The Demographic Diversity of Food Intake and Prevalence of Kidney Stone Diseases in the Indian Continent
Abstract
1. Introduction
2. Diverse Food Habits in India
2.1. Stone Forming Area in India
2.2. Food Habits with Stone Formation
2.2.1. Protein
2.2.2. Calcium-Rich Food
2.2.3. Carbohydrate-Rich Food
2.2.4. Sodium and Potassium
2.2.5. Oxalate-Rich Food
3. Mechanism of Different Types of Stones According to Food Habits
3.1. Impact of Food on the Mechanism of Stone Formation
3.1.1. Calcium Stone
3.1.2. Uric Acid
3.1.3. Cystine Stone
4. Food Diversity and Nutritional Effects in Indian Population
5. Concluding Remarks
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Romero, V.; Akpinar, H.; Assimos, D.G. Kidney stones: A global picture of prevalence, incidence, and associated risk factors. Rev. Urol. 2010, 12, e86–e96. [Google Scholar] [PubMed]
- Hesse, A.; Siener, R.; Heynck, H.; Jahnen, A. The influence of dietary factors on the risk of urinary stone formation. Scanning Microsc. 1993, 7, 1119–1127. [Google Scholar] [PubMed]
- Serio, A.; Fraioli, A. Epidemiology of nephrolithiasis. Nephron 1999, 81, 26–30. [Google Scholar] [CrossRef] [PubMed]
- Amato, M.; Lusini, M.L.; Nelli, F. Epidemiology of nephrolithiasis today. Urol. Int. 2004, 72, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Martín, F.M.; Millán Rodríguez, F.; Esquena Fernández, S.; Segarra Tomás, J.; Rousaud Barón, F.; Martínez-Rodríguez, R.; Villavicencio Mavrich, H. Incidence and prevalence of published studies about urolithiasis in Spain: A review. Actas Urol. Esp. 2007, 31, 511–520. [Google Scholar] [CrossRef]
- Lieske, J.C.; Peña de la Vega, L.S.; Slezak, J.M.; Bergstralh, E.J.; Leibson, C.L.; Ho, K.L.; Gettman, M.T. Renal stone epidemiology in Rochester, Minnesota: An update. Kidney Int. 2006, 69, 760–764. [Google Scholar] [CrossRef] [PubMed]
- Safarinejad, M.R. Adult urolithiasis in a population-based study in Iran: Prevalence, incidence, and associated risk factors. Urol. Res. 2007, 35, 73–82. [Google Scholar] [CrossRef]
- Yasui, T.; Iguchi, M.; Suzuki, S.; Kohri, K. Prevalence and epidemiological characteristics of urolithiasis in Japan: National trends between 1965 and 2005. Urology 2008, 71, 209–213. [Google Scholar] [CrossRef]
- Stechman, M.J.; Loh, N.Y.; Thakker, R.V. Genetic causes of hypercalciuric nephrolithiasis. Pediatr. Nephrol. 2009, 24, 2321–2332. [Google Scholar] [CrossRef]
- Goldfarb, D.S.; Fischer, M.E.; Keich, Y.; Goldberg, J. A twin study of genetic and dietary influences on nephrolithiasis: A report from the Vietnam Era Twin (VET) Registry. Kidney Int. 2005, 67, 1053–1061. [Google Scholar] [CrossRef]
- Devuyst, O.; Pirson, Y. Genetics of hypercalciuric stone forming diseases. Kidney Int. 2007, 72, 1065–1072. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Chonchol, M.B. Vitamin D and kidney stone disease. Curr. Opin. Nephrol. Hypertens. 2013, 22, 383–389. [Google Scholar] [CrossRef] [PubMed]
- Duchene, D.A.; Pearle, M.S. Stones and Endourology in Older Adults. In Geriatric Urology; Springer: New York, NY, USA, 2014; pp. 357–368. [Google Scholar]
- Borghi, L.; Schianchi, T.; Meschi, T.; Guerra, A.; Allegri, F.; Maggiore, U.; Novarini, A. Comparison of two diets for the prevention of recurrent stones in diopathic hypercalciuria. N. Engl. J. Med. 2002, 346, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Taylor, E.N.; Fung, T.T.; Curhan, G.C. DASH-style diet associates with reduced risk for kidney stones. JASN 2009, 20, 2253–2259. [Google Scholar] [CrossRef] [PubMed]
- Trinchieri, A. Epidemiology of urolithiasis. Arch. Ital. Urol. Androl. 1996, 68, 203–249. [Google Scholar] [PubMed]
- Robertson, W.G. Renal stones in the tropics. Semin. Nephrol. 2003, 23, 77–87. [Google Scholar] [CrossRef] [PubMed]
- Kosambi, D.D. The Culture and Civilisation of Ancient India in Historical Outline; Routledge and K. Paul: London, UK, 1965. [Google Scholar]
- Sofia, N.H.; Manickavasakam, K.; Walter, T.M. Prevalence and risk factors of kidney stone. GJRA 2016, 5, 183–187. [Google Scholar]
- Ganesamoni, R.; Singh, S.K. (Eds.) Epidemiology of stone disease in Northern India. In Urolithiasis; Springer: London, UK, 2012; pp. 39–46. [Google Scholar]
- Mikawlrawng, K.; Kumar, S.; Vandana, R. Current scenario of urolithiasis and the use of medicinal plants as antiurolithiatic agents in Manipur (North East India): A review. Int. J. Herb. Med. 2014, 2, 1–2. [Google Scholar]
- Maalouf, N.M.; Moe, O.W.; Adams-Huet, B.; Sakhaee, K. Hypercalciuria associated with high dietary protein intake is not due to acid load. J. Clin. Endocrinol. Metab. 2011, 96, 3733–3740. [Google Scholar] [CrossRef]
- Van den Berg, E.; Hospers, F.A.; Navis, G.; Engberink, M.F.; Brink, E.J.; Geleijnse, J.M.; van Baak, M.A.; Gans, R.O.; Bakker, S.J. Dietary acid load and rapid progression to end-stage renal disease of diabetic nephropathy in Westernized South Asian people. J. Nephrol. 2011, 24, 11–17. [Google Scholar] [CrossRef]
- Robertson, W.G.; Peacock, M.; Baker, M.; Marshall, D.H.; Pearlman, B.; Speed, R.; Sergeant, V.; Smith, A. Studies on the prevalence and epidemiology of urinary stone disease in men in Leeds. BJU Int. 1983, 55, 595–598. [Google Scholar] [CrossRef]
- Breslau, N.A.; Brinkley, L.; Hill, K.D.; Pak, C.Y. Relationship of animal protein-rich diet to kidney stone formation and calcium metabolism. J. Clin. Endocrinol. Metab. 1988, 66, 140–146. [Google Scholar] [CrossRef] [PubMed]
- Lekcharoensuk, C.; Osborne, C.A.; Lulich, J.P.; Pusoonthornthum, R.; Kirk, C.A.; Ulrich, L.K.; Koehler, L.A.; Carpenter, K.A.; Swanson, L.L. Association between dietary factors and calcium oxalate and magnesium ammonium phosphate urolithiasis in cats. J. Am. Vet. Med. Assoc. 2001, 219, 1228–1237. [Google Scholar] [CrossRef] [PubMed]
- Meschi, T.; Nouvenne, A.; Ticinesi, A.; Prati, B.; Guerra, A.; Allegri, F.; Pigna, F.; Soldati, L.; Vezzoli, G.; Gambaro, G.; et al. Dietary habits in women with recurrent idiopathic calcium nephrolithiasis. J. Transl. Med. 2012, 10, 63. [Google Scholar] [CrossRef] [PubMed]
- Goldfarb, D.S.; Coe, F.L. Prevention of recurrent nephrolithiasis. Am. Fam. Physician 1999, 60, 2269–2276. [Google Scholar] [PubMed]
- Finkielstein, V.A.; Goldfarb, D.S. Strategies for preventing calcium oxalate stones. CMAJ 2006, 174, 1407–1409. [Google Scholar] [CrossRef] [PubMed]
- Traxer, O.; Huet, B.; Poindexter, J.; Pak, C.Y.; Pearle, M.S. Effect of ascorbic acid consumption on urinary stone risk factors. J. Urol. 2003, 170, 397–401. [Google Scholar] [CrossRef]
- Meschi, T.; Maggiore, U.; Fiaccadori, E.; Schianchi, T.; Bosi, S.; Adorni, G.; Ridolo, E.; Guerra, A.; Allegri, F.; Novarini, A.; et al. The effect of fruits and vegetables on urinary stone risk factors. Kidney Int. 2004, 66, 2402–2410. [Google Scholar] [CrossRef]
- Thomas, L.D.; Elinder, C.G.; Tiselius, H.G.; Wolk, A.; Åkesson, A. Ascorbic acid supplements and kidney stone incidence among men: A prospective study. JAMA Intern. Med. 2013, 173, 386–388. [Google Scholar] [CrossRef]
- Lamarche, J.; Nair, R.; Peguero, A.; Courville, C. Vitamin C-induced oxalate nephropathy. Int. J. Nephrol. 2011, 2011, 146927. [Google Scholar] [CrossRef]
- Nishiura, J.L.; Martini, L.A.; Mendonça, C.O.G.; Schor, N.; Heilberg, I.P. Effect of calcium intake on urinary oxalate excretion in calcium stone-forming patients. Braz. J. Med. Biol. Res. 2002, 35, 669–675. [Google Scholar] [CrossRef]
- Curhan, G.C.; Willett, W.C.; Rimm, E.B.; Stampfer, M.J. A prospective study of dietary calcium and other nutrients and the risk of symptomatic kidney stones. N. Engl. J. Med. 1993, 328, 833–838. [Google Scholar] [CrossRef]
- Pendse, A.K.; Singh, P.P. The etiology of urolithiasis in Udaipur (Western part of India). Urol. Res. 1986, 14, 59–62. [Google Scholar] [CrossRef] [PubMed]
- Siener, R.; Ebert, D.; Nicolay, C.; Hesse, A. Dietary risk factors for hyperoxaluria in calcium oxalate stone formers. Kidney Int. 2003, 63, 1037–1043. [Google Scholar] [CrossRef] [PubMed]
- Holmes, R.P.; Goodman, H.O.; Assimos, D.G. Contribution of dietary oxalate to urinary oxalate excretion. Kidney Int. 2001, 59, 270–276. [Google Scholar] [CrossRef] [PubMed]
- Taylor, E.N.; Curhan, G.C. Oxalate intake and the risk for nephrolithiasis. J. Am. Soc. Nephrol. 2007, 18, 2198–2204. [Google Scholar] [CrossRef] [PubMed]
- Urivetzky, M.; Kessaris, D.; Smith, A.D. Ascorbic acid overdosing: A risk factor for calcium oxalate nephrolithiasis. J. Urol. 1992, 147, 1215–1218. [Google Scholar] [CrossRef]
- Trinchieri, A.; Nespoli, R.; Ostini, F.; Rovera, F.; Zanetti, G.; Pisani, E. A study of dietary calcium and other nutrients in idiopathic renal calcium stone formers with low bone mineral content. J. Urol. 1998, 159, 654–657. [Google Scholar] [CrossRef]
- Massey, L.K.; Liebman, M.; Kynast-Gales, S.A. Ascorbate increases human oxaluria and kidney stone risk. J. Nutr. 2005, 135, 1673–1677. [Google Scholar] [CrossRef]
- Ferraro, P.M.; Curhan, G.C.; Gambaro, G.; Taylor, E.N. Total, dietary, and supplemental vitamin C intake and risk of incident kidney stones. Am. J. Kidney Dis. 2016, 67, 400–407. [Google Scholar] [CrossRef]
- Bataille, P.; Charransol, G.; Gregoire, I.; Daigre, J.L.; Coevoet, B.; Makdassi, R.; Pruna, A.; Locquet, P.; Sueur, J.P.; Fournier, A. Effect of calcium restriction on renal excretion of oxalate and the probability of stones in the various pathophysiological groups with calcium stones. J. Urol. 1983, 130, 218–223. [Google Scholar] [CrossRef]
- Curhan, G.C.; Willett, W.C.; Speizer, F.E.; Spiegelman, D.; Stampfer, M.J. Comparison of dietary calcium with supplemental calcium and other nutrients as factors affecting the risk for kidney stones in women. Ann. Intern. Med. 1997, 126, 497–504. [Google Scholar] [CrossRef]
- Heaney, R.P.; Rafferty, K. Carbonated beverages and urinary calcium excretion. Am. J. Clin. Nutr. 2001, 74, 343–347. [Google Scholar] [CrossRef] [PubMed]
- Asselman, M.; Verkoelen, C.F. Fructose intake as a risk factor for kidney stone disease. Kidney Int. 2008, 73, 139–140. [Google Scholar] [CrossRef]
- Saldana, T.M.; Basso, O.; Darden, R.; Sandler, D.P. Carbonated beverages and chronic kidney disease. Epidemiology (Camb. Mass.) 2007, 18, 501–506. [Google Scholar] [CrossRef] [PubMed]
- Reddy, S.T.; Wang, C.Y.; Sakhaee, K.; Brinkley, L.; Pak, C.Y. Effect of low-carbohydrate high-protein diets on acid-base balance, stone-forming propensity, and calcium metabolism. Am. J. Kidney Dis. 2002, 40, 265–274. [Google Scholar] [CrossRef]
- Nguyen, N.U.; Dumoulin, G.T.H.M.; Henriet, M.T.; Regnard, J. Increase in urinary calcium and oxalate after fructose infusion. Horm. Metab. Res. 1995, 27, 155–158. [Google Scholar] [CrossRef]
- Awasthi, M.; Malhotra, S.R. Assessment of mineral intake by kidney stone patients of Kangra District, Himachal Pradesh with respect to their gender, age and income. Indian J. Pediatr. 2013, 80, 996–1001. [Google Scholar] [CrossRef] [PubMed]
- Park, S.M.; Jee, J.; Joung, J.Y.; Cho, Y.Y.; Sohn, S.Y.; Jin, S.; Hur, K.Y.; Kim, J.H.; Kim, S.W.; Chung, J.H.; et al. High dietary sodium intake assessed by 24-hour urine specimen increase urinary calcium excretion and bone resorption marker. J. Bone Metab. 2014, 21, 189–194. [Google Scholar] [CrossRef]
- Barzel, U.S.; Massey, L.K. Excess dietary protein can adversely affect bone. J. Nutr. 1998, 128, 1051–1053. [Google Scholar] [CrossRef]
- Giannini, S.; Nobile, M.; Sartori, L.; Dalle Carbonare, L.; Ciuffreda, M.; Corrò, P.; D'Angelo, A.; Calò, L.; Crepaldi, G. Acute effects of moderate dietary protein restriction in patients with idiopathic hypercalciuria and calcium nephrolithiasis. Am. J. Clin. Nutr. 1999, 69, 267–271. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Chen, X.; Yu, Z. The change of human Na+/dicarboxylate co-transporter 1 expression in the kidney and its relationship with pathogenesis of nephrolithiasis. Zhonghua Yi Xue Za Zhi 2001, 81, 1066–1069. [Google Scholar] [PubMed]
- Ambühl, P.M. Protein intake in renal and hepatic disease. Int. J. Vitam. Nutr. Res. 2011, 81, 162–172. [Google Scholar] [CrossRef]
- Speedy, A.W. Global production and consumption of animal source foods. J. Nutr. 2003, 133, 4048S–4053S. [Google Scholar] [CrossRef] [PubMed]
- Sorensen, M.D. Calcium intake and urinary stone disease. Transl. Androl. Urol. 2014, 3, 235–240. [Google Scholar] [PubMed]
- Nouvenne, A.; Meschi, T.; Guerra, A.; Allegri, F.; Prati, B.; Borghi, L. Dietary treatment of nephrolithiasis. Clin. Cases Miner. Bone Metab. 2008, 5, 135–141. [Google Scholar]
- Li, H.; Klett, D.E.; Littleton, R.; Elder, J.S.; Sammon, J.D. Role of insulin resistance in uric acid nephrolithiasis. World J. Nephrol. 2014, 3, 237–242. [Google Scholar] [CrossRef]
- Cox, C.L.; Stanhope, K.L.; Schwarz, J.M.; Graham, J.L.; Hatcher, B.; Griffen, S.C.; Bremer, A.A.; Berglund, L.; McGahan, J.P.; Keim, N.L.; et al. Consumption of fructose- but not glucose sweetened beverages for 10 weeks increases circulating concentrations of uric acid, retinol binding protein-4, and gamma-glutamyl transferase activity in overweight/obese humans. Nutr. Metab. 2012, 9, 68. [Google Scholar] [CrossRef]
- Gulati, S.; Misra, A. Sugar intake, obesity, and diabetes in India. Nutrients 2014, 6, 5955–5974. [Google Scholar] [CrossRef]
- Nouvenne, A.; Meschi, T.; Prati, B.; et al. Effects of a low-salt diet on idiopathic hypercalciuria in calcium-oxalate stone formers: A 3-mo randomized controlled trial. Am. J. Clin. Nutr. 2010, 91, 565–570. [Google Scholar] [CrossRef]
- Lemann, J., Jr. Pathogenesis of idiopathic hypercalciuria and nephrolithiasis. In Disorders of Bone and Mineral Metabolism; Coe, F.L., Favus, M.J., Eds.; Raven Press: New York, NY, USA, 1992; pp. 685–706. [Google Scholar]
- Sabto, J.; Powell, M.J.; Breidahl, M.J.; Gurr, F.W. Influence of urinary sodium on calcium excretion in normal individuals. A redefinition of hypercalciuria. Med. J. Aust. 1984, 140, 354–356. [Google Scholar] [PubMed]
- Blaine, J.; Chonchol, M.; Levi, M. Renal control of calcium, phosphate, and magnesium homeostasis. Clin. J. Am. Soc. Nephrol. 2015, 10, 1257–1272. [Google Scholar] [CrossRef] [PubMed]
- Lemann, J.; Pleuss, J.A.; Gray, R.W.; Hoffmann, R.G. Potassium administration increases and potassium deprivation reduces urinary calcium excretion in healthy adults. Kidney Int. 1991, 39, 973–983. [Google Scholar] [CrossRef] [PubMed]
- Muldowney, F.P.; Freaney, R.; Moloney, M.F. Importance of dietary sodium in the hypercalciuria syndrome. Kidney Int. 1982, 22, 292–296. [Google Scholar] [CrossRef] [PubMed]
- Silver, J.; Rubinger, D.; Friedlaender, M.M.; Popovtzer, M.M. Sodium dependent idiopathic hypercalciuria in renal-stone formers. Lancet 1983, 322, 484–486. [Google Scholar] [CrossRef]
- Xu, H.; Zisman, A.L.; Coe, F.L.; Worcester, E.M. Kidney stones: An update on current pharmacological management and future directions. Exp. Opin. Pharmacother. 2013, 14, 435–447. [Google Scholar] [CrossRef] [PubMed]
- Knight, J.; Jiang, J.; Wood, K.D.; Holmes, R.P.; Assimos, D.G. Oxalate and sucralose absorption in idiopathic calcium oxalate stone formers. Urology 2011, 78, 475.e9–475.e13. [Google Scholar] [CrossRef] [PubMed]
- Massey, L.K.; Roman-Smith, H.; Sutton, R.A. Effect of dietary oxalate and calcium on urinary oxalate and risk of formation of calcium oxalate kidney stones. J. Am. Diet Assoc. 1993, 93, 901–906. [Google Scholar] [CrossRef]
- Ngo, T.C.; Assimos, D.G. Uric acid nephrolithiasis: Recent progress and future directions. Rev. Urol. 2007, 9, 17–27. [Google Scholar]
- Hesse, A.; Siener, R. Current aspects of epidemiology and nutrition in urinary stone disease. World J. Urol. 1997, 15, 165–171. [Google Scholar] [CrossRef] [PubMed]
- Guha, M.; Bankura, B.; Ghosh, S.; Pattanayak, A.K.; Ghosh, S.; Pal, D.K.; Puri, A.; Kundu, A.K.; Das, M. Polymorphisms in CaSR and CLDN14 genes associated with increased risk of kidney stone disease in patients from the eastern part of India. PLoS ONE 2015, 10, e0130790. [Google Scholar] [CrossRef] [PubMed]
- Evan, A.P. Physiopathology and etiology of stone formation in the kidney and the urinary tract. Pediatr. Nephrol. 2010, 25, 831–841. [Google Scholar] [CrossRef] [PubMed]
- Trinchieri, A.; Mandressi, A.; Luongo, P.; Longo, G.; Pisani, E. The influence of diet on urinary risk factors for stones in healthy subjects and idiopathic renal calcium stone formers. BJU Int. 1991, 67, 230–236. [Google Scholar] [CrossRef]
- Tolbert, N.E. Microbodies-peroxisomes and glyoxysomes. Annu. Rev. Plant Biol. 1971, 22, 45–74. [Google Scholar] [CrossRef]
- Han, H.; Segal, A.M.; Seifter, J.L.; Dwyer, J.T. Nutritional management of kidney stones (nephrolithiasis). Clin. Nutr. Res. 2015, 4, 137–152. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, Q.V.; Kälin, A.; Drouve, U.; Casez, J.P.; Jaeger, P. Sensitivity to meat protein intake and hyperoxaluria in idiopathic calcium stone formers. Kidney Int. 2001, 59, 2273–2281. [Google Scholar] [CrossRef] [PubMed]
- Koh, E.T.; Reiser, S.; Fields, M. Dietary fructose as compared to glucose and starch increases the calcium content of kidney of magnesium-deficient rats. J. Nutr. 1989, 119, 1173–1178. [Google Scholar] [CrossRef]
- Koh, E.T.; Min, K.W. Fructose precipitates calcium phosphate in the kidneys of female rats fed magnesium-deficient diets. Magnes. Res. 1991, 4, 171–176. [Google Scholar]
- Yatabe, M.S.; Yatabe, J.; Takano, K.; Murakami, Y.; Sakuta, R.; Abe, S.; Sanada, H.; Kimura, J.; Watanabe, T. Effects of a high-sodium diet on renal tubule Ca2+ transporter and claudin expression in Wistar-Kyoto rats. BMC Nephrol. 2012, 13, 160. [Google Scholar] [CrossRef]
- Pak, C.Y.; Britton, F.; Peterson, R.; Ward, D.; Northcutt, C.; Breslau, N.A.; McGuire, J.; Sakhaee, K.; Bush, S.; Nicar, M.; et al. Ambulatory evaluation of nephrolithiasis: Classification, clinical presentation and diagnostic criteria. Am. J. Med. 1980, 69, 19–30. [Google Scholar] [CrossRef]
- Lewandowski, S.; Rodgers, A.L. Idiopathic calcium oxalate urolithiasis: Risk factors and conservative treatment. Clin. Chim. Acta 2004, 345, 17–34. [Google Scholar] [CrossRef] [PubMed]
- Pak, C.Y.; Kaplan, R.; Bone, H.; Townsend, J.; Waters, O. A simple test for the diagnosis of absorptive, resorptive and renal hypercalciurias. N. Engl. J. Med. 1975, 292, 497–500. [Google Scholar] [CrossRef] [PubMed]
- Herring, L.C. Observations on the analysis of ten thousand urinary calculi. J. Urol. 1962, 88, 545–562. [Google Scholar] [CrossRef]
- Taylor, E.N.; Curhan, G.C. Fructose consumption and the risk of kidney stones. Kidney Int. 2008, 73, 207–212. [Google Scholar] [CrossRef] [PubMed]
- Coe, F.L. Hyperuricosuric calcium oxalate nephrolithiasis. Kidney Int. 1978, 13, 418–426. [Google Scholar] [CrossRef]
- Martillo, M.A.; Nazzal, L.; Crittenden, D.B. The crystallization of monosodium urate. Curr. Rheumatol. Rep. 2014, 16, 400. [Google Scholar] [CrossRef]
- Villegas, R.; Xiang, Y.B.; Elasy, T.; Xu, W.H.; Cai, H.; Cai, Q.; Linton, M.F.; Fazio, S.; Zheng, W.; Shu, X.O. Purine-rich foods, protein intake, and the prevalence of hyperuricemia: The Shanghai Men’s Health Study. Nutr. Metab. Cardiovasc. Dis. 2012, 22, 409–416. [Google Scholar] [CrossRef]
- Robertson, W.G.; Heyburn, P.J.; Peacock, M.; Hanes, F.A.; Swaminathan, R. The effect of high animal protein intake on the risk of calcium stone-formation in the urinary tract. Clin. Sci. 1979, 57, 285–288. [Google Scholar] [CrossRef]
- Fox, I.H.; Palella, T.D.; Kelley, W.N. Hyperuricemia: A marker for cell energy crisis. N. Engl. J. Med. 1987, 111–112. [Google Scholar] [CrossRef]
- Rutchik, S.D.; Resnick, M.I. Cystine calculi: Diagnosis and management. Urol. Clin. N. Am. 1997, 24, 163–171. [Google Scholar] [CrossRef]
- Singh, S.K.; Agarwal, M.M.; Sharma, S. Medical therapy for calculus disease. BJU Int. 2011, 107, 356–368. [Google Scholar] [CrossRef] [PubMed]
- Lipkin, M.E.; Preminger, G.M. Demystifying the medical management of nephrolithiasis. Rev. Urol. 2011, 13, 34–38. [Google Scholar] [PubMed]
- Agarwal, M.M.; Singh, S.K.; Mavuduru, R.; Mandal, A.K. Preventive fluid and dietary therapy for urolithiasis: An appraisal of strength, controversies and lacunae of current literature. Indian J. Urol. 2011, 27, 310–319. [Google Scholar] [CrossRef] [PubMed]
- Zuckerman, J.M.; Assimos, D.G. Hypocitraturia: Pathophysiology and medical management. Rev. Urol. 2009, 11, 134–144. [Google Scholar] [PubMed]
- Santos, F.D.A.; Donzele, J.L.; de Oliveira Silva, F.C.; de Oliveira, R.F.M.; de Abreu, M.L.T.; Saraiva, A.; Haese, D.; Kill, J.L. Levels of digestible methionine+ cystine in diets for high genetic potential barrows from 95 to 125 kg. Rev. Bras. Zootech. 2011, 40, 581–586. [Google Scholar] [CrossRef]
- Worcester, E.M.; Coe, F.L.; Evan, A.P.; Parks, J.H. Reduced renal function and benefits of treatment in cystinuria vs other forms of nephrolithiasis. BJU Int. 2006, 97, 1285–1290. [Google Scholar] [CrossRef] [PubMed]
- Gul, Z.; Monga, M. Medical and dietary therapy for kidney stone prevention. Korean J. Urol. 2014, 55, 775–779. [Google Scholar] [CrossRef]
- Noce, A.; Vidiri, M.F.; Marrone, G.; Moriconi, E.; Bocedi, A.; Capria, A.; Rovella, V.; Ricci, G.; De Lorenzo, A.; Di Daniele, N. Is low-protein diet a possible risk factor of malnutrition in chronic kidney disease patients? Cell Death Discov. 2016, 2, 16026. [Google Scholar] [CrossRef]
- Fouque, D.; Laville, M. Low Protein Diets for CHRONIC kidney Disease in Non Diabetic Adults; The Cochrane Library: London, UK, 2009. [Google Scholar]
- Menon, V.; Kopple, J.D.; Wang, X.; Beck, G.J.; Collins, A.J.; Kusek, J.W.; Greene, T.; Levey, A.S.; Sarnak, M.J. Effect of a very low protein diet on outcomes: Long term follow-up of the modification of diet in renal disease (MDRD) Study. Am. J. Kidney Dis. 2009, 53, 208–217. [Google Scholar] [CrossRef]
- Kovesdy, C.P.; Kopple, J.D.; Kalantar-Zadeh, K. Management of protein energy wasting in non dialysis-dependent chronic kidney disease: Reconciling low protein intake with nutritional therapy. Am. J. Clin. Nutr. 2013, 97, 1163–1177. [Google Scholar] [CrossRef]
- Needham, E. Management of acute renal failure. Injury 2005, 1, 7. [Google Scholar]
- Rule, A.D.; Krambeck, A.E.; Lieske, J.C. Chronic kidney disease in kidney stone formers. Clin. J. Am. Soc. Nephrol. 2011, 6, 2069–2075. [Google Scholar] [CrossRef] [PubMed]
- Juraschek, S.P.; Appel, L.J.; Anderson, C.A.; Miller, E.R. Effect of a high-protein diet on kidney function in healthy adults: Results from the Omni Heart trial. Am. J. Kidney Dis. 2013, 61, 547–554. [Google Scholar] [CrossRef] [PubMed]
- Jadeja, Y.P.; Kher, V. Protein energy wasting in chronic kidney disease: An update with focus on nutritional interventions to improve outcomes. Indian J. Endocr. Metab. 2012, 16, 246–251. [Google Scholar] [CrossRef] [PubMed]
- Avesani, C.M.; Kamimura, M.A.; Cuppari, L. Energy expenditure in chronic kidney disease patients. J. Ren. Nutr. 2011, 21, 27–30. [Google Scholar] [CrossRef]
- Shah, A.; Bross, R.; Shapiro, B.B.; Morrison, G.; Kopple, J.D. Dietary energy requirements in relatively healthy maintenance hemodialysis patients estimated from long-term metabolic studies. Am. J. Clin. Nutr. 2016, 103, 757–765. [Google Scholar] [CrossRef]
- Subramanyam, M.A.; Kawachi, I.; Berkman, L.F.; Subramanian, S.V. Is economic growth associated with reduction in child under nutrition in India? PLoS Med. 2011, 8, e1000424. [Google Scholar] [CrossRef]
- Nazar, C.M.J. Significance of diet in chronic kidney disease. J. Nephropharmacol. 2013, 2, 37–43. [Google Scholar]
- Bartoletti, R.; Cai, T.; Mondaini, N.; Melone, F.; Travaglini, F.; Carini, M.; Rizzo, M. Epidemiology and risk factors in urolithiasis. Urol. Int. 2007, 79 (Suppl. 1), 3–7. [Google Scholar] [CrossRef]
- Wesson, J.A.; Johnson, R.J.; Mazzali, M.; Beshensky, A.M.; Stietz, S.; Giachelli, C.; Liaw, L.; Alpers, C.E.; Couser, W.G.; Kleinman, J.G.; et al. Osteopontin is a critical inhibitor of calcium oxalate crystal formation and retention in renal tubules. JASN 2003, 14, 139–147. [Google Scholar] [CrossRef]






| Food Content | Impact on Stone Formation | Studied Zone | Reference |
|---|---|---|---|
| Dietary oxalate | Intestinal hyperabsorption of oxalate increased urinary oxalate excretion | Western part of India | Pendse et al., 1986 [36] |
| Germany | Hesse et al., 1993 [2]; Siener et al., 2003 [37] | ||
| North Carolina, USA | Holmes et al., 2001 [38] | ||
| Italy | Meschi et al., 2004 [31] | ||
| Boston | Taylor and Curhan, 2007 [39] | ||
| Eastern India | Mikawlrawng et al., 2014 [21] | ||
| Dietary ascorbic acid | Increases urinary oxalate excretion | New York | Urivetzky et al., 1992 [40] |
| Italy | Trinchieri et al., 1998 [41] | ||
| Washington | Massey et al., 2005 [42] | ||
| Sweden | Thomas et al., 2013 [32] | ||
| Boston | Ferraro et al., 2016 [43] | ||
| High dietary calcium | Reduces calcium oxalate stone formation | France | Bataille et al., 1983 [44] |
| Boston | Curhan et al., 1993 [35] | ||
| Germany | Siener et al., 2003 [37] | ||
| High intake of carbonated beverage | Increases urinary oxalate | Boston | Curhan et al., 1997 [45] |
| Women of Omaha | Heaneyand Rafferty, 2001 [46] | ||
| Netherland | Asselman and Verkoelen, 2008 [47] | ||
| Boston | Taylor et al., 2009 [15] | ||
| North Carolina | Saldana et al., 2007 [48] | ||
| Protein rich diet | Increases acid load in the kidney increases risk of stone formation | Boston | Curhan et al., 1997 [45] |
| Chicago, USA | Reddy et al., 2002 [49] | ||
| Reduce the bone’s ability to absorb calcium | Switzerland | Nguyen et al., 2001 [50] | |
| Increases urinary calcium | Italy | Borghi et al., 2002 [14] | |
| High intake of sodium | Increases urinary calcium | Northern India | Awasthi and Malhotra, 2013 [51] |
| Post-menopausal women of Korea | Park et al., 2014 [52] | ||
| Southern India | Sofia et al., 2016 [19] |
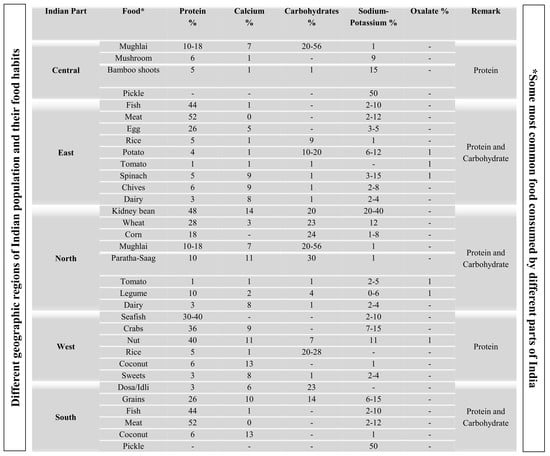
| Indian Part | Food * | Protein % | Calcium % | Carbohydrates % | Sodium-Potassium % | Oxalate % | Dominant Food Content Related to KSD |
|---|---|---|---|---|---|---|---|
| Central | Mughlai | 10–18 | 7 | 20–56 | 1 | - | Protein |
| Mushroom | 6 | 1 | - | 9 | - | ||
| Bamboo shoots | 5 | 1 | 1 | 15 | - | ||
| Pickle | - | - | - | 50 | - | ||
| East | Fish | 44 | 1 | - | 2–10 | - | Protein and Carbohydrate |
| Meat | 52 | 0 | - | 2–12 | - | ||
| Egg | 26 | 5 | - | 3–5 | - | ||
| Rice | 5 | 1 | 9 | 1 | - | ||
| Potato | 4 | 1 | 10–20 | 6–12 | 1 | ||
| Tomato | 1 | 1 | 1 | - | 1 | ||
| Spinach | 5 | 9 | 1 | 3–15 | 1 | ||
| Chives | 6 | 9 | 1 | 2–8 | - | ||
| Dairy | 3 | 8 | 1 | 2–4 | - | ||
| North | Kidney bean | 48 | 14 | 20 | 20–40 | - | Protein and Carbohydrate |
| Wheat | 28 | 3 | 23 | 12 | - | ||
| Corn | 18 | - | 24 | 1–8 | - | ||
| Mughlai | 10–18 | 7 | 20–56 | 1 | - | ||
| Paratha-Saag | 10 | 11 | 30 | 1 | - | ||
| Tomato | 1 | 1 | 1 | 2–5 | 1 | ||
| Legume | 10 | 2 | 4 | 0–6 | 1 | ||
| Dairy | 3 | 8 | 1 | 2–4 | - | ||
| West | Seafish | 30–40 | - | - | 2–10 | - | Protein |
| Crabs | 36 | 9 | - | 7–15 | - | ||
| Nut | 40 | 11 | 7 | 11 | 1 | ||
| Rice | 5 | 1 | 20–28 | - | - | ||
| Coconut | 6 | 13 | - | 1 | - | ||
| Sweets | 3 | 8 | 1 | 2–4 | - | ||
| South | Dosa/Idli | 3 | 6 | 23 | - | - | Protein and Carbohydrate |
| Grains | 26 | 10 | 14 | 6–15 | - | ||
| Fish | 44 | 1 | - | 2–10 | - | ||
| Meat | 52 | 0 | - | 2–12 | - | ||
| Coconut | 6 | 13 | - | 1 | - | ||
| Pickle | - | - | - | 50 | - |
| Macromolecules/Nutrients | Potential Level in KSD |
|---|---|
| Protein rich food | High |
| Calcium rich food | High, sometimes low |
| Carbohydrate rich food | High |
| Sodium Potassium | High |
| Oxalate rich food | High |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guha, M.; Banerjee, H.; Mitra, P.; Das, M. The Demographic Diversity of Food Intake and Prevalence of Kidney Stone Diseases in the Indian Continent. Foods 2019, 8, 37. https://doi.org/10.3390/foods8010037
Guha M, Banerjee H, Mitra P, Das M. The Demographic Diversity of Food Intake and Prevalence of Kidney Stone Diseases in the Indian Continent. Foods. 2019; 8(1):37. https://doi.org/10.3390/foods8010037
Chicago/Turabian StyleGuha, Manalee, Hritwick Banerjee, Pubali Mitra, and Madhusudan Das. 2019. "The Demographic Diversity of Food Intake and Prevalence of Kidney Stone Diseases in the Indian Continent" Foods 8, no. 1: 37. https://doi.org/10.3390/foods8010037
APA StyleGuha, M., Banerjee, H., Mitra, P., & Das, M. (2019). The Demographic Diversity of Food Intake and Prevalence of Kidney Stone Diseases in the Indian Continent. Foods, 8(1), 37. https://doi.org/10.3390/foods8010037