Influence of Clitoria ternatea Flower Extract on the In Vitro Enzymatic Digestibility of Starch and Its Application in Bread
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Extraction
2.3. Preparation of Flour and Extract
2.4. Inhibition of Pancreatic α-Amylase
2.5. In Vitro Starch Digestibility and Predicted Glycemic Index (pGI)
2.6. Estimation of Starch Fraction
2.7. Bread Preparation
2.8. Statistical Analysis
3. Results
3.1. Inhibition of Pancreatic α-Amylase
3.2. In Vitro Starch Digestibility and Predicted Glycemic Index (pGI)
3.3. Starch Fraction
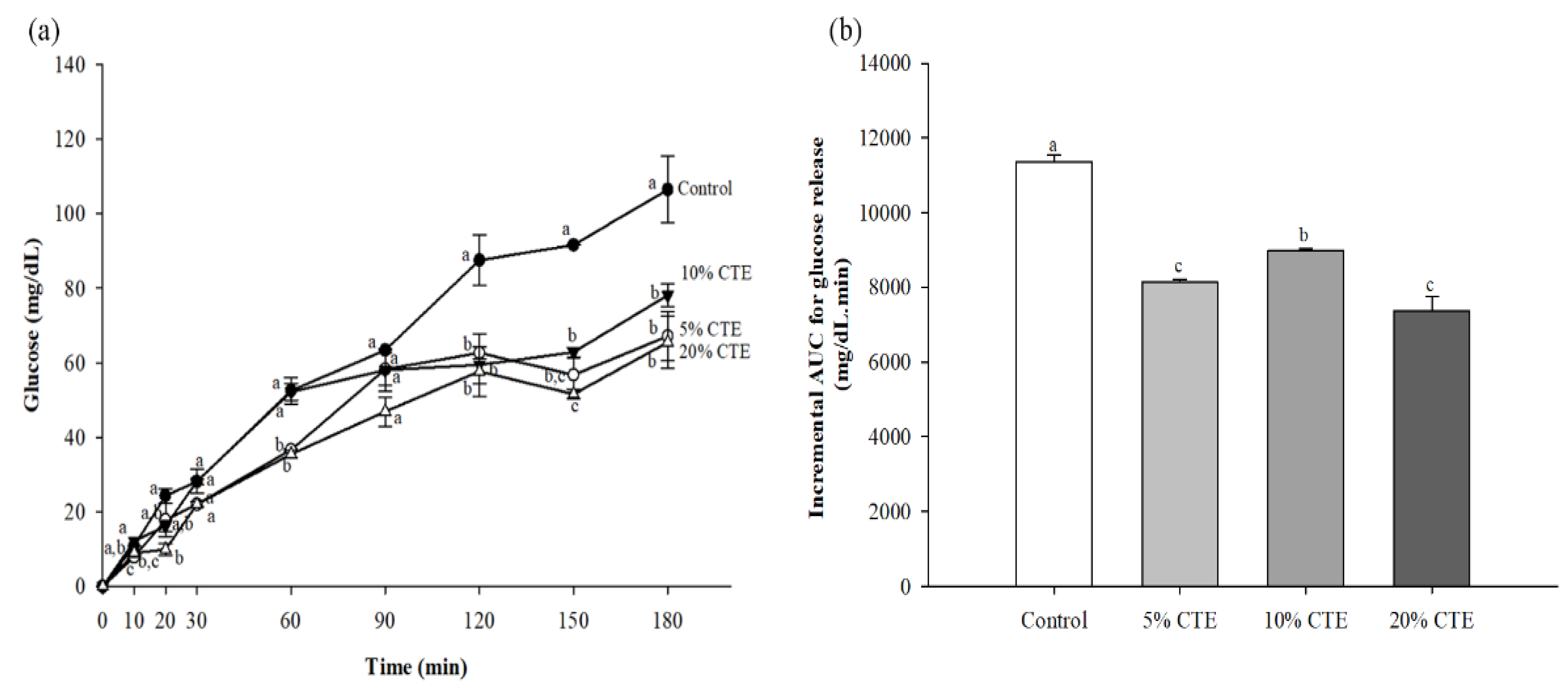
3.4. In Vitro Digistibility of Bread
4. Discussion
5. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Lafiandra, D.; Riccardi, G.; Shewry, P.R. Improving cereal grain carbohydrates for diet and health. J. Cereal Sci. 2014, 59, 312–326. [Google Scholar] [CrossRef] [PubMed]
- O’Keefe, J.H.; Gheewala, N.M.; O’Keefe, J.O. Dietary strategies for improving post-prandial glucose, lipids, inflammation, and cardiovascular health. J. Am. Coll. Cardiol. 2008, 51, 249–255. [Google Scholar] [CrossRef] [PubMed]
- Eleazu, C.O. The concept of low glycemic index and glycemic load foods as panacea for type 2 diabetes mellitus; prospects, challenges and solutions. Afr. Health Sci. 2016, 16, 468–479. [Google Scholar] [CrossRef] [PubMed]
- Goñi, I.; Garcia-Alonso, A.; Saura-Calixto, F. A starch hydrolysis procedure to estimate glycemic index. Nutr. Res. 1997, 17, 427–437. [Google Scholar] [CrossRef] [Green Version]
- Jenkins, D.; Wolever, T.; Taylor, R.H.; Barker, H.; Fielden, H.; Baldwin, J.M.; Bowling, A.C.; Newman, H.C.; Jenkins, A.L.; Goff, D.V. Glycemic index of foods: A physiological basis for carbohydrate exchange. Am. J. Clin. Nutr. 1981, 34, 362–366. [Google Scholar] [CrossRef] [PubMed]
- O'dea, K.; Snow, P.; Nestel, P. Rate of starch hydrolysis in vitro as a predictor of metabolic responses to complex carbohydrate in vivo. Am. J. Clin. Nutr. 1981, 34, 1991–1993. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Englyst, H.N.; Veenstra, J.; Hudson, G.J. Measurement of rapidly available glucose (RAG) in plant foods: A potential in vitro predictor of the glycaemic response. Br. J. Nutr. 1996, 75, 327–337. [Google Scholar] [CrossRef] [PubMed]
- Dewettinck, K.; Van Bockstaele, F.; Kühne, B.; Van de Walle, D.; Courtens, T.; Gellynck, X. Nutritional value of bread: Influence of processing, food interaction and consumer perception. J. Cereal Sci. 2008, 48, 243–257. [Google Scholar] [CrossRef]
- Reshmi, S.; Sudha, M.; Shashirekha, M. Starch digestibility and predicted glycemic index in the bread fortified with pomelo (Citrus maxima) fruit segments. Food Chem. 2017, 237, 957–965. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.; Huang, Y.N.; Gao, B.; Xu, P.Y.; Inagaki, C.; Kawabata, J. α-Glucosidase inhibitory effect by the flower buds of Tussilago farfara L. Food Chem. 2008, 106, 1195–1201. [Google Scholar] [CrossRef]
- Jeng, T.L.; Chiang, Y.C.; Lai, C.C.; Liao, T.C.; Lin, S.Y.; Lin, T.C.; Sung, J.M. Sweet potato leaf extract inhibits the simulated in vitro gastrointestinal digestion of native starch. J. Food Drug Anal. 2015, 23, 399–406. [Google Scholar] [CrossRef] [PubMed]
- Barrett, A.; Ndou, T.; Hughey, C.A.; Straut, C.; Howell, A.; Dai, Z.; Kaletunc, G. Inhibition of α-amylase and glucoamylase by tannins extracted from cocoa, pomegranates, cranberries, and grapes. J. Agric. Food Chem. 2013, 61, 1477–1486. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, P.K.; Kumar, V.; Kumar, N.S.; Heinrich, M. The Ayurvedic medicine Clitoria ternatea—From traditional use to scientific assessment. J. Ethnopharmacol. 2008, 120, 291–301. [Google Scholar] [CrossRef] [PubMed]
- Talpate, K.A.; Bhosale, U.A.; Zambare, M.R.; Somani, R. Antihyperglycemic and antioxidant activity of Clitorea ternatea Linn. on streptozotocin-induced diabetic rats. Ayu 2013, 34, 433–439. [Google Scholar] [PubMed]
- Parimaladevi, B.; Boominathan, R.; Mandal, S.C. Evaluation of antipyretic potential of Clitoria ternatea L. extract in rats. Phytomedicine 2004, 11, 323–326. [Google Scholar] [CrossRef] [PubMed]
- Shyamkumar; Ishwar, B. Anti-inflammatory, analgesic and phytochemical studies of Clitoria ternatea Linn flower extract. Int. Res. J. Pharm. 2012, 3, 208–210. [Google Scholar]
- Kamilla, L.; Mnsor, S.M.; Ramanathan, S.; Sasidharan, S. Antimicrobial activity of Clitoria ternatea (L.) extracts. Pharmacologyonline 2009, 1, 731–738. [Google Scholar]
- Terahara, N.; Oda, M.; Matsui, T.; Osajima, Y.; Saito, N.; Toki, K.; Honda, T. Five new anthocyanins, ternatins A3, B4, B3, B2, and D2, from Clitoria ternatea flowers. J. Nat. Prod. 1996, 59, 139–144. [Google Scholar] [CrossRef] [PubMed]
- Adisakwattana, S.; Ruengsamran, T.; Kampa, P.; Sompong, W. In vitro inhibitory effects of plant-based foods and their combinations on intestinal α-glucosidase and pancreatic α-amylase. BMC Complement. Altern. Med. 2012, 12, 110. [Google Scholar] [CrossRef] [PubMed]
- Chayaratanasin, P.; Barbieri, M.A.; Suanpairintra, N.; Adisakwattana, S. Inhibitory effect of Clitoria ternatea flower petal extract on fructose-induced protein glycation and oxidation-dependent damages to albumin in vitro. BMC Complement. Altern. Med. 2015, 15, 27. [Google Scholar] [CrossRef] [PubMed]
- Englyst, H.N.; Kingman, S.; Cummings, J. Classification and measurement of nutritionally important starch fractions. Eur. J. Clin. Nutr. 1992, 46, S33–S50. [Google Scholar] [PubMed]
- Gularte, M.A.; Gómez, M.; Rosell, C.M. Impact of legume flours on quality and in vitro digestibility of starch and protein from gluten-free cakes. Food Bioprocess Technol. 2012, 5, 3142–3150. [Google Scholar] [CrossRef] [Green Version]
- Mishra, S.; Monro, J.A. Digestibility of starch fractions in wholegrain rolled oats. J. Cereal Sci. 2009, 50, 61–66. [Google Scholar] [CrossRef]
- Zhang, G.; Hamaker, B.R. Slowly digestible starch: Concept, mechanism, and proposed extended glycemic index. Crit. Rev. Food Sci. Nutr. 2009, 49, 852–867. [Google Scholar] [CrossRef] [PubMed]
- Englyst, K.N.; Englyst, H.N.; Hudson, G.J.; Cole, T.J.; Cummings, J.H. Rapidly available glucose in foods: An in vitro measurement that reflects the glycemic response. Am. J. Clin. Nutr. 1999, 69, 448–454. [Google Scholar] [CrossRef] [PubMed]
- Suganya, G.; Sampath Kumar, P.; Dheeba, B.; Sivakumar, R. In vitro antidiabetic, antioxidant and anti-inflammatory activity of Clitoria ternatea L. Int. J. Pharm. Pharm. Sci. 2014, 6, 342–347. [Google Scholar]
- Oboh, G.; Ademosun, A.O.; Ayeni, P.O.; Omojokun, O.S.; Bello, F. Comparative effect of quercetin and rutin on α-amylase, α-glucosidase, and some pro-oxidant-induced lipid peroxidation in rat pancreas. Comp. Clin. Pathol. 2015, 24, 1103–1110. [Google Scholar] [CrossRef]
- Sheng, Z.; Ai, B.; Zheng, L.; Zheng, X.; Xu, Z.; Shen, Y.; Jin, Z. Inhibitory activities of kaempferol, galangin, carnosic acid and polydatin against glycation and α-amylase and α-glucosidase enzymes. Int. J. Food Sci. Technol. 2018, 53, 755–766. [Google Scholar] [CrossRef]
- Homoki, J.R.; Nemes, A.; Fazekas, E.; Gyémánt, G.; Balogh, P.; Gál, F.; Al-Asri, J.; Mortier, J.; Wolber, G.; Babinszky, L.; et al. Anthocyanin composition, antioxidant efficiency, and α-amylase inhibitor activity of different Hungarian sour cherry varieties (Prunus cerasus L.). Food Chem. 2016, 194, 222–229. [Google Scholar] [CrossRef] [PubMed]
- Everette, J.D.; Walker, R.B.; Islam, S. Inhibitory activity of naturally occurring compounds towards rat intestinal α-glucosidase using p-nitrophenyl-α-d-glucopyranoside (PNP-G) as a substrate. Am. J. Food Technol. 2013, 8, 65–73. [Google Scholar]
- Podsedek, A.; Majewska, I.; Kucharska, A.Z. Inhibitory potential of red cabbage against digestive enzymes linked to obesity and type 2 diabetes. J. Agric. Food Chem. 2017, 65, 7192–7199. [Google Scholar] [CrossRef] [PubMed]
- Zhu, F. Interactions between starch and phenolic compound. Trends Food Sci. Technol. 2015, 43, 129–143. [Google Scholar] [CrossRef]
- Camelo-Méndez, G.A.; Agama-Acevedo, E.; Sanchez-Rivera, M.M.; Bello-Pérez, L.A. Effect on in vitro starch digestibility of Mexican blue maize anthocyanins. Food Chem. 2016, 211, 281–284. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Lin, Q.; Chen, Z.; Xiao, H. The interaction between tea polyphenols and rice starch during gelatinization. Food Sci. Technol. Int. 2011, 17, 569–577. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Englyst, K.N.; Liu, S.; Englyst, H.N. Nutritional characterization and measurement of dietary carbohydrates. Eur. J. Clin. Nutr. 2007, 61, S19–S39. [Google Scholar] [CrossRef] [PubMed]
- Calixto, F.S.; Abia, R. Resistant starch: An indigestible fraction of foods. Grasas Aceites 1991, 42, 239–242. [Google Scholar] [CrossRef]
- Cone, J.W.; Wolters, M.G. Some properties and degradability of isolated starch granules. Starch-Stärke 1990, 42, 298–301. [Google Scholar] [CrossRef]
- Alcázar-Alay, S.C.; Almeida-Meireles, M.A. Physicochemical properties, modifications and applications of starches from different botanical sources. Food Sci. Technol. 2015, 35, 215–236. [Google Scholar] [CrossRef] [Green Version]
- Meynier, A.; Goux, A.; Atkinson, F.; Brack, O.; Vinoy, S. Postprandial glycaemic response: How is it influenced by characteristics of cereal products? Br. J. Nutr. 2015, 113, 1931–1939. [Google Scholar] [CrossRef] [PubMed]
- Slavin, J.L. Carbohydrates, dietary fiber, and resistant starch in white vegetables: Links to health outcomes. Adv. Nutr. 2013, 4, 351S–355S. [Google Scholar] [CrossRef] [PubMed]
- Ells, L.J.; Seal, C.J.; Kettlitz, B.; Bal, W.; Mathers, J.C. Postprandial glycaemic, lipaemic and haemostatic responses to ingestion of rapidly and slowly digested starches in healthy young women. Br. J. Nutr. 2005, 94, 948–955. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Keeratipibul, S.; Luangsakul, N.; Lertsatchayarn, T. The effect of Thai glutinous rice cultivars, grain length and cultivating locations on the quality of rice cracker (arare). LWT-Food Sci. Technol. 2008, 41, 1934–1943. [Google Scholar] [CrossRef]
- Hu, P.; Zhao, H.; Duan, Z.; Linlin, Z.; Wu, D. Starch digestibility and the estimated glycemic score of different types of rice differing in amylose contents. J. Cereal Sci. 2004, 40, 231–237. [Google Scholar] [CrossRef]
- Chan, H.; Brand-Miller, J.; Holt, S.; Wilson, D. The glycaemic index values of Vietnamese foods. Eur. J. Clin. Nutr. 2001, 55, 1076–1083. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ranawana, D.; Henry, C.; Lightowler, H.; Wang, D. Glycaemic index of some commercially available rice and rice products in Great Britain. Int. J. Food Sci. Nutr. 2009, 60, 99–110. [Google Scholar] [CrossRef] [PubMed]
- Lemlioglu-Austin, D.; Turner, N.D.; McDonough, C.M.; Rooney, L.W. Effects of sorghum [Sorghum bicolor (L.) Moench] crude extracts on starch digestibility, estimated glycemic index (EGI), and resistant starch (RS) contents of porridges. Molecules 2012, 17, 11124–11138. [Google Scholar] [CrossRef] [PubMed]
- Dupuis, J.H.; Lu, Z.H.; Yada, R.Y.; Liu, Q. The effect of thermal processing and storage on the physicochemical properties and in vitro digestibility of potatoes. In. J. Food Sci. Technol. 2016, 51, 2233–2241. [Google Scholar] [CrossRef]
- Goh, R.; Gao, J.; Ananingsih, V.K.; Ranawana, V.; Henry, C.J.; Zhou, W. Green tea catechins reduced the glycaemic potential of bread: An in vitro digestibility study. Food Chem. 2015, 180, 203–210. [Google Scholar] [CrossRef] [PubMed]
- Coe, S.; Fraser, A.; Ryan, L. Polyphenol bioaccessibility and sugar reducing capacity of black, green, and white teas. Int. J. Food Sci. 2013, 2013, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Sivam, A.S.; Sun-Waterhouse, D.; Quek, S.; Perera, C.O. Properties of bread dough with added fiber polysaccharides and phenolic antioxidants: A review. J. Food Sci. 2010, 75, R163–R174. [Google Scholar] [CrossRef] [PubMed]
- Han, H.M.; Koh, B.K. Effect of phenolic acids on the rheological properties and proteins of hard wheat flour dough and bread. J. Sci. Food Agric. 2011, 91, 2495–2499. [Google Scholar] [CrossRef] [PubMed]
- Pathak, D.; Majumdar, J.; Raychaudhuri, U.; Chakraborty, R. Study on enrichment of whole wheat bread quality with the incorporation of tropical fruit by-product. Int. Food Res. J. 2017, 24, 238–246. [Google Scholar]
- Huang, G.; Guo, Q.; Wang, C.; Ding, H.H.; Cui, S.W. Fenugreek fibre in bread: Effects on dough development and bread quality. LWT-Food Sci. Technol. 2016, 71, 274–280. [Google Scholar] [CrossRef]






| CTE | % Inhibition | |||||
|---|---|---|---|---|---|---|
| Potato | Rice | Glutinous Rice | Wheat | Corn | Cassava | |
| 0.5% (w/v) | 7.1 ± 4.7 | 23.8 ± 9.2 | 20.8 ± 9.2 | 17.4 ± 6.5 | 24.1 ± 9.5 | 12.9 ± 1.4 |
| 1% (w/v) | 56.7 ± 7.8 | 82.1 ± 8.1 | 51.7 ± 8.5 | 50.1 ± 7.7 | 48.3 ± 8.2 | 34.5 ± 8.9 |
| 2% (w/v) | 93.4 ± 5.7 | 87.3 ± 13.4 | 89.7 ± 11.9 | 85.2 ± 4.3 | 81.6 ± 10.6 | 79.9 ± 19.7 |
| CTE | Hydrolysis Index (HI) | |||||
| Potato | Rice | Glutinous rice | Wheat | Corn | Cassava | |
| Control | 85.2 ± 0.5 a | 74.1 ± 4.3 a | 86.9 ± 2.4 a | 74.3 ± 2.5 a | 94.4 ± 3.6 a | 81.0 ± 1.3 a |
| 0.5% (w/v) | 71.0 ± 1.6 b | 63.4 ± 2.4 a,b | 65.5 ± 4.1 b | 62.4 ± 3.7 b | 69.5 ± 3.2 b | 65.5 ± 2.2 b |
| 1% (w/v) | 68.8 ± 3.7 b,c | 57.2 ± 4.9 b | 63.7 ± 5.2 b | 61.0 ± 1.4 b | 68.5 ± 2.2 b | 61.3 ± 3.4 b,c |
| 2% (w/v) | 62.2 ± 2.1 c | 55.2 ± 3.3 b | 50.3 ± 5.1 c | 50.4 ± 4.8 c | 59.0 ± 2.3 c | 51.5 ± 6.0 c |
| CTE | Predicted Glycemic Index (pGI) | |||||
| Potato | Rice | Glutinous rice | Wheat | Corn | Cassava | |
| Control | 86.5 ± 0.3 a | 80.4 ± 2.4 a | 87.2 ± 1.3 a | 80.5 ± 1.4 a | 91.2 ± 2.0 a | 84.2 ± 0.7 a |
| 0.5% (w/v) | 78.7 ± 0.9 b | 74.5 ± 1.3 a,b | 75.7 ± 2.2 b | 74.0 ± 2.0 b | 77.9 ± 1.8 b | 75.7 ± 1.2 b |
| 1% (w/v) | 77.5 ± 2.0 b,c | 71.1 ± 2.7 b | 74.7 ± 2.8 b | 73.2 ± 0.8 b | 77.3 ± 1.2 b | 73.4 ± 1.9 b,c |
| 2% (w/v) | 73.8 ± 1.1 c | 70.0 ± 1.8 b | 67.4 ± 2.8 c | 67.4 ± 2.6 c | 72.1 ± 1.3 c | 68.0 ± 3.3 c |
| Potato | Rice | Glutinous Rice | Wheat | Corn | Cassava | |
|---|---|---|---|---|---|---|
| CTE | −0.040 | 0.511 | 0.486 | 0.650 * | 0.373 | 0.758 * |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chusak, C.; Henry, C.J.; Chantarasinlapin, P.; Techasukthavorn, V.; Adisakwattana, S. Influence of Clitoria ternatea Flower Extract on the In Vitro Enzymatic Digestibility of Starch and Its Application in Bread. Foods 2018, 7, 102. https://doi.org/10.3390/foods7070102
Chusak C, Henry CJ, Chantarasinlapin P, Techasukthavorn V, Adisakwattana S. Influence of Clitoria ternatea Flower Extract on the In Vitro Enzymatic Digestibility of Starch and Its Application in Bread. Foods. 2018; 7(7):102. https://doi.org/10.3390/foods7070102
Chicago/Turabian StyleChusak, Charoonsri, Christiani Jeyakumar Henry, Praew Chantarasinlapin, Varanya Techasukthavorn, and Sirichai Adisakwattana. 2018. "Influence of Clitoria ternatea Flower Extract on the In Vitro Enzymatic Digestibility of Starch and Its Application in Bread" Foods 7, no. 7: 102. https://doi.org/10.3390/foods7070102






