age, gender, race, tanner score, total energy intake and physical activity | Phospholipid dairy fatty acids, elevated by dairy consumption, were inversely associated with CRP, 8-iso-PGF2α and urinary 15-keto-dihydro-PGF2α in overweight but not in normal weight adolescents (all pinteraction < 0.05). However, higher PL dairy fatty acid levels were associated with lower IL-6 among all adolescents. Adjustment for dietary intake of calcium, vitamin D, protein, total flavonoids and ω-3 fatty acids did not alter the findings | Dairy-specific saturated fats C15:0 and 17:0 fatty acids, may contribute to the potential health benefits of dairy products, especially for overweight adolescents |
| Gadotti, 2017 [162] | Brazil | Cross-sectional | To assess the effect of dairy consumption and plasma inflammatory markers in 259 participants. Subjects were assigned groups depending on inflammatory status and multiple logistic regression tests were conducted to estimate the odds ratio (OR) for the inflammatory cluster across tertiles of dairy consumption | The highest tertile of yoghurt consumption was 0.34 [95% CI: (0.14–0.81)] relative to the reference tertile, demonstrating a linear effect (ptrend = 0.015). Cheese consumption exhibited an OR of 2.49 (95% CI: (1.09–5.75)) relative to the reference | Increasing yoghurt consumption might have a protective effect on inflammation, while cheese consumption seems to be associated with a pro-inflammatory status |
| van Meijl, 2010 [163] | Netherlands | Randomised crossover | Effects of low-fat milk and yoghurt intake on inflammatory markers in 35 overweight or obese participants versus carbohydrate controls for 8 weeks | No significant effects on IL-6, MCP-1, ICAM-1 or VCAM-1 versus control. TNF-α index decreased by 53 (p = 0.015) | Low-fat dairy consumption may increase concentrations of s-TNFR but it has no effects on other inflammatory markers of chronic inflammation and endothelial function |
| Zemel, 2010 [164] | USA | Randomised crossover | Effects of a dairy-rich, high calcium diet on oxidative and inflammatory stress in 10 overweight and 10 obese individuals compared with soy supplemented eucaloric diets | After 7 days, dairy intake decreased oxidative stress by lowering 8-isoprostane-F2α (12%, p < 0.0005), plasma malondialdehyde (22%, p < 0.0005). Adiponectin increased significantly (20%, p < 0.002). Inflammatory markers were significantly reduced versus the control diet: IL-6 (13%, p < 0.01); TNF-α (15%, p < 0.002); MCP-1 (10%, p < 0.0006) | An increase in dairy food intake produces significant and substantial suppression of the oxidative and inflammatory stress associated with overweight and obesity |
| Nestel, 2013 [165] | Australia | Randomised crossover | Consumption of full-fat versus low-fat dairy on biomarkers of inflammation in 12 overweigh individuals | 75% of those who consumed low-fat products versus full-fat fermented products tended to have higher levels of inflammatory markers tested (CRP, IL-13, TNF-α, VCAM-1 and others; ptrend < 0.001) | Short-term diets of low-fat dairy products did not lead to a favourable biomarker profile associated with CVD risk compared with the full-fat dairy products. Full-fat fermented dairy products are more favourable |
| Labonté, 2014 [166] | Canada | Randomised crossover | Assessing the impact of dairy intake versus energy equivalent products on inflammatory markers in 112 healthy participants with systemic inflammation | After dairy consumption, no significant changes in CRP (7.3%, p = 0.47). However, both the control and dairy diet reduced IL-6 (17.6% and 19.9%, respectively; p < 0.0001 for both, p = 0.77 for between-diet comparison | Short-term consumption of a combination of low- and high-fat dairy products as part of a healthy diet has no adverse effects on inflammation |
| Dugan, 2016 [167] | USA | Randomised crossover | Effect of low-fat dairy consumption on hepatic enzymes and inflammation in 37 participants with metabolic syndrome versus a carbohydrate control | Lower levels of TNF-α (p = 0.028) and MCP-1 (p = 0.001) were observed in women after low-fat dairy intake versus the control group. The hepatic steatosis index was also reduced (p = 0.001) | Three servings of dairy per day improved both liver function and systemic inflammation in subjects with metabolic syndrome |
| Zemel, 2008 [168] | USA | Randomised controlled longitudinal | Evaluation of feeding calcium rich high-dairy eucaloric diet and hypocaloric diet versus low dairy group intake in obese participants over 24 weeks | High-dairy eucaloric diet and a hypocaloric diet resulted in an 11% (p < 0.03) and 29% (p < 0.01) decrease in CRP, respectively (post-test vs. pre-test), whereas there was no significant change in the low-dairy groups. Adiponectin decreased by 8% in subjects fed the eucaloric high-dairy diet (p = 0.003) and 18% for the hypocaloric high-dairy diet (p = 0.05) | Dietary calcium suppresses adipose tissue oxidative and inflammatory stress |
| de Aguilar-Nascimento, 2011 [169] | Brazil | Randomised controlled longitudinal | Effects of an early enteral formula on the levels of glutathione and inflammatory markers in 25 aged patients with acute ischemic stroke. Group 1 consumed whey, group 2, the control consumed casein | Mortality was similar between groups (33%; p = 1.00) and was associated with higher IL-6 levels (group 1: 73.7 ± 24.7; versus group 2: 16.6 ± 2.4 pg/dL; p = 0.04) and CRP (82.0 ± 35.6 vs. 48.3 ± 14.5 mg/L; p = 0.02). Serum IL-6 was lower (p = 0.03) and glutathione was higher (p = 0.03) in whey protein-fed patients versus the casein group | Enteral formula containing whey protein may decrease inflammation and increase antioxidant defences in elderly patients with ischemic stroke |
| Jones, 2013 [170] | Canada | Randomised controlled longitudinal | Assessing a diet rich in calcium and dairy products on weight loss and appetite during energy restriction in 49 overweight and obese individuals for 12 weeks, versus a suitable control. A meal tolerance test was carried out in week 12 | MCP-1 was reduced after 30 mins with Dairy/Calcium group compared with the control in the meal tolerance test (p = 0.04). No change was observed for IL-6, TNF-α, or IL-1β | Modest reduction in MCP-1 |
| Pei, 2017 [171] | USA | Randomised controlled | Premenopausal women (BMI 18.5–27 and 30–40 kg/m2) were randomised to consume 339 g of low-fat yoghurt (yoghurt non-obese (YN); yoghurt obese (YO)) or 324 g of soya pudding (control non-obese; control obese (CO)) daily for 9 weeks (n 30/group). Fasting blood samples were analysed for various inflammatory markers | After 9-week yoghurt consumption, YO and YN had decreased TNF-α/sTNFR RII. Yoghurt consumption increased plasma IgM EndoCAb regardless of obesity status. sCD14 was not affected by diet but LBP/sCD14 was lowered in both YN and YO. Yoghurt intervention increased plasma 2-arachidonoylglycerol in YO but not YN. YO peripheral blood mononuclear cells expression of NF-κB inhibitor α and transforming growth factor β1 increased relative to CO at 9 weeks | Consumption of low-fat yoghurt for 9 weeks reduced biomarkers of chronic inflammation and endotoxin exposure in premenopausal women compared with a non-dairy control food |
| Wannamethee, 2018 [172] | UK | Prospective cohort study | This study investigated serum CLA (measured as a % of total fatty acids) and the risk of incident heart failure in 3806 older men aged between 60 and 79 years using metabolomics. The men were without prevalent HF and were followed up for an average of 13 years, during which there were 295 incident HF cases | CLA was adversely associated with cholesterol levels but was inversely associated with CRP and NT-proBNP. No association between CLA and CHD. High CLA was associated with reduced risk of HF (hazard ratio [95% confidence interval], 0.64 [0.43–0.96]; quartile 4 versus quartile 1). Elevated CLA was associated with reduced HF risk only in those with higher dairy fat intake, a major dietary source of CLA (p = 0.03) | The reduced risk of HF was partially explained by NT-proBNP. High dairy fat intake was not associated with incident coronary heart disease but was associated with reduced risk of HF, largely because of the inverse effect of CLA |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lordan, R.; Tsoupras, A.; Mitra, B.; Zabetakis, I. Dairy Fats and Cardiovascular Disease: Do We Really Need to Be Concerned? Foods 2018, 7, 29. https://doi.org/10.3390/foods7030029
Lordan R, Tsoupras A, Mitra B, Zabetakis I. Dairy Fats and Cardiovascular Disease: Do We Really Need to Be Concerned? Foods. 2018; 7(3):29. https://doi.org/10.3390/foods7030029
Chicago/Turabian StyleLordan, Ronan, Alexandros Tsoupras, Bhaskar Mitra, and Ioannis Zabetakis. 2018. "Dairy Fats and Cardiovascular Disease: Do We Really Need to Be Concerned?" Foods 7, no. 3: 29. https://doi.org/10.3390/foods7030029







