Suppression of Pancreatin-Induced Digestion of Starch in Starch Granules by Starch/Fatty Acid and Starch/Flavonoid Complexes in Retrograding Rice Flour
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ingredients and Reagents
2.2. Preparation of Adzuki Extract
2.3. Preparation of Retrograding Joshinko
2.4. Estimation of Starch Digestion
2.5. Measurements of Total Sugar
2.6. Estimation of Flavonoid and Fatty Acid Contents by HPLC
2.7. Reconstitution Experiments
2.8. Presentation of Data
3. Results and Discussion
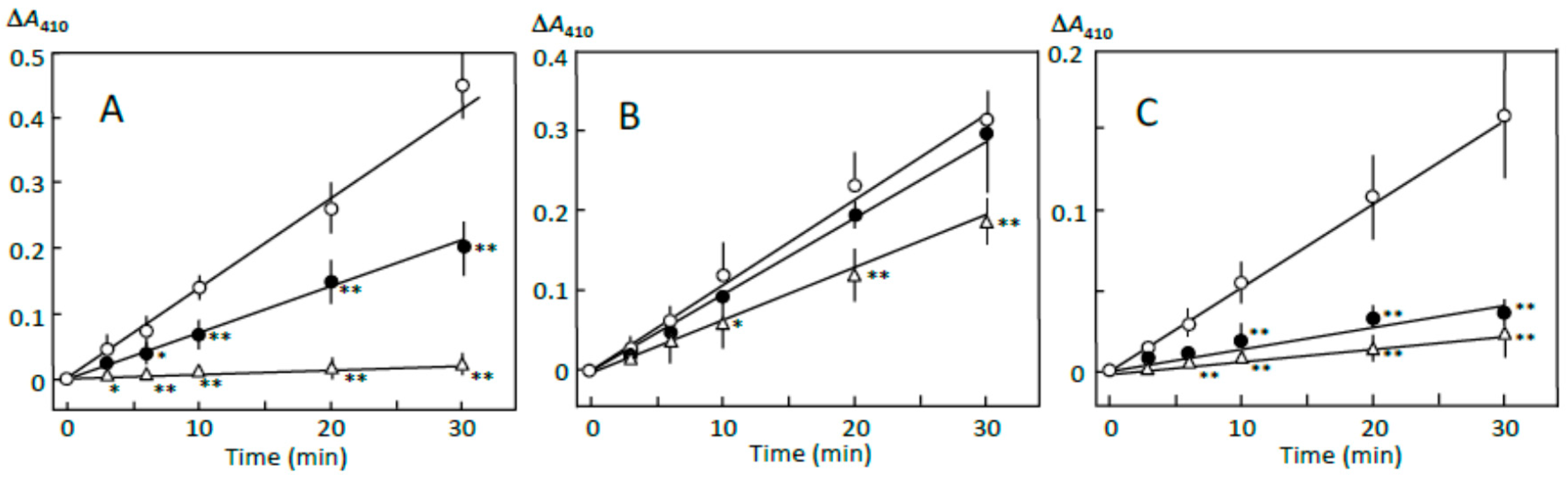
3.1. Starch Digestion Estimated by Reducing Sugar Formation
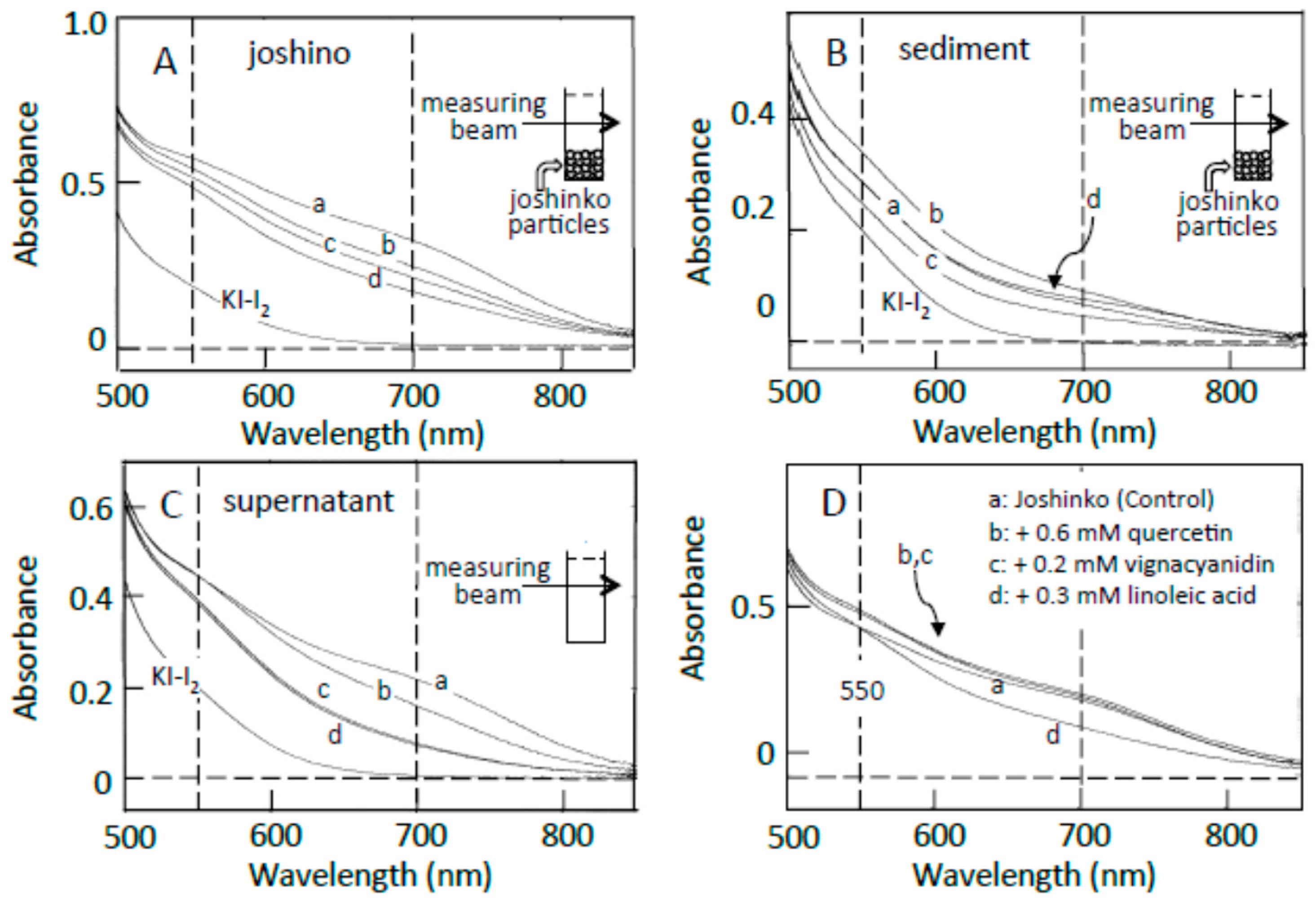
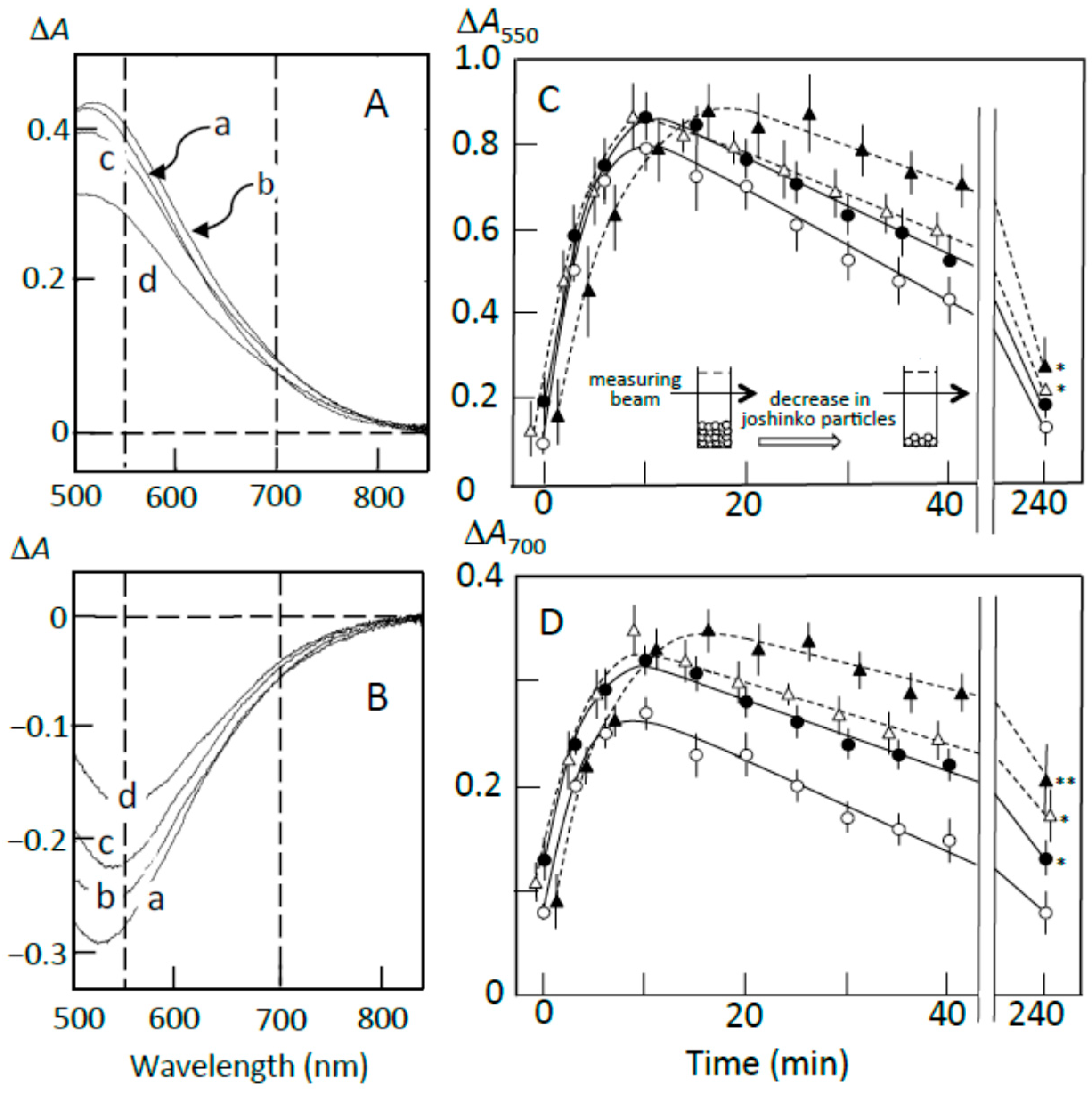
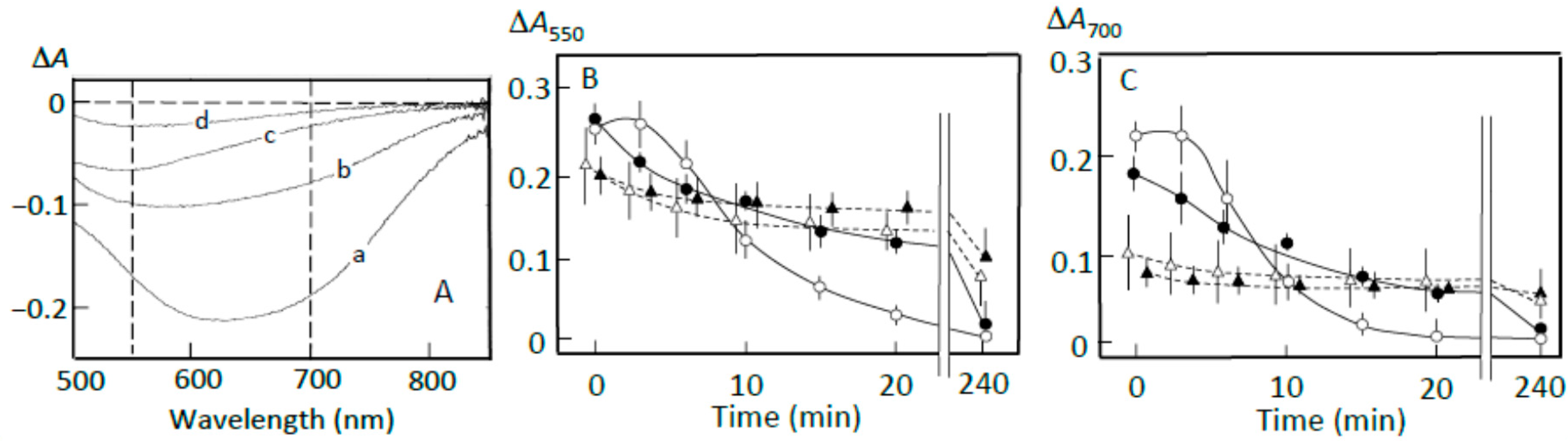
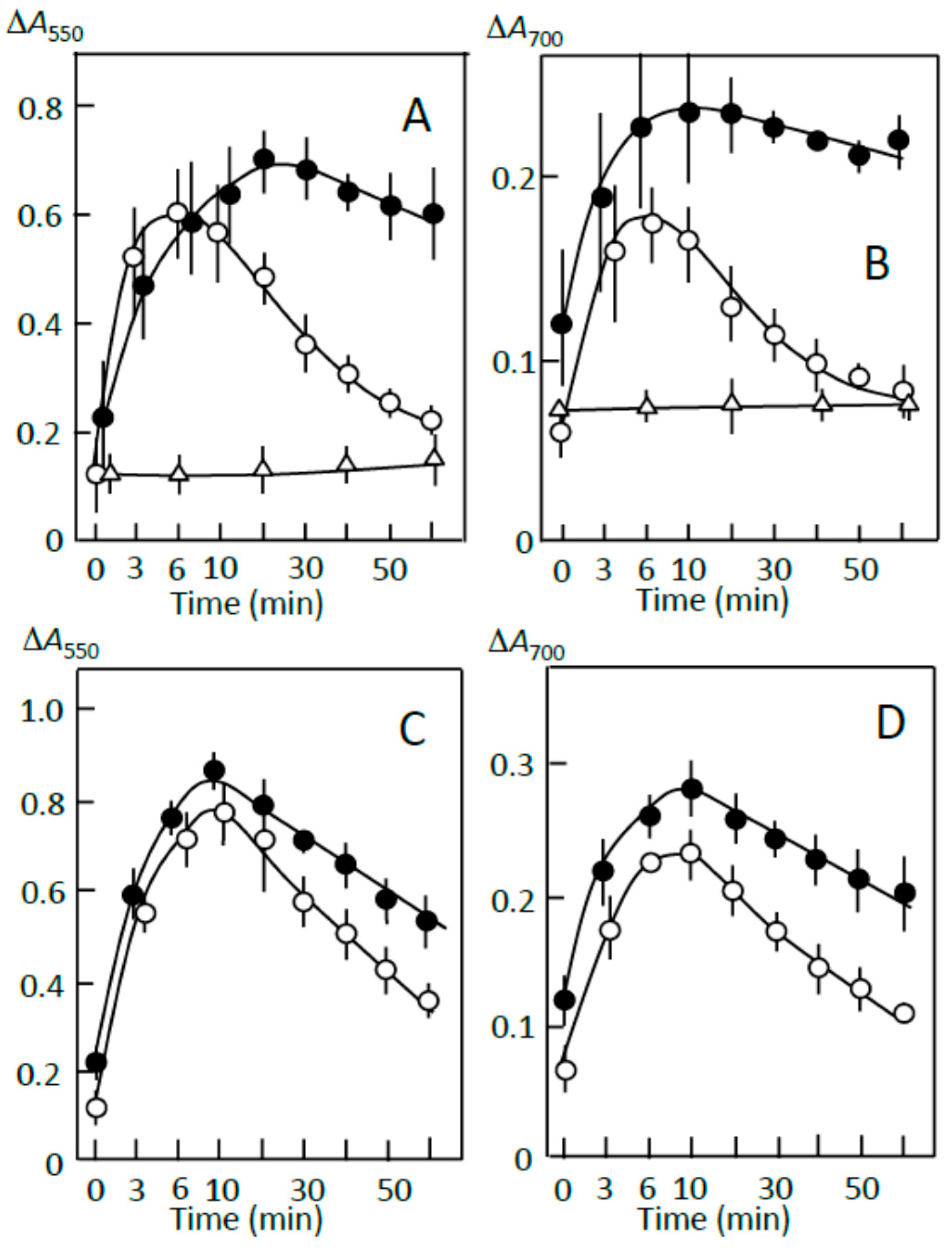
3.2. Starch-Iodine Complex Formation in Retrograding Joshinko
3.3. Starch Digestion of Retrograding Joshinko Estimated Using Iodine
3.4. Starch Digestion of the Sediment Estimated Using Iodine
3.5. Starch Digestion of the Supernatant Estimated Using Iodine
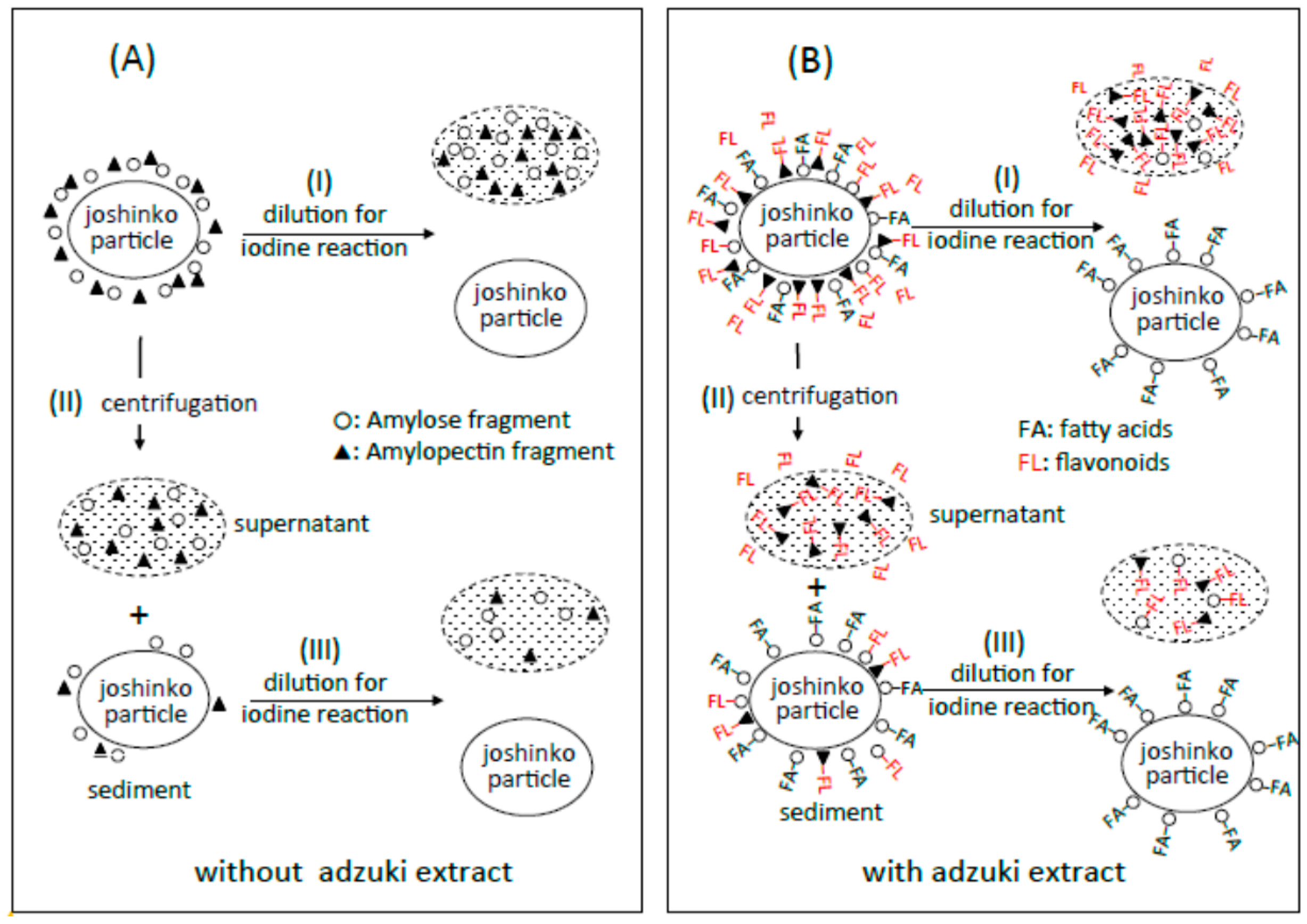
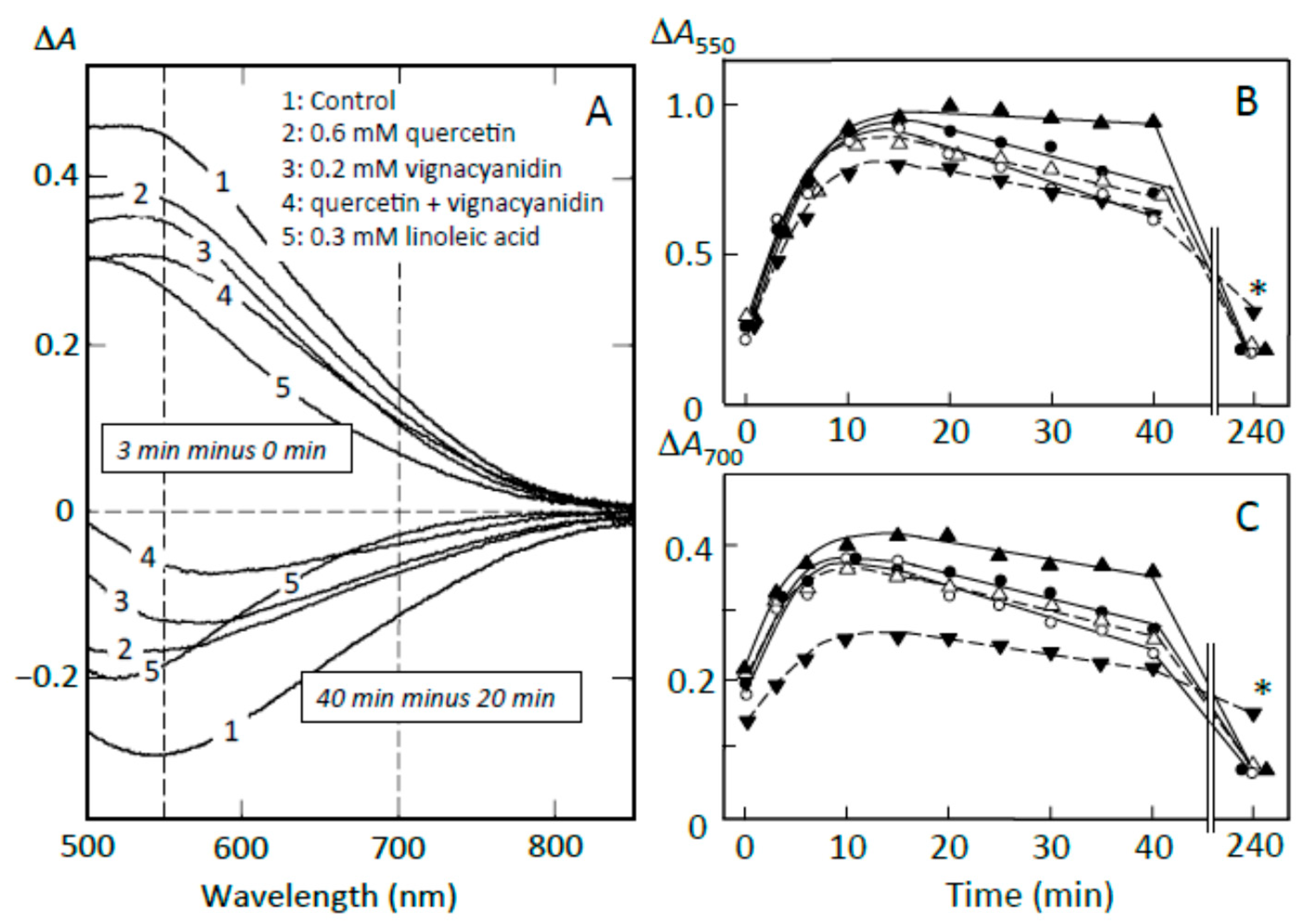
3.6. Suppression of Starch Digestion by Amylose and Amylopectin Combined with Adzuki Components
3.7. Suppression of Starch Digestion by Fatty Acids and Flavonoids in Adzuki Extract
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Gohara, A.K.; Pereira de Souza, A.H.; Gomes, S.T.M.; Evelázio de Souza, N.; Visentainer, J.V.; Matsushita, M. Nutritional and bioactive compounds of adzuki bean cultivars using chemometric approach. Cienc. Agrotecnol. 2016, 40, 104–113. [Google Scholar] [CrossRef]
- Lin, P.Y.; Lai, H.M. Bioactive compounds in legumes and their germinated products. J. Agric. Food Chem. 2006, 54, 3807–3814. [Google Scholar] [CrossRef] [PubMed]
- Sato, S.; Yamate, J.; Hori, Y.; Hatai, A.; Nozawa, M.; Sagai, M. Protective effect of polyphenol-containing azuki bean (Vigna angularis) seed coats on the renal cortex in streptozotocin-induced diabetic rats. J. Nutr. Biochem. 2005, 16, 547–553. [Google Scholar] [CrossRef] [PubMed]
- Takahama, U.; Yamauchi, R.; Hirota, S. Antioxidative flavonoids in adzuki-meshi (rice boiled with adzuki bean) react with nitrite under simulated stomach conditions. J. Funct. Food 2016, 26, 657–666. [Google Scholar] [CrossRef]
- Takahama, U.; Yamauchi, R.; Hirota, S. Interactions of starch with a cyanidin-catechin pigment (vignacyanidin) isolated from Vigna angularis bean. Food Chem. 2013, 141, 2600–2605. [Google Scholar] [CrossRef] [PubMed]
- Hirota, S.; Takahama, U. Inhibition of pancreatin-induced digestion of cooked rice starch by adzuki (Vigana angularis) bean flavonoids and the possibility of a decrease in the inhibitory effects in the stomach. J. Agric. Food Chem. 2017, 65, 2172–2179. [Google Scholar] [CrossRef] [PubMed]
- Takahama, U.; Hirota, S. Fatty acids, epicatechin-dimethylgallate, and rutin interact with buckwheat starch inhibiting its digestion by amylase: Implications for the decrease in glycemic index by buckwheat flour. J. Agric. Food Chem. 2010, 58, 12431–12439. [Google Scholar] [CrossRef] [PubMed]
- Morina, F.; Takahama, U.; Yamauchi, R.; Hirota, S.; Veljovic-Jovanovic, S. Quercetin 7-O-glucoside suppresses nitrite-induced formation of dinitrosocatechins and their quinones in catechin/nitrite systems under stomach simulating conditions. Food Funct. 2015, 6, 218–229. [Google Scholar] [CrossRef] [PubMed]
- Takahama, U.; Yamauchi, R.; Hirota, S. Isolation and characterization of a cyanidin-catechin pigment from adzuki bean (Vigna angularis). Food Chem. 2013, 141, 282–288. [Google Scholar] [CrossRef] [PubMed]
- Lever, A. A new reaction for colorimetric determination of carbohydrates. Anal. Biochem. 1972, 47, 273–279. [Google Scholar] [CrossRef]
- DuBois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric method for determination of sugars and related substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Goesaert, H.; Brijs, K.; Veraverbeke, W.S.; Courtin, C.M.; Gebruers, K.; Delcour, J.A. Wheat flour constituents: How they impact bread quality, and how to impact their functionality. Trends Food Sci. Technol. 2005, 16, 12–30. [Google Scholar] [CrossRef]
- Xie, F.; Pollet, E.; Halley, P.J.; Avérous, L. Advanced nano-biocomposites based on starch. In Polysaccharides; Ramawat, K.G., Mérillon, J.-M., Eds.; Springer International Publishing: Cham, Switzerland, 2014; pp. 1–75. [Google Scholar] [CrossRef]
- Wang, S.; Li, C.; Copeland, L.; Niu, Q.; Wang, S. Starch retrogradation: A comprehensive review. Comp. Rev. Food Sci. Food Saf. 2015, 14, 568–585. [Google Scholar] [CrossRef]
- Nakano, M.; Ito, S.; Fujino, Y. Lipids and the Component Fatty Acids of Beans. Available online: http://id.nii.ac.jp/1588/00002481/ (accessed on 9 August 2018).
- Mikus, F.F.; Hixon, R.M.; Rundle, R.E. The complexes of fatty acids with amylose. J. Am. Chem. Soc. 1946, 68, 1115–1123. [Google Scholar] [CrossRef] [PubMed]
- Mohri, Z. Interaction between starch and fatty acid esters in frozen starch noodles. Agric. Biol. Chem. 1980, 44, 1455–1459. [Google Scholar] [CrossRef]
- Mercier, C.; Charbonniere, R.; Grebaut, J.; de la Gueriviere, F.J. Formation of amylose-lipid complexes by twin-screw extrusion cooking of manioc starch. Cereal Chem. 1980, 57, 4–9. [Google Scholar]
- Bhatnagar, S.; Hanna, M.A. Amylose-lipid complex formation during single-screw extrusion of various corn starches. Cereal Chem. 1994, 71, 582–587. [Google Scholar]
- Godet, M.C.; Bouchet, B.; Colonna, P.; Gallant, D.J.; Buleon, A. Crystalline amylose-fatty acid complexes: Morphology and crystal thickness. J. Food Sci. 1996, 61, 1196–1201. [Google Scholar] [CrossRef]
- Putseys, J.A.; Lamberts, L.; Delcour, J.A. Amylose-inclusion complexes: Formation, identity and physic-chemical properties. J. Cereal Sci. 2010, 51, 238–247. [Google Scholar] [CrossRef]
- Marinopoulou, A.; Kalogianni, E.P.; Raphaelides, S.N. Amylose-fatty acid inclusion complexes as examined by interfacial tension measurements. Colloids Surf. B 2016, 137, 133–137. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, H.; Yoshida, N.; Tomiyama, Y.; Mizushina, Y. Fatty acid characteristics of triacylglycerols and phospholipids in adzuki beans (Vigna angularis). Food Sci. Technol. Res. 2010, 16, 209–214. [Google Scholar] [CrossRef]
- Li, L.; Liu, B.; Zheng, X. Bioactive ingredients in adzuki bean sprouts. J. Med. Plants Res. 2011, 5, 5894–5898. [Google Scholar]
- Shen, W.; Xu, Y.; Lu, Y.-H. Inhibitory effects of Citrus flavonoids on starch digestion and antihyperglycemic effects in HepG2 cells. J. Agric. Food Chem. 2012, 60, 9609–9619. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.-P.; He, H.; Lu, Y.-H. Four flavonoid compounds from Phyllostachys edulis leaf extract retard the digestion of starch and its working mechanisms. J. Agric. Food Chem. 2014, 62, 7760–7770. [Google Scholar] [CrossRef] [PubMed]
- Crowe, T.C.; Seligman, S.A.; Copeland, L. Inhibition of enzymic digestion of amylose by free fatty acids in vitro contributes to resistant starch formation. J. Nutr. 2000, 130, 2006–2008. [Google Scholar] [CrossRef] [PubMed]
- Nebesny, E.; Rosicka, J.; Tkaczyk, M. Effect of enzymatic hydrolysis of wheat starch on amylose-lipid complexes stability. Starch 2002, 54, 603–608. [Google Scholar] [CrossRef]
- Zhang, B.; Huang, Q.; Luo, F.; Fu, X. Structural characterizations and digestibility of debranched high-amylose maize starch complexed with lauric acid. Food Hydrocoll. 2012, 28, 174–181. [Google Scholar] [CrossRef]
- Yilmazer-Musa, M.; Griffith, A.M.; Michels, A.J.; Schneider, E.; Frei, B. Grape seed and tea extracts and catechin 3-gallates are potent inhibitors of α-amylase and α-glucosidase activity. J. Agric. Food Chem. 2012, 60, 8924–8929. [Google Scholar] [CrossRef] [PubMed]
- Takahama, U.; Hirota, S. Interactions of Flavonoids with α-amylase and starch slowing down its digestion. Food Funct. 2018, 9, 677–687. [Google Scholar] [CrossRef] [PubMed]
- Takahama, U.; Hirota, S. Effects of starch on nitrous acid-induced oxidation of kaempferol and inhibition of α-amylase-catalyzed digestion of starch by kaempferol under conditions simulating the stomach and the intestine. Food Chem. 2013, 141, 313–319. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Yang, X.; Li, S.; Gao, W. Preparation, physicochemical characterization and in vitro digestibility on solid complex of maize starches with quercetin. LWT-Food Sci. Technol. 2011, 44, 787–792. [Google Scholar] [CrossRef]

| (+)-Catechin | Taxifolin | Quercetin 7-Glucoside | Rutin | Quercetin | Vignacyanidins | |
|---|---|---|---|---|---|---|
| Retention time (min) | 13.8 | 23.1 | 27.3 | 29.6 | 34.7 | 36.8–42.7 a |
| Concentration (mM) b | 33.5 ± 2.1 | 26.2 ± 2.7 | 11.1 ± 0.7 | 3.2 ± 0.2 | 36.0 ± 2.1 | 5.9 ± 0.3 c |
| Ratio (A/B) d | 0.16 | 0.11 | 0.26 | 0.12 | 0.78 | 1.83 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hirota, S.; Takahama, U. Suppression of Pancreatin-Induced Digestion of Starch in Starch Granules by Starch/Fatty Acid and Starch/Flavonoid Complexes in Retrograding Rice Flour. Foods 2018, 7, 128. https://doi.org/10.3390/foods7080128
Hirota S, Takahama U. Suppression of Pancreatin-Induced Digestion of Starch in Starch Granules by Starch/Fatty Acid and Starch/Flavonoid Complexes in Retrograding Rice Flour. Foods. 2018; 7(8):128. https://doi.org/10.3390/foods7080128
Chicago/Turabian StyleHirota, Sachiko, and Umeo Takahama. 2018. "Suppression of Pancreatin-Induced Digestion of Starch in Starch Granules by Starch/Fatty Acid and Starch/Flavonoid Complexes in Retrograding Rice Flour" Foods 7, no. 8: 128. https://doi.org/10.3390/foods7080128




