Antimicrobial and Antioxidative Effects of Plant Powders in Raw and Cooked Minced Pork
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.2. Sensory Evaluation
2.3. Color Measurement
2.4. pH Determination
2.5. Determination of the Total Phenolic Content by HPLC–MS/MS
2.6. Microbial Enumeration
2.7. Challenge Test
2.8. Determination of the Lipid Oxidation Level by HPLC–MS/MS
2.9. Determination of the Lipid Oxidation Level by the Thiobarbituric Acid Reactive Substances (TBARS) Method
2.10. Statistical Analysis
3. Results
3.1. Sensory Evaluation
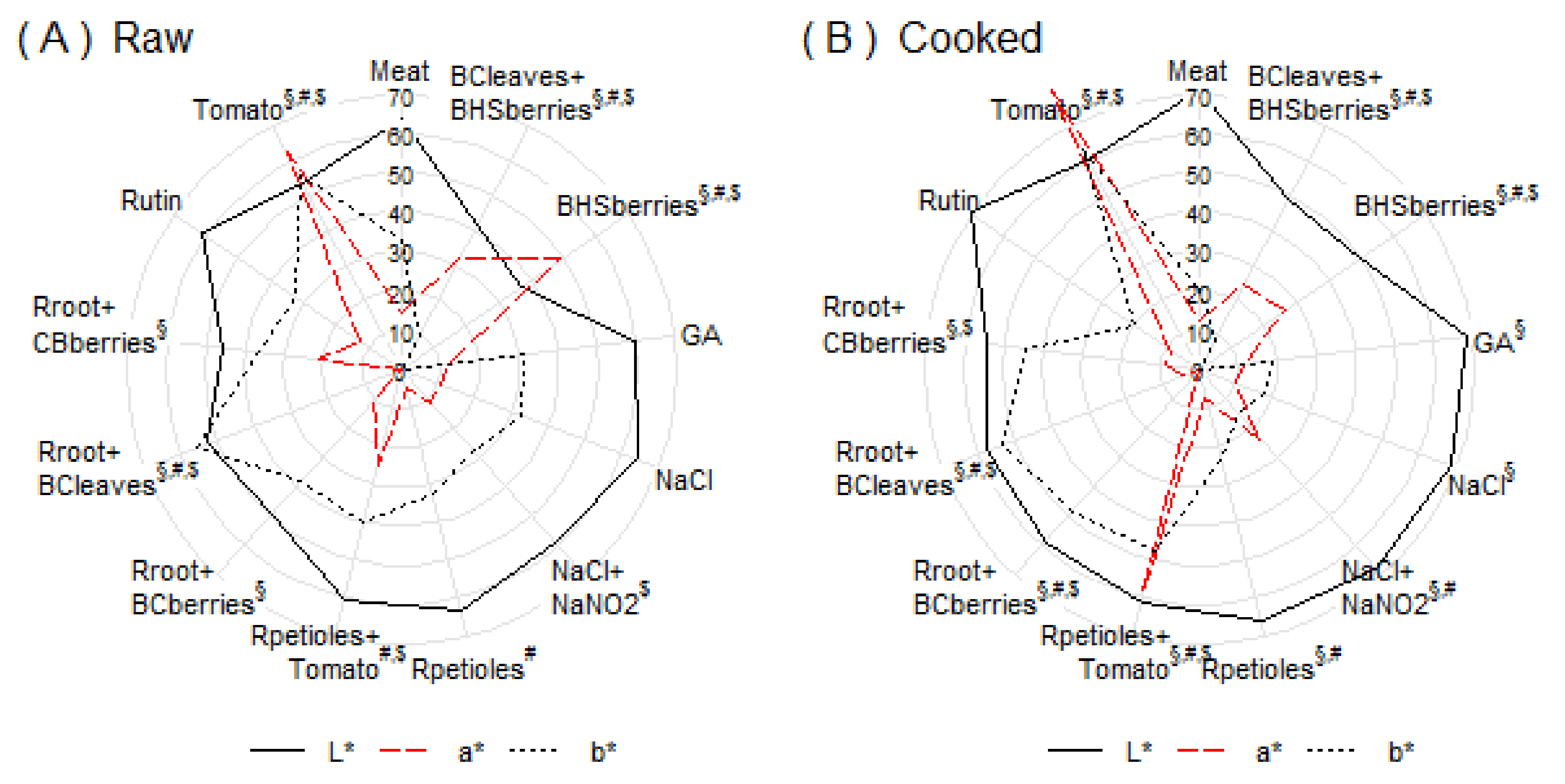
3.2. Color Measurements
3.3. Changes in pH Values
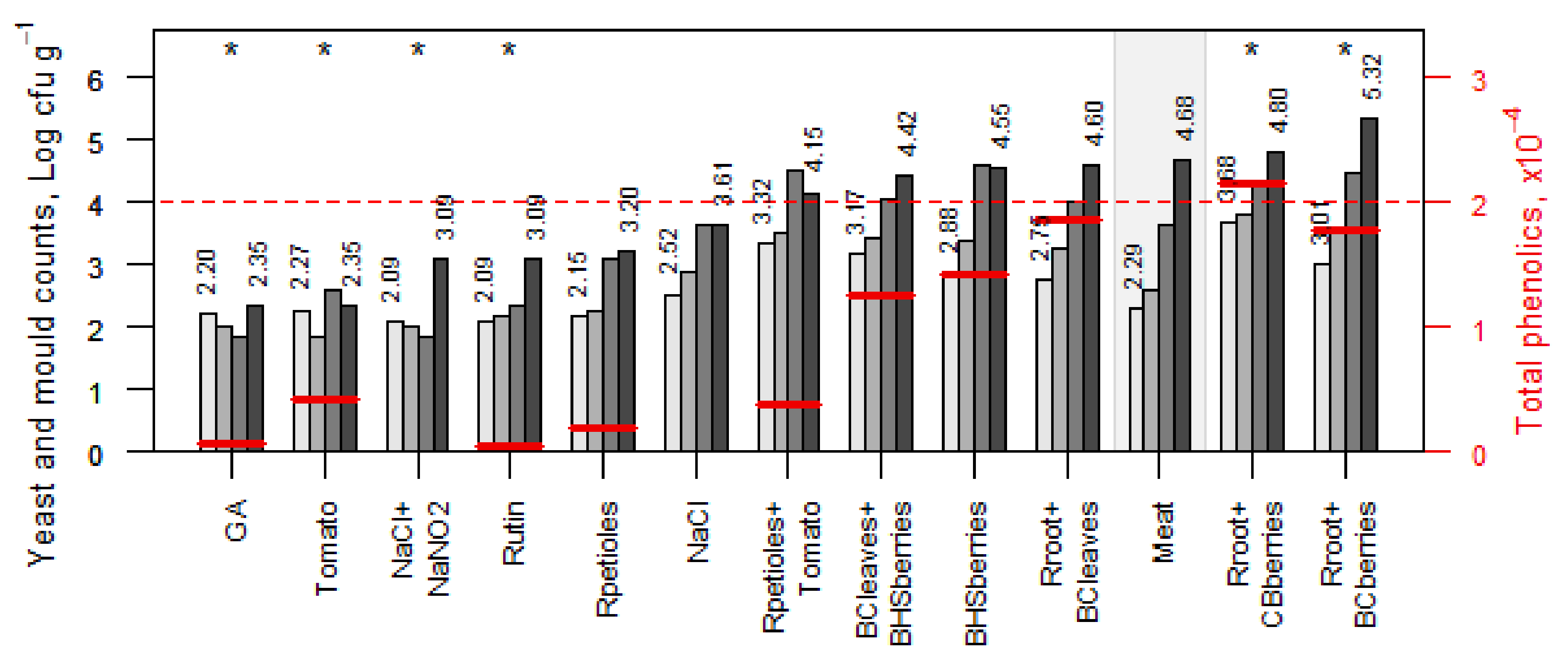
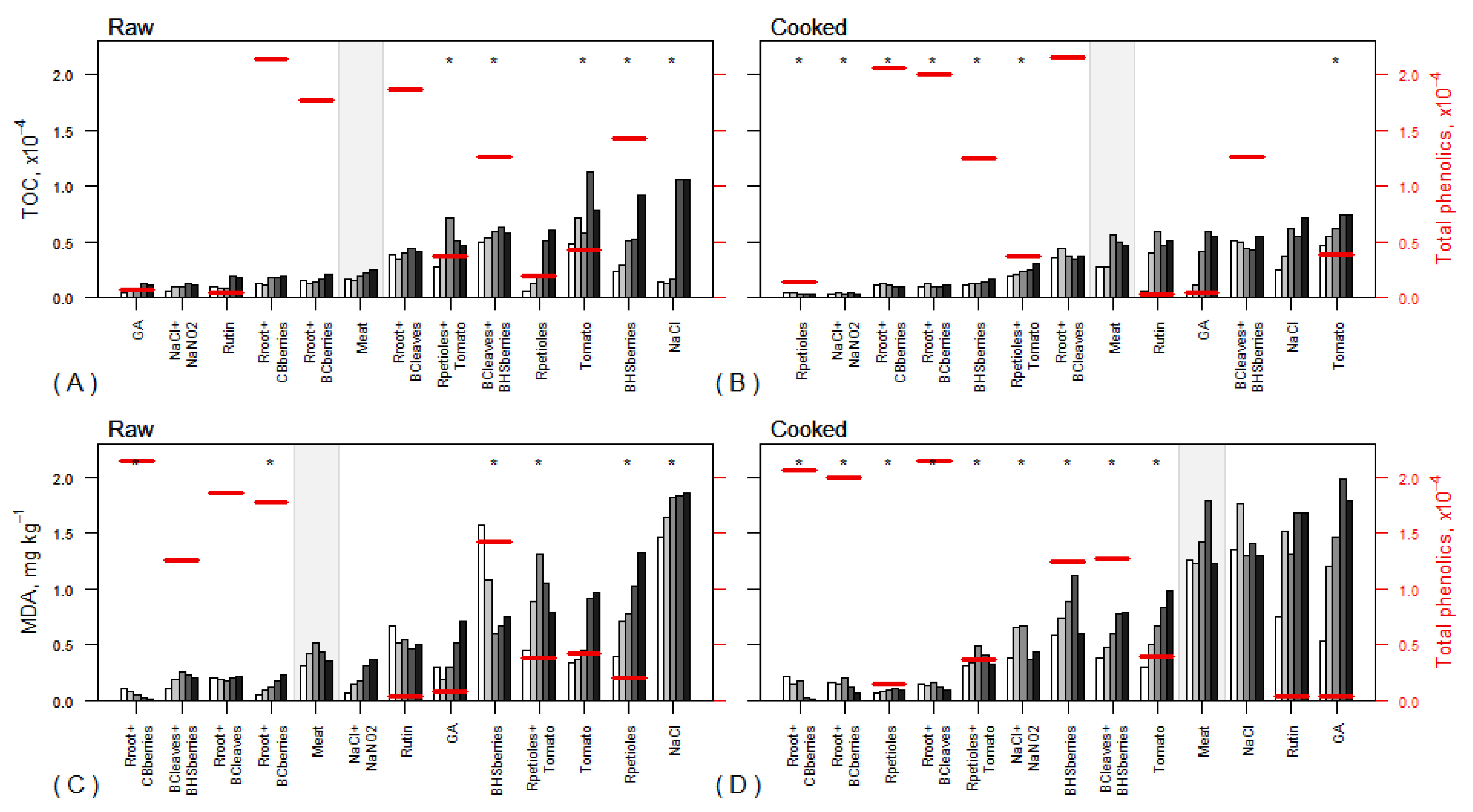
3.4. Total Phenolic Content
3.5. Antimicrobial Activity
3.6. Challenge Test
3.7. Fatty Acid Oxidation
3.8. Antimicrobial and Antioxidant Effect
4. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Al-Zoreky, N.S. Antimicrobial activity of pomegranate (Punica granatum L.) fruit peels. Int. J. Food Microbiol. 2009, 134, 244–248. [Google Scholar] [CrossRef] [PubMed]
- Sultana, T.; Rana, J.; Chakraborty, S.R.; Das, K.K.; Rahman, T.; Noor, R. Microbiological analysis of common preservatives used in food items and demonstration of their in vitro antibacterial activity. Asian Pac. J. Trop. Dis. 2014, 4, 452–456. [Google Scholar] [CrossRef]
- Lorenzo, J.M.; Pateiro, M.; Domínguez, R.; Barba, F.J.; Putnik, P.; Kovačević, D.B.; Shpigelman, A.; Granato, D.; Franco, D. Berries extracts as natural antioxidants in meat products: A review. Food Res. Int. 2018, 106, 1095–1104. [Google Scholar] [CrossRef] [PubMed]
- Gahruie, H.H.; Hosseini, S.M.H.; Taghavifard, M.H.; Eskandari, M.H.; Golmakani, M.-T.; Shad, E. Lipid oxidation, color changes, and microbiological quality of frozen beef burgers incorporated with shirazi thyme, cinnamon, and rosemary extracts. Hindawi J. Food Qual. 2017, 6350156. [Google Scholar] [CrossRef]
- Kumar, Y.; Yadav, D.N.; Ahmad, T.; Narsaiah, K. Recent trends in the use of natural antioxidants for meat and meat products. Compr. Rev. Food Sci. Food Saf. 2015, 14, 796–812. [Google Scholar] [CrossRef] [Green Version]
- Weiss, J.; Gibis, M.; Schuh, V.; Salminen, H. Advances in ingredient and processing systems for meat and meat products. Meat Sci. 2010, 86, 196–213. [Google Scholar] [CrossRef] [PubMed]
- Deda, M.S.; Bloukas, J.G.; Fista, G.A. Effect of tomato paste and nitrite level on processing and quality characteristics of frankfurters. Meat Sci. 2007, 76, 501–508. [Google Scholar] [CrossRef]
- Mariutti, L.R.B.; Bragagnolo, N. Influence of salt on lipid oxidation in meat and seafood products: A review. Food Res. Int. 2017, 94, 90–100. [Google Scholar] [CrossRef]
- Pisoschi, A.M.; Pop, A.; Georgescu, C.; Turcus, V.; Olah, N.K.; Mathe, E. An overview of natural antimicrobials role in food. Eur. J. Med. Chem. 2018, 143, 922–935. [Google Scholar] [CrossRef]
- Raudsepp, P.; Anton, D.; Roasto, M.; Meremäe, K.; Pedastsaar, P.; Mäesaar, M.; Raal, A.; Laikoja, K.; Püssa, T. The antioxidative and antibacterial properties of the blue honeysuckle (Lonicera caerulea L.), Siberian rhubarb (Rheum rhaponticum L.) and some other plants. Food Control. 2013, 31, 129–135. [Google Scholar] [CrossRef]
- Taguri, T.; Tanaka, T.; Kouno, I. Antibacterial spectrum of plant polyohneols and extracts depending upon hydroxyphenyl structure. Biol. Pharm. Bull. 2006, 29, 2226–2235. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jurikova, T.; Rop, O.; Mlcek, J.; Sochor, J.; Balla, S.; Szekeres, L.; Hegedusova, A.; Hubalek, J.; Adam, V.; Kizek, R. Phenolic profile of edible honeysuckle berries (Genus Lonicera) and their biological effects. Molecules 2012, 17, 61–79. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raudsepp, P.; Koskar, J.; Anton, D.; Meremäe, K.; Kapp, K.; Laurson, P.; Bleive, U.; Kaldmäe, H.; Roasto, M.; Püssa, T. Antibacterial and antioxidative properties of different parts of garden rhubarb blackcurrant, chokeberry and blue honeysuckle. J. Sci. Food Agric. 2019, 99, 2311–2320. [Google Scholar] [CrossRef] [PubMed]
- Colla, G.; Kim, H.-J.; Kyriacou, M.C.; Rouphael, Y. Nitrate in fruits and vegetables. Sci. Hortic. 2018, 237, 221–238. [Google Scholar] [CrossRef]
- Püssa, T.; Raudsepp, P.; Kuzina, K.; Raal, A. Polyphenolic composition of roots and petioles of Rheum rhaponticum L. Phytochem. Anal. 2009, 20, 98–103. [Google Scholar] [CrossRef] [PubMed]
- Gironés-Vilaplana, A.; Valentão, P.; Andrade, P.B.; Ferreres, F.; Moreno, D.A.; García-Viguera, C. Phytochemical profile of a blend of black chokeberry and lemon juice with cholinesterase inhibitory effect and antioxidant potential. Food Chem. 2012, 134, 2090–2096. [Google Scholar] [CrossRef] [PubMed]
- Eyiler, E.; Oztan, A. Production of frankfurters with tomato powder as a natural additive. LWT Food Sci. Technol. 2011, 44, 307–311. [Google Scholar] [CrossRef]
- García, M.L.; Calvo, M.M.; Selgas, M.D. Beef hamburgers enriched in lycopene using dry tomato peel as an ingredient. Meat Sci. 2009, 83, 45–49. [Google Scholar] [CrossRef]
- Hayes, J.E.; Canonico, I.; Allen, P. Effects of organic tomato pulp powder and nitrite level on the physicochemical, textural and sensory properties of pork luncheon roll. Meat Sci. 2013, 95, 755–762. [Google Scholar] [CrossRef]
- Savadkoohi, S.; Hoogenkamp, H.; Shamsi, K.; Farahnaky, A. Color, sensory and textural attributes of beef frankfurter, beef ham and meat-free sausage containing tomato pomace. Meat Sci. 2014, 97, 410–418. [Google Scholar] [CrossRef]
- Zhang, Y.; Holman, B.W.B.; Ponnampalam, E.N.; Kerr, M.G.; Bailes, K.L.; Kilgannon, A.K.; Collins, D.; Hopkins, D.I. Understanding beef flavour and overall liking traits using two different methods for determination of thiobarbituric acid reactive substance (TBARS). Meat Sci. 2019, 149, 114–119. [Google Scholar] [CrossRef] [PubMed]
- Modzelewska-Kapituła, M. Effects of tomato powder on colour, lipid oxidation and sensory properties of comminuted meat products. J. Food Qual. 2012, 35, 323–330. [Google Scholar] [CrossRef]
- Püssa, T.; Raudsepp, P.; Toomik, P.; Pällin, R.; Mäeorg, U.; Kuusik, S.; Soidla, R.; Rei, M. Study of oxidation products of free polyunsaturated fatty acids in mechanically deboned meat. J. Food Compos. Anal. 2009, 22, 307–314. [Google Scholar] [CrossRef]
- Björkroth, J. Microbiological ecology of marinated meat products. Meat Sci. 2005, 70, 477–480. [Google Scholar] [CrossRef] [PubMed]
- Hmelak Gorenjak, A.; Cencič, A. Nitrate in vegetables and their impact on human health. A review. Acta Aliment. 2013, 42, 158–172. [Google Scholar] [CrossRef]
- Gyawali, R.; Ibrahim, S.A. Natural products as antimicrobial agents. Food Control. 2014, 46, 412–429. [Google Scholar] [CrossRef]
- Tiwari, B.; Valdramidis, V.; O’Donell, C.; Muthukumarappan, K.; Cullen, P.; Bourke, P. Application of natural antimicrobials for food preservation. J. Agric. Food Chem. 2009, 57, 5987–6000. [Google Scholar] [CrossRef] [Green Version]
- Brewer, M.S. Natural antioxidants: Sources, compounds, mechanisms of action and potential applications. Compr. Rev. Food Sci. Food Saf. 2011, 10, 221–247. [Google Scholar] [CrossRef]
- Guéraud, F.; Atalay, M.; Bresgen, N.; Cipak, A.; Eckl, P.M.; Huc, L.; Jouanin, I.; Siems, W.; Uchida, K. Chemistry and biochemistry of lipid peroxidation products. Free Radic. Res. 2010, 44, 1098–1124. [Google Scholar] [CrossRef]
- Khoo, H.E.; Azlan, A.; Tang, S.T.; Lim, S.M. Anthocyanidins and anthocyanins: Colored pigments as food, pharmaceutical ingredients, and the potential health benefits. Food Nutr. Res. 2017, 61, 1361779. [Google Scholar] [CrossRef] [Green Version]
- Matsuda, H.; Morikawa, T.; Toguchida, I.; Park, J.-Y.; Harima, S.; Yoshikawa, M. Antioxidant constituents from rhubarb: Structural requirements of stilbenes for the activity and structures of two new anthraquinone glucosides. Bioorg. Med. Chem. 2001, 9, 41–50. [Google Scholar] [CrossRef]
- López, M.; Martínez, F.; Del Valle, C.; Ferrit, M.; Luque, R. Study of phenolic compounds as natural antioxidants by a fluorescence method. Talanta 2003, 60, 609–616. [Google Scholar] [CrossRef] [Green Version]
- Velderrain-Rodriguez, G.; Torres-Moreno, H.; Villegas-Ochoa, M.A.; Ayala-Zavala, F.; Robles-Zepeda, R.E.; Wall-Medrano, A.; González-Aguilar, G.A. Gallic acid content and an antioxidant mechanism are responsible for the antiproliferative activity of ‘Ataulfo’ mango peel on LS180 cells. Molecules 2018, 23, 695. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Demirbas, A. Oil, micronutrient and heavy metal contents of tomatoes. Food Chem. 2010, 118, 504–507. [Google Scholar] [CrossRef]
- Csepregi, K.; Neugart, S.; Schreiner, M.; Hideg, E. Comparative evaluation of total antioxidant capacities of plant polyphenols. Molecules 2016, 21, 208. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burri, S.C.M.; Ekholm, A.; Håkansson, Å.; Tornberg, E.; Rumpunen, K. Antioxidant capacity and major phenol compounds of horticultural plant materials not usually used. J. Funct. Foods. 2017, 38, 119–127. [Google Scholar] [CrossRef] [PubMed]
- Miguel, M.M. Anthocyanins: Antioxidant and/or anti-inflammatory activities. J. Appl. Pharm. Sci. 2011, 1, 7–15. [Google Scholar]



| No | Abbreviation | Composition |
|---|---|---|
| 1 | Meat (Control) | Minced meat |
| 2 | NaCl | Minced meat with 1% sodium chloride |
| 3 | NaCl+NaNO2 | Minced meat with 1% sodium chloride and 150 mg/kg sodium nitrite |
| 4 | Rroot+BCberries | Minced meat with 1% rhubarb root+1% blackcurrant berries |
| 5 | Rroot+BCleaves | Minced meat with 1% rhubarb root+1% blackcurrant leaves |
| 6 | Rroot+CBberries | Minced meat with 1% rhubarb root+1% chokeberry berries |
| 7 | BCleaves+BHSberries | Minced meat with 1% blackcurrant leaves+1% blue honeysuckle berries |
| 8 | Rpetioles+Tomato | Minced meat with 1% rhubarb petioles+1% tomato |
| 9 | BHSberries | Minced meat with 2% blue honeysuckle berries |
| 10 | Rpetioles | Minced meat with 2% rhubarb petioles |
| 11 | Tomato | Minced meat with 2% tomato |
| 12 | Rutin | Minced meat with 80 mg/kg rutin |
| 13 | GA | Minced meat with 80 mg/kg gallic acid |
| Sample | Storage Day | pH * | Water Activity * aw | Total Count (cfu·g−1) | Growth Potential δ (log cfu·g−1) ** |
|---|---|---|---|---|---|
| Meat | 0 | 6.19 ± 0.021 | 0.986 ± 0.003 | 2 | 5.72 |
| 7 | 6.19 ± 0.021 | 0.986 | 2.2 × 10² | ||
| 14 | 6.19 ± 0.007 | 0.982 ± 0.001 | 9.4 × 10³ | ||
| NaCl+NaNO2 | 0 | 6.17 | 0.972 ± 0.001 | <1 | 5.97 |
| 7 | 6.22 ± 0.014 | 0.976 ± 0.001 | 7.5 × 10¹ | ||
| 14 | 6.18 ± 0.007 | 0.976 | <1 | ||
| Rpetioles+Tomato | 0 | 5.58 ± 0.021 | 0.985 | 9 | 4.54 |
| 7 | 5.50 ± 0.014 | 0.984 | 2 | ||
| 14 | 5.37 | 0.982 ± 0.001 | 4 | ||
| Rroot+BCberries | 0 | 5.66 ± 0.014 | 0.984 | 6.1 × 10² | 5.30 |
| 7 | 5.68 ± 0.007 | 0.983 ± 0.001 | 4.5 × 10¹ | ||
| 14 | 5.71 | 0.981 | 7.1 × 10¹ | ||
| Tomato | 0 | 5.90 ± 0.007 | 0.983 | 3 | 5.67 |
| 7 | 5.84 ± 0.007 | 0.982 | 2 | ||
| 14 | 5.85 ± 0.007 | 0.978 ± 0.001 | 1 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Anton, D.; Koskar, J.; Raudsepp, P.; Meremäe, K.; Kaart, T.; Püssa, T.; Roasto, M. Antimicrobial and Antioxidative Effects of Plant Powders in Raw and Cooked Minced Pork. Foods 2019, 8, 661. https://doi.org/10.3390/foods8120661
Anton D, Koskar J, Raudsepp P, Meremäe K, Kaart T, Püssa T, Roasto M. Antimicrobial and Antioxidative Effects of Plant Powders in Raw and Cooked Minced Pork. Foods. 2019; 8(12):661. https://doi.org/10.3390/foods8120661
Chicago/Turabian StyleAnton, Dea, Julia Koskar, Piret Raudsepp, Kadrin Meremäe, Tanel Kaart, Tõnu Püssa, and Mati Roasto. 2019. "Antimicrobial and Antioxidative Effects of Plant Powders in Raw and Cooked Minced Pork" Foods 8, no. 12: 661. https://doi.org/10.3390/foods8120661





