SPME Method Optimized by Box-Behnken Design for Impact Odorants in Reduced Alcohol Wines
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Instrumentation
2.3. Compound Identification and Elution Profiles
- RI = relative index of compound i;
- z = carbon number of the alkane z;
- tR(i), tR(z), and tR(z+1) = retention times of the compound i, the compound z, and the alkane z + 1, respectively.
2.4. Optimization of Sample Extraction Conditions
- ŷ = predicted response; b0 is the intercept or average response;
- b1x1 + b2x2 + b3x3 = linear terms associated with each factor (temp, time, sample vol.);
- b12x1x2 + b13x1x3 + b23x2x3 = second-order interaction terms between each factor;
- b11x12 + b22x22 + b33x32 = quadratic terms for each factor;
- x1 = factor extraction temperature;
- x2 = factor extraction time;
- x3 = factor sample volume in a 20 mL vial.
2.5. Calibration Curves
2.6. Natural and Reduced Alcohol Wines
2.7. Reconstituted Wines
2.8. GC-MS Analysis of Wine Samples
3. Results and Discussion
3.1. Optimization of SPME Factors
3.2. Volatile Changes after RO-EP Treatment
3.3. Effect of Ethanol Content Reduction
4. Conclusions
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- WHO. Global Strategy to Reduce the Harmful Use of Alcohol. Available online: http://www.who.int/substance_abuse/activities/gsrhua/en/ (accessed on 3 January 2018).
- Sharma, A.; Sinha, K.; Vandenberg, B. Pricing as a means of controlling alcohol consumption. Br. Med. Bull. 2017, 123, 149–158. [Google Scholar] [CrossRef] [PubMed]
- Annunziata, A.; Pomarici, E.; Vecchio, R.; Mariani, A. Do consumers want more nutritional and health information on wine labels? Insights from the EU and USA. Nutrients 2016, 8, 416. [Google Scholar] [CrossRef] [PubMed]
- Schmidtke, L.M.; Blackman, J.W.; Agboola, S.O. Production technologies for reduced alcoholic wines. J. Food Sci. 2012, 77, 25–41. [Google Scholar] [CrossRef] [PubMed]
- Varela, C.; Dry, P.; Kutyna, D.; Francis, I.; Henschke, P.; Curtin, C.; Chambers, P. Strategies for reducing alcohol concentration in wine. Aust. J. Grape Wine Res. 2015, 21, 670–679. [Google Scholar] [CrossRef]
- Longo, R.; Blackman, J.W.; Antalick, G.; Torley, P.J.; Rogiers, S.Y.; Schmidtke, L.M. Harvesting and blending options for lower alcohol wines: A sensory and chemical investigation. J. Sci. Food Agric. 2018, 98, 33–42. [Google Scholar] [CrossRef] [PubMed]
- Longo, R.; Blackman, J.W.; Torley, P.J.; Rogiers, S.Y.; Schmidtke, L.M. Changes in volatile composition and sensory attributes of wines during alcohol content reduction. J. Sci. Food Agric. 2017, 97, 8–16. [Google Scholar] [CrossRef] [PubMed]
- Sáenz-Navajas, M.-P.; Avizcuri, J.-M.; Ballester, J.; Fernández-Zurbano, P.; Ferreira, V.; Peyron, D.; Valentin, D. Sensory-active compounds influencing wine experts’ and consumers’ perception of red wine intrinsic quality. LWT-Food Sci. Technol. 2015, 60, 400–411. [Google Scholar] [CrossRef]
- Bindon, K.; Holt, H.; Williamson, P.O.; Varela, C.; Herderich, M.; Francis, I.L. Relationships between harvest time and wine composition in Vitis vinifera L. cv. Cabernet sauvignon 2. Wine sensory properties and consumer preference. Food Chem. 2014, 154, 90–101. [Google Scholar] [CrossRef] [PubMed]
- Muñoz-Redondo, J.M.; Cuevas, F.J.; León, J.M.; Ramírez, P.; Moreno-Rojas, J.M.; Ruiz-Moreno, M.J. Quantitative profiling of ester compounds using HS-SPME-GC-MS and chemometrics for assessing volatile markers of the second fermentation in bottle. J. Agric. Food Chem. 2017, 65, 2768–2775. [Google Scholar] [CrossRef] [PubMed]
- Avellone, G.; Salvo, A.; Costa, R.; Saija, E.; Bongiorno, D.; Di Stefano, V.; Calabrese, G.; Dugo, G. Investigation on the influence of spray-drying technology on the quality of Sicilian Nero d’Avola wines. Food Chem. 2018, 240, 222–230. [Google Scholar] [CrossRef] [PubMed]
- Rocha, S.; Ramalheira, V.; Barros, A.; Delgadillo, I.; Coimbra, M.A. Headspace solid phase microextraction (SPME) analysis of flavor compounds in wines. Effect of the matrix volatile composition in the relative response factors in a wine model. J. Agric. Food. Chem. 2001, 49, 5142–5151. [Google Scholar] [CrossRef] [PubMed]
- Rebiere, L.; Clark, A.C.; Schmidtke, L.M.; Prenzler, P.D.; Scollary, G.R. A robust method for quantification of volatile compounds within and between vintages using headspace-solid-phase micro-extraction coupled with GC-MS application on Semillon wines. Anal. Chim. Acta 2010, 660, 149–157. [Google Scholar] [CrossRef] [PubMed]
- Longo, R.; Blackman, J.W.; Antalick, G.; Torley, P.J.; Rogiers, S.Y.; Schmidtke, L.M. Volatile and sensory profiling of Shiraz wine in response to alcohol management: Comparison of harvest timing versus technological approaches. Food Res. Int. 2018, 109, 561–571. [Google Scholar] [CrossRef] [PubMed]
- Villamor, R.R.; Evans, M.A.; Mattinson, D.S.; Ross, C.F. Effects of ethanol, tannin and fructose on the headspace concentration and potential sensory significance of odorants in a model wine. Food Res. Int. 2013, 50, 38–45. [Google Scholar] [CrossRef]
- Whiton, R.; Zoecklein, B. Optimization of headspace solid-phase microextraction for analysis of wine aroma compounds. Am. J. Enol. Viticult. 2000, 51, 379–382. [Google Scholar]
- Candioti, L.V.; De Zan, M.M.; Cámara, M.S.; Goicoechea, H.C. Experimental design and multiple response optimization. Using the desirability function in analytical methods development. Talanta 2014, 124, 123–138. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, S.L.C.; Lemos, V.A.; de Carvalho, V.S.; da Silva, E.G.P.; Queiroz, A.F.S.; Felix, C.S.A.; da Silva, D.L.F.; Dourado, G.B.; Oliveira, R.V. Multivariate optimization techniques in analytical chemistry—An overview. Microchem. J. 2018, 140, 176–182. [Google Scholar] [CrossRef]
- Siebert, T.E.; Smyth, H.E.; Capone, D.L.; Neuwöhner, C.; Pardon, K.H.; Skouroumounis, G.K.; Herderich, M.J.; Sefton, M.A.; Pollnitz, A.P. Stable isotope dilution analysis of wine fermentation products by HS-SPME-GC-MS. Anal. Bioanal. Chem. 2005, 381, 937–947. [Google Scholar] [CrossRef] [PubMed]
- Francis, I.L.; Newton, J.L. Determining wine aroma from compositional data. Aust. J. Grape Wine Res. 2005, 11, 114–126. [Google Scholar] [CrossRef]
- Antalick, G.; Perello, M.-C.; de Revel, G. Development, validation and application of a specific method for the quantitative determination of wine esters by headspace-solid-phase microextraction-gas chromatography–mass spectrometry. Food Chem. 2010, 121, 1236–1245. [Google Scholar] [CrossRef]
- Sadoughi, N.; Schmidtke, L.M.; Antalick, G.; Blackman, J.W.; Steel, C.C. Gas chromatography-mass spectrometry method optimized using response surface modeling for the quantitation of fungal off-flavors in grapes and wine. J. Agric. Food. Chem. 2015, 63, 2877–2885. [Google Scholar] [CrossRef] [PubMed]
- Kondjoyan, N.; Berdagué, J.-L. A Compilation of Relative Retention Indices for the Analysis of Aromatic Compounds; Laboratoire Flaveur: Alger, France, 1996. [Google Scholar]
- Brereton, R.G. Chemometrics: Data Analysis for the Laboratory and Chemical Plant; John Wiley & Sons: Hoboken, NJ, USA, 2003. [Google Scholar]
- Longo, R.; Blackman, J.W.; Antalick, G.; Torley, P.J.; Rogiers, S.Y.; Schmidtke, L.M. A comparative study of partial dealcoholisation versus early harvest: Effects on wine volatile and sensory profiles. Food Chem. 2018, 261, 21–29. [Google Scholar] [CrossRef] [PubMed]
- Burin, V.M.; Marchand, S.; de Revel, G.; Bordignon-Luiz, M.T. Development and validation of method for heterocyclic compounds in wine: Optimization of HS-SPME conditions applying a response surface methodology. Talanta 2013, 117, 87–93. [Google Scholar] [CrossRef] [PubMed]
- Damerau, A.; Kamlang-ek, P.; Moisio, T.; Lampi, A.M.; Piironen, V. Effect of spme extraction conditions and humidity on the release of volatile lipid oxidation products from spray-dried emulsions. Food Chem. 2014, 157, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Ma, Q.L.; Hamid, N.; Bekhit, A.E.D.; Robertson, J.; Law, T.F. Optimization of headspace solid phase microextraction (HS-SPME) for gas chromatography mass spectrometry (GC-MS) analysis of aroma compounds in cooked beef using response surface methodology. Microchem. J. 2013, 111, 16–24. [Google Scholar] [CrossRef]
- Azzi-Achkouty, S.; Estephan, N.; Ouaini, N.; Rutledge, D.N. Headspace solid-phase microextraction for wine volatile analysis. Crit. Rev. Food Sci. Nutr. 2017, 57, 2009–2020. [Google Scholar] [CrossRef] [PubMed]
- Lord, H.; Pawliszyn, J. Evolution of solid-phase microextraction technology. J. Chromatogr. A 2000, 885, 153–193. [Google Scholar] [CrossRef]
- Ferreira, V.; López, R.; Cacho, J.F. Quantitative determination of the odorants of young red wines from different grape varieties. J. Sci. Food Agric. 2000, 80, 1659–1667. [Google Scholar] [CrossRef]
- Motta, S.; Guaita, M.; Petrozziello, M.; Ciambotti, A.; Panero, L.; Solomita, M.; Bosso, A. Comparison of the physicochemical and volatile composition of wine fractions obtained by two different dealcoholization techniques. Food Chem. 2017, 221, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Bencomo, J.J.; Muñoz-González, C.; Andújar-Ortiz, I.; Martín-Álvarez, P.J.; Moreno-Arribas, M.V.; Pozo-Bayón, M.Á. Assessment of the effect of the non-volatile wine matrix on the volatility of typical wine aroma compounds by headspace solid phase microextraction/gas chromatography analysis. J. Sci. Food Agric. 2011, 91, 2484–2494. [Google Scholar] [CrossRef] [PubMed]
- Robinson, A.L.; Ebeler, S.E.; Heymann, H.; Boss, P.K.; Solomon, P.S.; Trengove, R.D. Interactions between wine volatile compounds and grape and wine matrix components influence aroma compound headspace partitioning. J. Agric. Food Chem. 2009, 57, 10313–10322. [Google Scholar] [CrossRef] [PubMed]
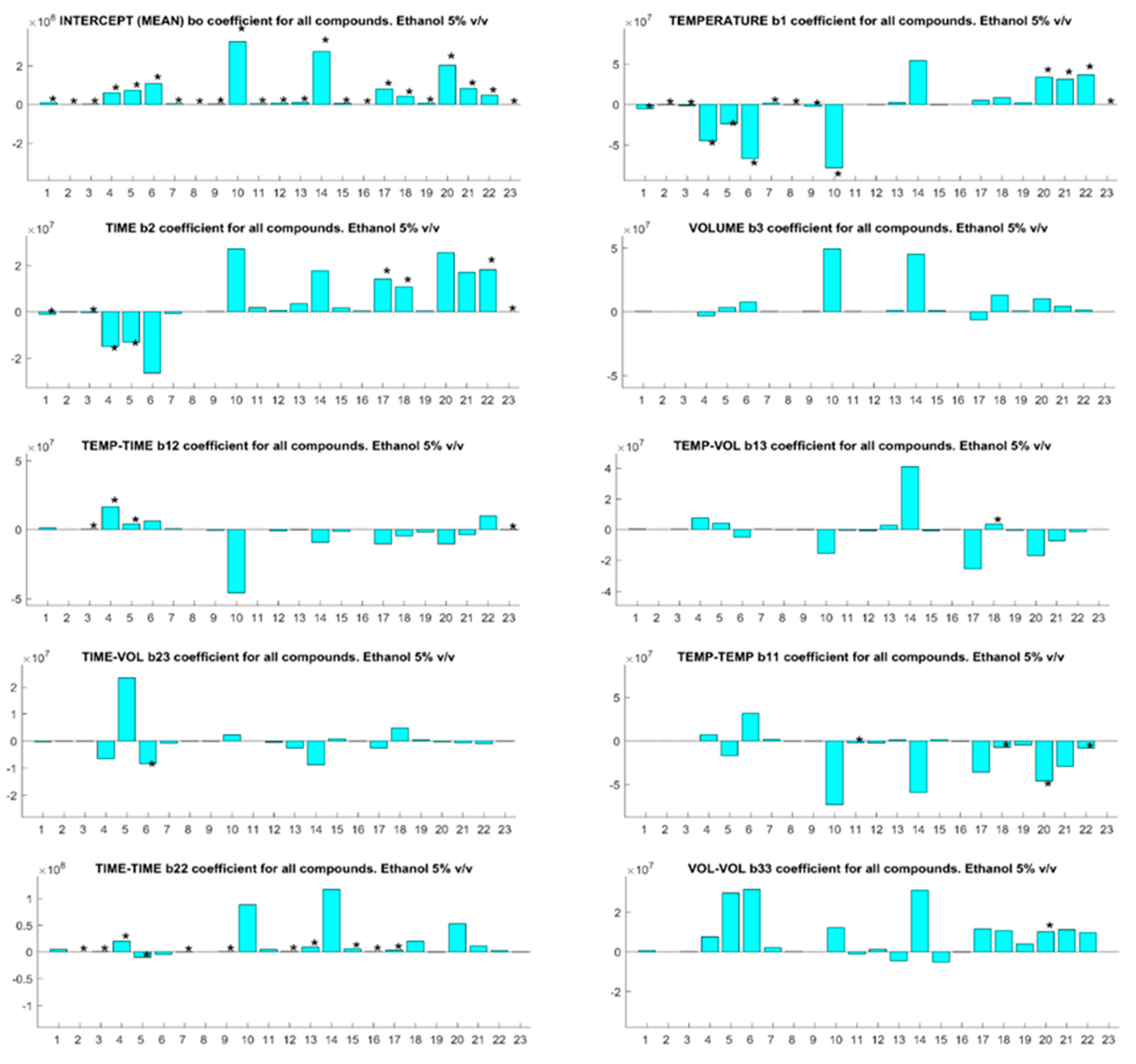

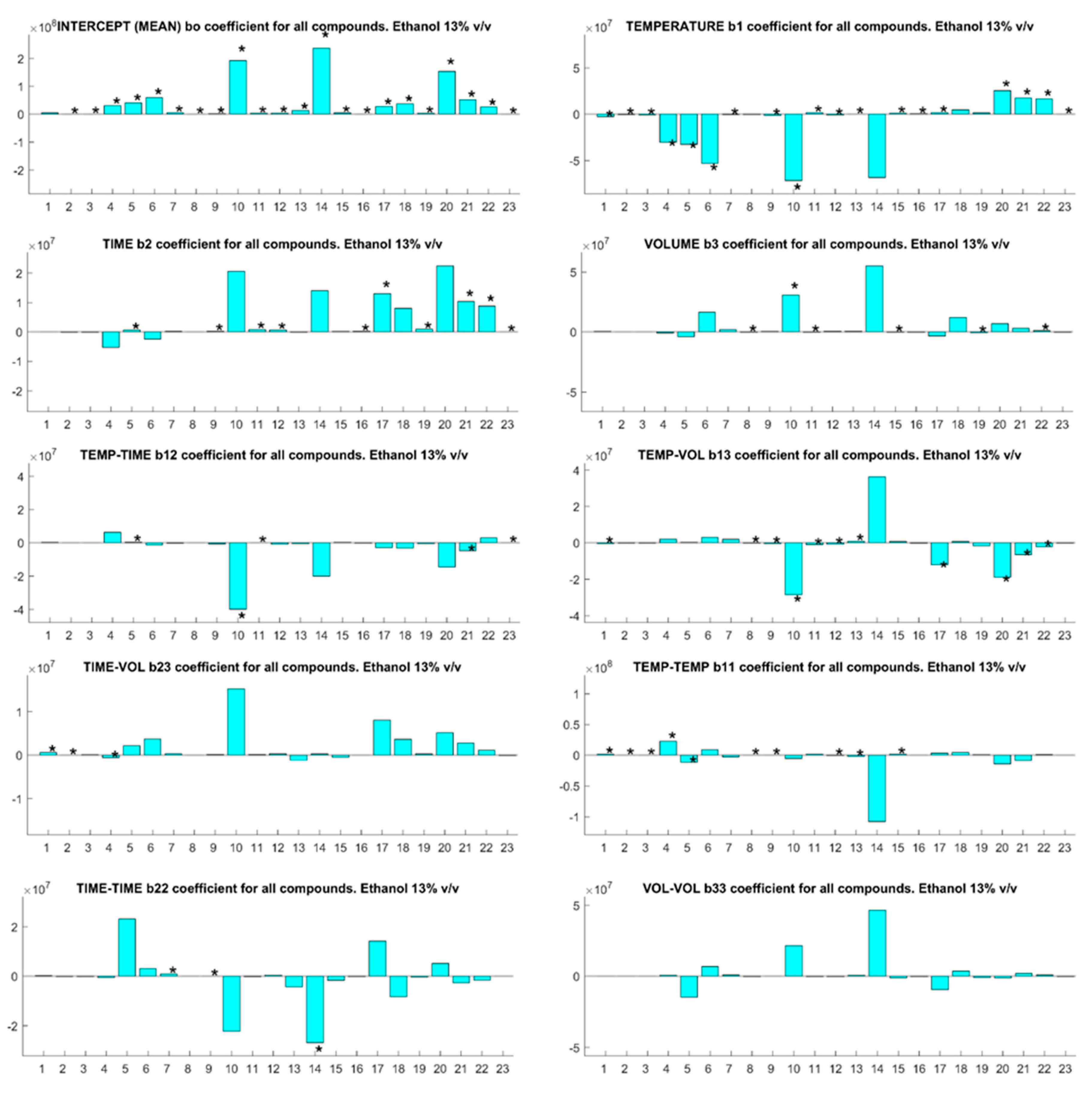
| Code | Compounds | Odors 1 | OT 1 (µg/L) | BP (°C) |
|---|---|---|---|---|
| 1 | ethyl butyrate | apple | 20 | 121 |
| 2 | ethyl-2-methyl butyrate | apple | 1–18 | 138 |
| 3 | ethyl-3-methyl butyrate | sweat, acid, rancid | 33.4 | 134 |
| 4 | isoamyl acetate | banana | 30 | 130 |
| 5 | 3-methyl-1-butanol | whiskey, malt, burnt | 30,000 | 132 |
| 6 | ethyl hexanoate | apple peel, fruit | 2–14 | 167 |
| 7 | ethyl-s-lactate | fruit, milk 2 | 154,000 | 154 |
| 8 | (z)-3-hexenol | green (cut grass) | 400 | 156 |
| 9 | methyl octanoate | waxy, apple peel 2 | - | 192 |
| 10 | ethyl octanoate | fruit, fat | 2–5 | 207 |
| 11 | propanoic acid | pungent, rancid, soy | 8100 | 141 |
| 12 | linalool | flower, lavender | 25.2 | 198 |
| 13 | methyl decanoate | wax, soap, fruit 2 | - | 108 |
| 14 | ethyl decanoate | grape | 200 | 245 |
| 15 | isoamyl octanoate | wax, soap, pear 2 | - | 267 |
| 16 | 3-(methylthio)-1-propanol | sweet, potato | 1000 | 90 |
| 17 | β-phenyl ethyl acetate | rose, honey | 250 | 229 |
| 18 | ethyl dodecanoate | wax, soap 2 | - | 269 |
| 19 | geraniol | rose, geranium | 30 | 230 |
| 20 | β-phenyl ethanol | honey, rose | 10,000–14,000 | 219 |
| 21 | octanoic acid | sweat, cheese | 500 | 240 |
| 22 | decanoic acid | rancid, fat | 1000 | 268 |
| 23 | vanillin | vanilla | 200 | 285 |
| Parameter/Conditions | Levels |
|---|---|
| Extraction temperature (°C) | 30, 50, 70 |
| Extraction time (min) | 15, 30, 45 |
| Sample volume (mL) in 20 mL vial | 7, 10, 13 |
| Compound (µg L−1) | Dealcoholized and Natural Wines | Reconstituted Wines | |||
|---|---|---|---|---|---|
| 5% v/v | 8% v/v | 13.7% v/v | Storage Time | ||
| 24 h | 14 Days | ||||
| ethyl butyrate | 0.90 ± 0.01 a | 1.72 ± 0.08 b | 2.69 ± 0.06 c | 0.75 ± 0.01 a | 0.76 ± 0.02 a |
| ethyl-2-methyl butyrate | 0.33 ± 0.00 b | 0.46 ± 0.03 c | 0.47 ± 0.01 c | 0.23 ± 0.01 a | 0.17 ± 0.00 a |
| ethyl-3-methyl butyrate | 0.05 ± 0.00 b | 0.08 ± 0.01 c | 0.08 ± 0.00 c | 0.04 ± 0.00 ab | 0.03 ± 0.00 a |
| isoamyl acetate | 0.21 ± 0.01 b | 0.43 ± 0.03 c | 0.51 ± 0.03 c | 0.08 ± 0.01 a | 0.08 ± 0.01 a |
| 3-methyl-1-butanol | 6.18 ± 0.06 ab | 6.64 ± 0.41 b | 9.05 ± 0.24 c | 5.62 ± 0.17 ab | 5.38 ± 0.13 a |
| ethyl hexanoate | 1.72 ± 0.02 a | 2.34 ± 0.23 a | 5.41 ± 0.88 b | 1.38 ± 0.11 a | 1.51 ± 0.05 a |
| ethyl-s-lactate | 33,500 ± 720 a | 32,300 ± 2600 a | 47,300 ± 3060 b | 41,900 ± 1870 ab | 33,900 ± 1560 a |
| (z)-3-hexenol | 5.32 ± 0.10 c | 5.42 ± 0.04 c | 6.83 ± 0.05 d | 3.61 ± 0.14 a | 4.49 ± 0.08 b |
| methyl octanoate | 0.01 ± 0.00 | 0.01 ± 0.00 | 0.02 ± 0.00 | BLQ | BLQ |
| ethyl octanoate | 3.17 ± 0.01 b | 4.90 ± 0.15 c | 7.32 ± 0.15 d | 2.31 ± 0.02 a | 2.17 ± 0.05 a |
| propanoic acid | 600 ± 20 c | 250 ± 10 ab | 220 ± 0.00 a | 240 ± 0.00 a | 290 ± 10 b |
| linalool | 0.53 ± 0 b | 0.59 ± 0.01 b | 0.25 ± 0.06 a | 0.27 ± 0.01 a | 0.27 ± 0.00 a |
| methyl decanoate | BLQ | 0.01 ± 0.00 | 0.01 ± 0.00 | 0.01 ± 0.00 | 0.01 ± 0.00 |
| ethyl decanoate | 1.66 ± 0.02 bc | 1.39 ± 0.10 b | 2.64 ± 0.46 c | 0.19 ± 0.13 a | 0.74 ± 0.09 ab |
| isoamyl octanoate | 0.02 ± 0.00 b | 0.02 ± 0.00 b | 0.02 ± 0.00 b | 0.01 ± 0.00 a | 0.01 ± 0.00 a |
| 3-(methylthio)-1-propanol | 103 ± 0.42 a | 101 ± 0.87 a | 103 ± 12.0 a | 92.1 ± 3.44 a | 90.7 ± 9.40 a |
| β-phenyl ethyl acetate | 0.03 ± 0.00 a | 0.04 ± 0.00 a | 0.05 ± 0.00 b | 0.02 ± 0.00 a | 0.03 ± 0.00 a |
| ethyl dodecanoate | 0.04 ± 0.00 a | 0.03 ± 0.00 a | 0.12 ± 0.01 b | 0.02 ± 0.00 a | 0.02 ± 0.00 a |
| geraniol | 0.09 ± 0.00 a | 0.09 ± 0.00 a | 0.25 ± 0.03 b | 0.06 ± 0.00 a | 0.06 ± 0.00 a |
| β-phenyl ethanol | 280 ± 0.00 d | 320 ± 0.00 e | 240 ± 0.00 c | 230 ± 0.00 b | 200 ± 0.00 a |
| octanoic acid | 8.00 ± 0.12 c | 7.10 ± 0.19 b | 9.01 ± 0.3 d | 6.58 ± 0.08 b | 5.36 ± 0.10 a |
| decanoic acid | 0.94 ± 0.01 a | 0.90 ± 0.02 a | 2.16 ± 0.22 b | 1.16 ± 0.05 a | 0.96 ± 0.05 a |
| vanillin | 0.80 ± 0.06 a | 1.16 ± 0.1 ab | 1.13 ± 0.09 ab | 2.01 ± 0.40 b | 0.92 ± 0.08 a |
| Compound (µg L−1) | Dealcoholized and Natural Wines | Reconstituted Wines | |||
|---|---|---|---|---|---|
| 5% v/v | 8% v/v | 12.2% v/v | Storage Time | ||
| 24 h | 14 Days | ||||
| ethyl butyrate | 0.96 ± 0.01 a | 2.42 ± 0.23 b | 4.12 ± 0.05 c | 0.87 ± 0.02 a | 0.88 ± 0.01 a |
| ethyl-2-methyl butyrate | 0.11 ± 0.00 b | 0.20 ± 0.01 c | 0.25 ± 0.00 d | 0.09 ± 0.00 ab | 0.07 ± 0.00 a |
| ethyl-3-methyl butyrate | 0.02 ± 0.00 a | 0.04 ± 0.01 b | 0.05 ± 0.00 b | 0.02 ± 0.00 a | 0.01 ± 0.00 a |
| isoamyl acetate | 0.04 ± 0.01 a | 0.23 ± 0.01 b | 0.21 ± 0.00 b | BLQ | BLQ |
| 3-methyl-1-butanol | 3.92 ± 0.04 ab | 5.56 ± 0.26 bc | 6.68 ± 0.74 c | 2.78 ± 0.11 a | 2.92 ± 0.07 a |
| ethyl hexanoate | 3.79 ± 0.04 a | 5.77 ± 0.70 b | 11.6 ± 0.33 c | 2.62 ± 0.12 a | 2.84 ± 0.03 a |
| ethyl-s-lactate | 4790 ± 80 ab | 5230 ± 250 ab | 6560 ± 930 b | 4360 ± 260 a | 4280 ± 130 a |
| (z)-3-hexenol | 3.75 ± 0.06 c | 4.08 ± 0.03 c | 4.68 ± 0.14 d | 2.35 ± 0.07 a | 3.07 ± 0.03 b |
| methyl octanoate | 0.01 ± 0.00 | 0.01 ± 0.00 | 0.03 ± 0.00 | BLQ | BLQ |
| ethyl octanoate | 8.82 ± 0.09 b | 15.5 ± 0.71 c | 32.3 ± 0.65 d | 5.23 ± 0.02 a | 5.82 ± 0.07 a |
| propanoic acid | 200 ± 20 | 230 ± 10 | 180 ± 0.00 | 180 ± 0.00 | 210 ± 0.00 |
| linalool | 0.29 ± 0.00 c | 0.37 ± 0.00 d | 0.08 ± 0.00 b | 0.05 ± 0.00 a | 0.06 ± 0.00 a |
| methyl decanoate | BLQ | 0.01 ± 0.00 | 0.02 ± 0.00 | 0.01 ± 0.00 | 0.01 ± 0.00 |
| ethyl decanoate | 4.18 ± 0.06 b | 3.59 ± 0.31 b | 12.4 ± 0.33 c | 0.87 ± 0.42 a | 1.82 ± 0.03 a |
| isoamyl octanoate | 0.04 ± 0.00 b | 0.03 ± 0.00 b | 0.06 ± 0.00 c | 0.01 ± 0.00 a | 0.01 ± 0.00 a |
| 3-(methylthio)-1-propanol | 40.9 ± 0.22 b | 43.3 ± 1.76 b | 38.2 ± 1.46 b | 29.1 ± 1.61 a | 40.4 ± 1.27 b |
| β-phenyl ethyl acetate | 0.03 ± 0.00 ab | 0.04 ± 0.00 bc | 0.04 ± 0.00 c | 0.03 ± 0.00 a | 0.03 ± 0.00 a |
| ethyl dodecanoate | 0.06 ± 0.00 b | 0.07 ± 0.00 b | 0.3 ± 0.01 c | 0.02 ± 0.00 a | 0.02 ± 0.00 a |
| geraniol | 0.20 ± 0.00 c | 0.12 ± 0.00 b | 0.67 ± 0.01 d | 0.14 ± 0.02 b | 0.06 ± 0.00 a |
| β-phenyl ethanol | 90 ± 0.00 b | 90 ± 0.00 b | 50 ± 0.00 a | 50 ± 0.00 a | 50 ± 0.00 a |
| octanoic acid | 30.3 ± 0.14 b | 35.7 ± 0.67 c | 35.2 ± 1.12 c | 24.6 ± 0.12 a | 22.1 ± 0.12 a |
| decanoic acid | 3.37 ± 0.04 a | 3.72 ± 0.34 a | 15.7 ± 0.60 b | 3.69 ± 0.16 a | 2.61 ± 0.08 a |
| vanillin | 0.08 ± 0.01 a | 0.12 ± 0.01 a | 0.19 ± 0.01 ab | 0.29 ± 0.05 b | 0.15 ± 0.02 a |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saha, B.; Longo, R.; Torley, P.; Saliba, A.; Schmidtke, L. SPME Method Optimized by Box-Behnken Design for Impact Odorants in Reduced Alcohol Wines. Foods 2018, 7, 127. https://doi.org/10.3390/foods7080127
Saha B, Longo R, Torley P, Saliba A, Schmidtke L. SPME Method Optimized by Box-Behnken Design for Impact Odorants in Reduced Alcohol Wines. Foods. 2018; 7(8):127. https://doi.org/10.3390/foods7080127
Chicago/Turabian StyleSaha, Bithika, Rocco Longo, Peter Torley, Anthony Saliba, and Leigh Schmidtke. 2018. "SPME Method Optimized by Box-Behnken Design for Impact Odorants in Reduced Alcohol Wines" Foods 7, no. 8: 127. https://doi.org/10.3390/foods7080127
APA StyleSaha, B., Longo, R., Torley, P., Saliba, A., & Schmidtke, L. (2018). SPME Method Optimized by Box-Behnken Design for Impact Odorants in Reduced Alcohol Wines. Foods, 7(8), 127. https://doi.org/10.3390/foods7080127






