Critical Review on the Utilization of Handheld and Portable Raman Spectrometry in Meat Science
Abstract
:1. Introduction
2. Application of Raman Spectroscopy in Meat Quality Prediction
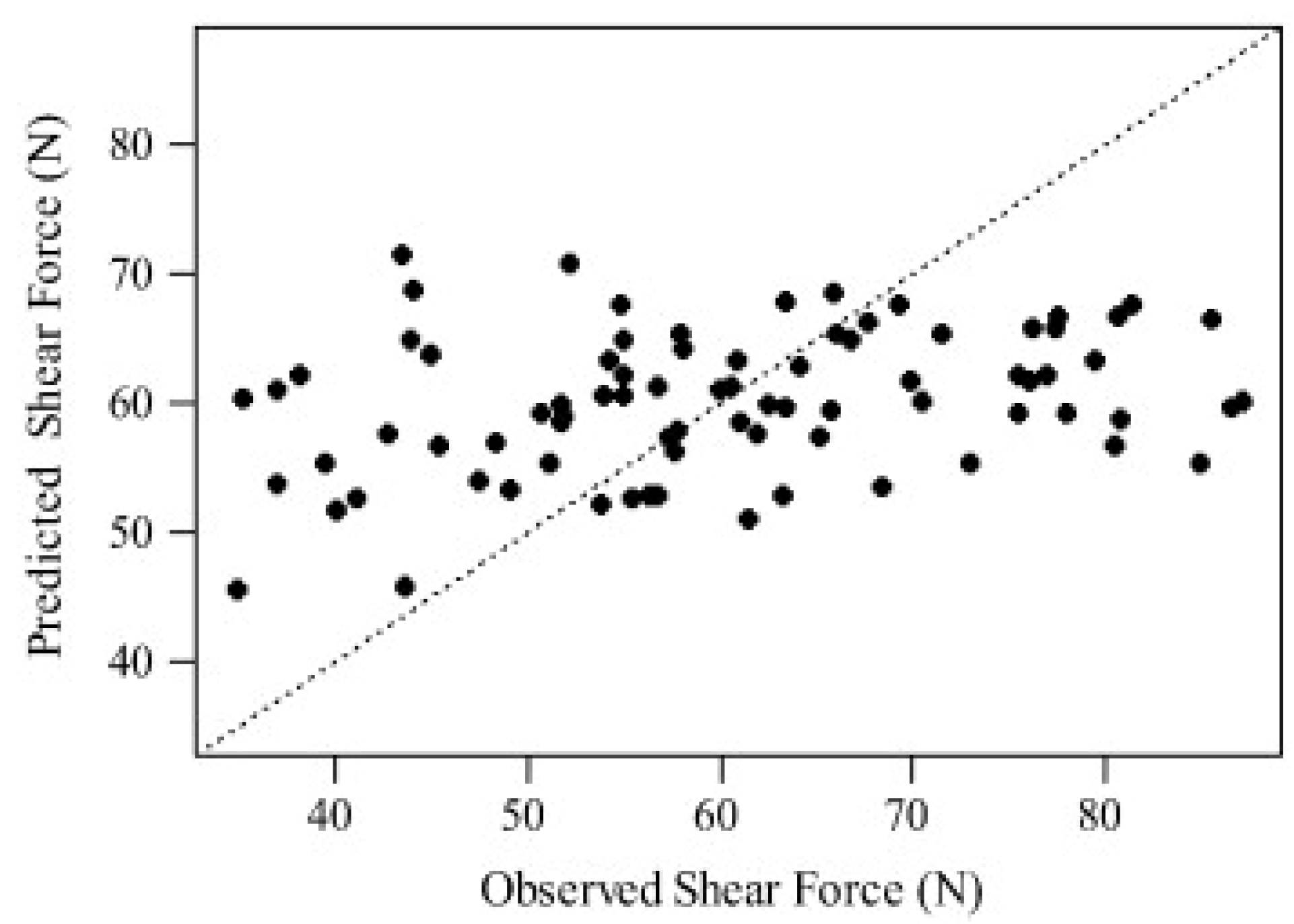
2.1. Prediction of Eating Quality Traits with Raman Spectroscopy
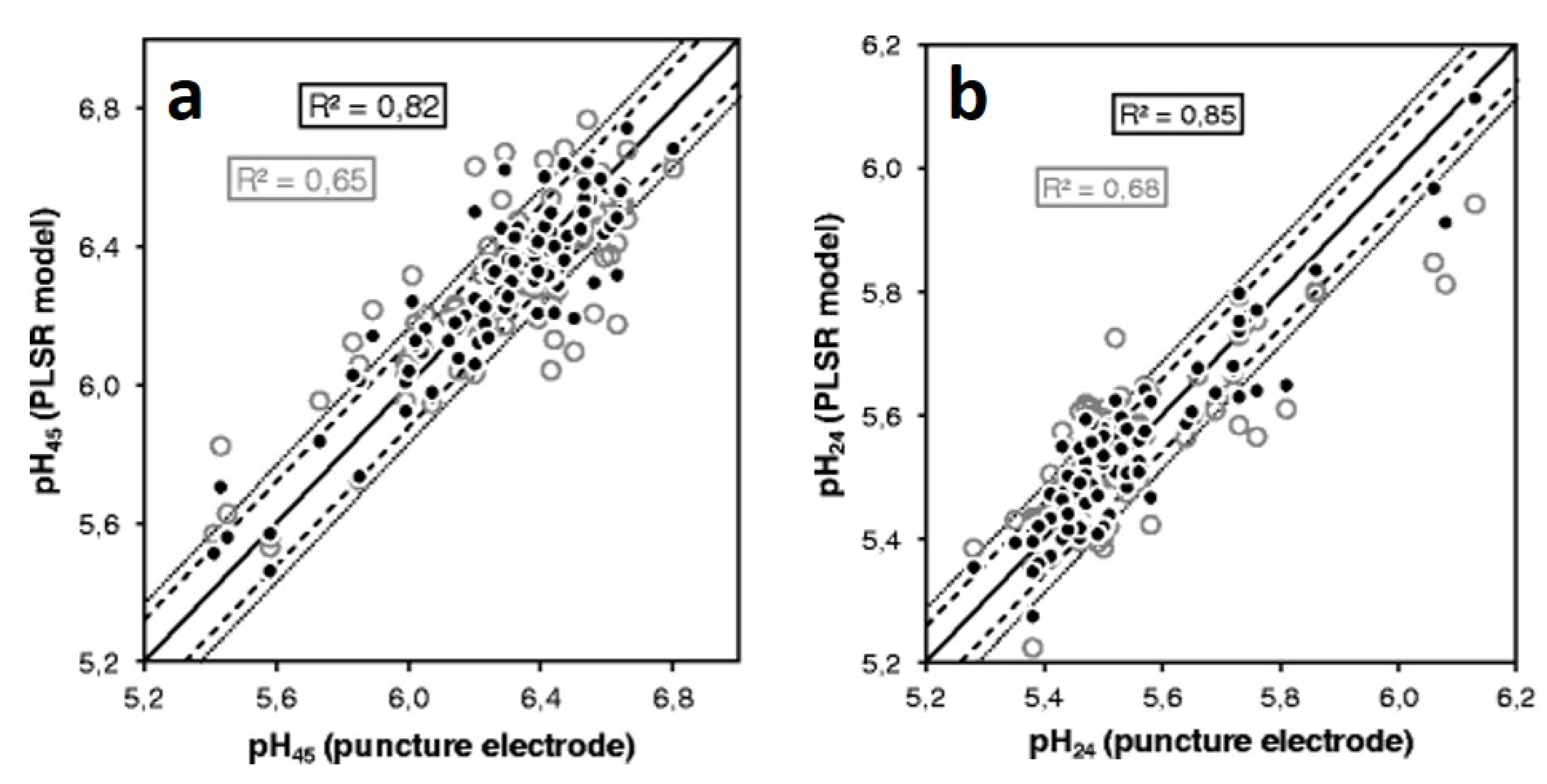
2.2. Prediction of pH Values with Raman Spectroscopy
2.3. Prediction of L* Values and Drip Loss with Raman Spectroscopy
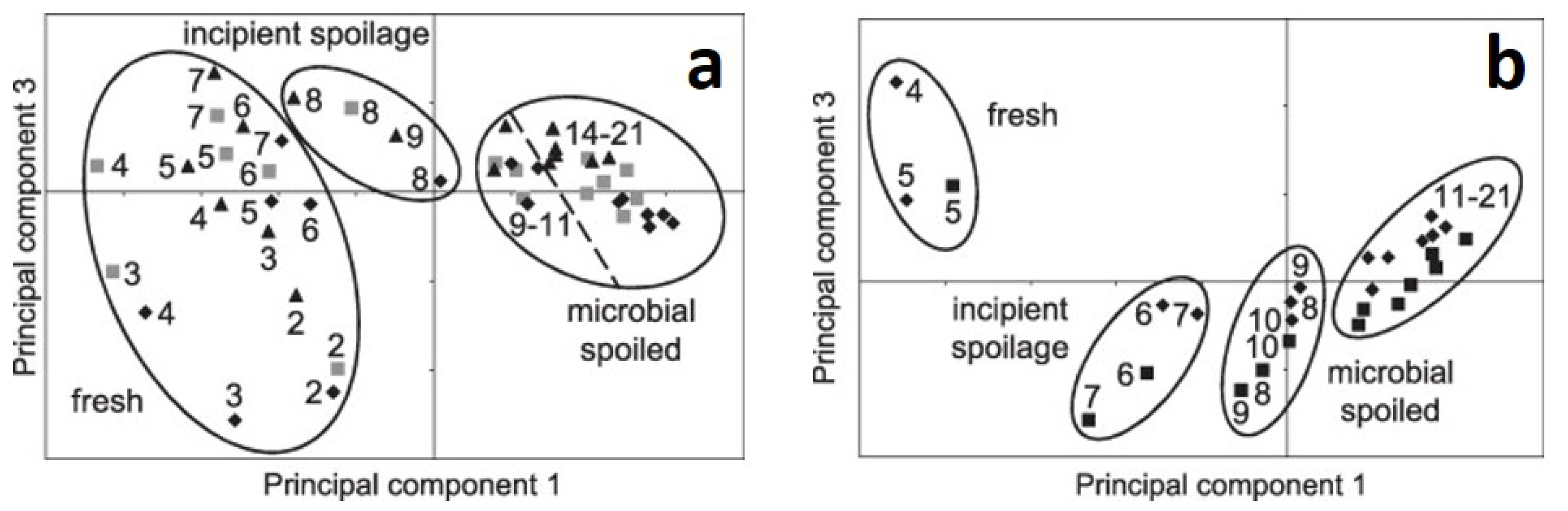
2.4. Meat Spoilage Identification with Raman Spectroscopy
2.5. Other Applications of Raman Spectroscopy in Meat Science
3. Conclusions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| ATR-MIR | attenuated total reflection mid-infrared |
| CAL | calibration |
| cfu | colony-forming units |
| CV | cross validation |
| DFD | dark, firm and dry |
| FA | fatty acid |
| GC | gas chromatography |
| IL | inner layer |
| IMF | intramuscular fat |
| LD | m. longissimus dorsi |
| LDA | linear discriminant analysis |
| LIF | laser induced fluorescence |
| LOO-CV | leave-one-out cross validation |
| LV | latent variable |
| MLR | multiple linear regression |
| NIRS | near-infrared spectroscopy |
| NMR | nuclear magnetic resonance |
| NRMSEC | normalized root mean square error of calibration |
| NRMSECV | normalized root mean square error of cross validation |
| NRMSEP | normalized root mean square error of prediction |
| OL | outer layer |
| PC | principal component |
| PCA | principal component analysis |
| PFN | pale, firm and non-exudative |
| PLS-DA | partial least squares discriminant analysis |
| PLS-R | partial least squares regression |
| PSE | pale, soft and exudative |
| RFN | reddish, firm and non-exudative |
| RMSEC | root mean square error of calibration |
| RMSECV | root mean square error of cross validation |
| RMSEP | root mean square error of prediction |
| RSE | reddish, soft and exudative |
| SM | m. semimembranosus |
| SVM-C | support vector machine classification |
| TVC | total viable count |
| VAL | validation |
| Vis | visible |
| VOCs | volatile organic compounds |
| WHC | water-holding capacity |
| WSBF | Warner-Bratzler shear force |
References
- Müller, A.; Steinhart, H. Recent developments in instrumental analysis for food quality. Food Chem. 2007, 102, 436–444. [Google Scholar] [CrossRef]
- Damez, J.L.; Clerjon, S. Recent Advances in Meat Quality Assessment. In Handbook of Meat and Meat Processing; CRC Press: Boca Raton, FL, USA, 2011; pp. 161–176. [Google Scholar]
- European Commission. Horse Meat—Questions and Answers. 2013. Available online: https://ec.europa.eu/food/safety/official_controls/food_fraud/horse_meat/q-ans_en (accessed on 15 May 2018).
- Premanandh, J. Horse meat scandal—A wake-up call for regulatory authorities. Food Control 2013, 34, 568–569. [Google Scholar] [CrossRef]
- European Parliament. Parliamentary Questions—Brazilian Rotten Meat Scandal. 2017. Available online: http://www.europarl.europa.eu/sides/getDoc.do?pubRef=-//EP//TEXT+WQ+E-2017-002022+0+DOC+XML+V0//EN (accessed on 15 May 2018).
- BBC News. Brazil Meat-Packing Giants ‘Exported Rotten Beef’. 2017. Available online: https://www.bbc.com/news/world-latin-america-39311336 (accessed on 16 May 2018).
- The Guardian. Fear of Meat Scandal as Data Shows Hygiene Breaches at over Half UK Plants. 2018. Available online: https://www.theguardian.com/world/2018/feb/23/fear-of-uk-meat-scandal-as-data-shows-hygiene-breaches-at-most-plants (accessed on 16 May 2018).
- Hunt, M.R.; Garmyn, A.J.; O’Quinn, T.G.; Corbin, C.H.; Legako, J.F.; Rathmann, R.J.; Brooks, J.C.; Miller, M.F. Consumer assessment of beef palatability from four beef muscles from USDA Choice and Select graded carcasses. Meat Sci. 2014, 98, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Van Wezemael, L.; De Smet, S.; Ueland, Ø.; Verbeke, W. Relationships between sensory evaluations of beef tenderness, shear force measurements and consumer characteristics. Meat Sci. 2014, 97, 310–315. [Google Scholar] [CrossRef]
- Damez, J.L.; Clerjon, S. Meat quality assessment using biophysical methods related to meat structure. Meat Sci. 2008, 80, 132–149. [Google Scholar] [CrossRef]
- Karumendu, L.U.; van de Ven, R.; Kerr, M.J.; Lanza, M.; Hopkins, D.L. Particle size analysis of lamb meat: Effect of homogenization speed, comparison with myofibrillar fragmentation index and its relationship with shear force. Meat Sci. 2009, 82, 425–431. [Google Scholar] [CrossRef]
- Shackelford, S.D.; Wheeler, T.L.; Koohmaraie, M. Relationship between shear force and trained sensory panel tenderness ratings of 10 major muscles from Bos indicus and Bos taurus cattle. J. Anim. Sci. 1995, 73, 3333–3340. [Google Scholar] [CrossRef]
- Warner, R.D.; Kauffman, R.G.; Russel, R.L. Quality attributes of major porcine muscles: A comparison with the Longissimus Lumborum. Meat Sci. 1993, 33, 359–372. [Google Scholar] [CrossRef]
- Kauffman, R.G.; Sybesma, W.; Smulders, F.J.; Eikelenboom, G.; Engel, B.; van Laack, R.L.; Hoving-Bolink, A.H.; Sterrenburg, P.; Nordheim, E.V.; Walstra, P.; et al. The effectiveness of examining early post-mortem musculature to predict ultimate pork quality. Meat Sci. 1993, 34, 283–300. [Google Scholar] [CrossRef]
- Van Laack, R.L.; Kauffman, R.G.; Sybesma, W.; Smulders, F.J.; Eikelenboom, G.; Pinheiro, J.C. Is colour brightness (L-value) a reliable indicator of water-holding capacity in porcine muscle? Meat Sci. 1994, 38, 193–201. [Google Scholar] [CrossRef]
- Warner, R.D.; Kauffman, R.G.; Greaser, M.L. Muscle protein changes post mortem in relation to pork quality traits. Meat Sci. 1997, 45, 339–352. [Google Scholar] [CrossRef]
- Honikel, K.O.; Fischer, C. A rapid method for the detection of PSE and DFD porcine muscles. J. Food Sci. 1977, 42, 1633–1636. [Google Scholar] [CrossRef]
- European Parliament; Council of the European Union. Regulation (EU) No 1308/2013 of the European Parliament and of the Council of 17 December 2013 establishing a common organisation of the markets in agricultural products and repealing Council Regulations (EEC) No 922/72, (EEC) No 234/79, (EC) No 1037/2001 and (EC) No 1234/2007. Off. J. Eur. Union 2013, 56, 802–803. [Google Scholar]
- AOAC International. Official method 991.36—Fat (crude) in meat and meat products. J. AOAC Int. 1992, 75, 289. [Google Scholar]
- Folch, J.; Lees, M.; Stanley, G.S. A simple method for the isolation and purification of total lipides from animal tissue. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar] [CrossRef] [PubMed]
- Lepage, G.; Roy, C.C. Direct transesterification of all classes of lipids in a one-step reaction. J. Lipid Res. 1986, 27, 114–120. [Google Scholar] [PubMed]
- O’Fallon, J.V.; Busboom, J.R.; Nelson, M.L.; Gaskins, C.T. A direct method for fatty acid methyl ester synthesis: Application to wet meat tissues, oils, and feedstuffs. J. Anim. Sci. 2007, 85, 1511–1521. [Google Scholar] [CrossRef]
- Díaz, P.; Nieto, G.; Garrido, M.D.; Bañón, S. Microbial, physical-chemical and sensory spoilage during the refrigerated storage of cooked pork loin processed by the sous vide method. Meat Sci. 2008, 80, 287–292. [Google Scholar] [CrossRef]
- ISO 21527-1:2008. Microbiology of Food and Animal Feeding Stuffs—Horizontal Method for the Enumeration of Yeasts and Moulds—Part 1: Colony Count Technique in Products with Water Activity Greater than 0.95. 2008. Available online: www.ios.org (accessed on 15 May 2018).
- Mayr, D.; Margesin, R.; Klingsbichel, E.; Hartungen, E.; Jenewein, D.; Schinner, F.; Mark, T.D. Rapid detection of meat spoilage by measuring volatile organic compounds by using proton transfer reaction mass spectrometry. Appl. Environ. Microbiol. 2003, 69, 4697–4705. [Google Scholar] [CrossRef]
- Lovestead, T.M.; Bruno, T.J. Detection of poultry spoilage markers from headspace analysis with cryoadsorption on a short alumina PLOT column. Food Chem. 2010, 121, 1274–1282. [Google Scholar] [CrossRef]
- Blixt, Y.; Borch, E. Using an electronic nose for determining the spoilage of vacuum-packaged beef. Int. J. Food Microbiol. 1999, 46, 123–134. [Google Scholar] [CrossRef]
- Balasubramanian, S.; Panigrahi, S.; Logue, C.M.; Gu, H.; Marchello, M. Neural networks-integrated metal oxide-based artificial olfactory system for meat spoilage identification. J. Food Eng. 2009, 91, 91–98. [Google Scholar] [CrossRef]
- Jia, W.; Liang, G.; Wang, Y.; Wang, J. Electronic Noses as a Powerful Tool for Assessing Meat Quality: A Mini Review. Food Anal. Methods 2018, 11, 2916–2924. [Google Scholar] [CrossRef]
- Prieto, N.; Pawluczyk, O.; Edward, M.; Dugan, R.; Aalhus, J.L. A Review of the Principles and Applications of Near-Infrared Spectroscopy to Characterize Meat, Fat, and Meat Products. Appl. Spectrosc. 2017, 71, 1403–1426. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Yu, H.; Xu, H.; Ying, Y. Near infrared spectroscopy for on/in-line monitoring of quality in foods and beverages: A review. J. Food Eng. 2008, 87, 303–313. [Google Scholar] [CrossRef]
- Prieto, N.; Roehe, R.; Lavín, P.; Batten, G.; Andrés, S. Application of near infrared reflectance spectroscopy to predict meat and meat products quality: A review. Meat Sci. 2009, 83, 175–186. [Google Scholar] [CrossRef] [PubMed]
- Schmutzler, M.; Beganovic, A.; Böhler, G.; Huck, C.W. Methods for detection of pork adulteration in veal product based on FT-NIR spectroscopy for laboratory, industrial and on-site analysis. Food Control 2015, 57, 258–267. [Google Scholar] [CrossRef]
- Ellis, D.I.; Broadhurst, D.; Clarke, S.J.; Goodacre, R. Rapid identification of closely related muscle foods by vibrational spectroscopy and machine learning. Analyst 2005, 130, 1648. [Google Scholar] [CrossRef]
- Ammor, M.S.; Argyri, A.; Nychas, G.J.E. Rapid monitoring of the spoilage of minced beef stored under conventionally and active packaging conditions using Fourier transform infrared spectroscopy in tandem with chemometrics. Meat Sci. 2009, 81, 507–514. [Google Scholar] [CrossRef]
- Alexandrakis, D.; Downey, G.; Scannell, A.G.M. Rapid Non-destructive Detection of Spoilage of Intact Chicken Breast Muscle Using Near-infrared and Fourier Transform Mid-infrared Spectroscopy and Multivariate Statistics. Food Bioprocess Technol. 2012, 5, 338–347. [Google Scholar] [CrossRef]
- Cozzolino, D.; Murray, I. Identification of animal meat muscles by visible and near infrared reflectance spectroscopy. LWT Food Sci. Technol. 2004, 37, 447–452. [Google Scholar] [CrossRef]
- Balage, J.M.; da Luz e Silva, S.; Gomide, C.A.; Bonin, M.d.N.; Figueira, A.C. Predicting pork quality using Vis/NIR spectroscopy. Meat Sci. 2015, 108, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Andrés, S.; Murray, I.; Navajas, E.A.; Fisher, A.V.; Lambe, N.R.; Bünger, L. Prediction of sensory characteristics of lamb meat samples by near infrared reflectance spectroscopy. Meat Sci. 2007, 76, 509–516. [Google Scholar] [CrossRef] [PubMed]
- Renou, J.; Bielicki, G.; Bonny, J.; Donnat, J.; Foucat, L. Assessment of meat quality by NMR. Spec. Publ. R. Soc. Chem. 2003, 286, 161–171. [Google Scholar]
- Bertram, H.C.; Ersen, H.J. Applications of NMR in Meat Science. Annu. Rep. NMR Spectrosc. 2004, 53, 157–202. [Google Scholar] [CrossRef]
- Straadt, I.K.; Aaslyng, M.D.; Bertram, H.C. Assessment of meat quality by NMR-an investigation of pork products originating from different breeds. Magn. Reson. Chem. 2011, 49, S71–S78. [Google Scholar] [CrossRef]
- Hassing, S.; Jernshøj, K. Benefits and challenges in applying Raman spectroscopy. Agro FOOD Ind. Hi Tech 2014, 25, 2. [Google Scholar]
- Herrero, A.M. Raman spectroscopy a promising technique for quality assessment of meat and fish: A review. Food Chem. 2008, 107, 1642–1651. [Google Scholar] [CrossRef]
- Ozaki, Y.; Šašić, S. Introduction to Raman Spectroscopy. In Pharmaceutical Applications of Raman Spectroscopy; Šašić, S., Ed.; John Wiley & Sons: Hoboken, NJ, USA, 2008; Chapter 1; pp. 1–28. [Google Scholar]
- Esbensen, K.H.; Guyot, D.; Westad, F.; Houmoller, L.P. Multivariate Data Analysis: In Practice: An Introduction to Multivariate Data Analysis and Experimental Design, 5th ed.; CAMO Software AS: Oslo, Norway, 2009. [Google Scholar]
- Næs, T.; Isaksson, T.; Fearn, T.; Davies, T. A User Friendly Guide to Multivariate Calibration And Classification; NIR Publications: Chichester, UK, 2004. [Google Scholar]
- Schmidt, H.; Scheier, R.; Hopkins, D.L. Preliminary investigation on the relationship of Raman spectra of sheep meat with shear force and cooking loss. Meat Sci. 2013, 93, 138–143. [Google Scholar] [CrossRef]
- Fowler, S.M.; Schmidt, H.; van de Ven, R.; Wynn, P.; Hopkins, D.L. Raman spectroscopy compared against traditional predictors of shear force in lamb m. longissimus lumborum. Meat Sci. 2014, 98, 652–656. [Google Scholar] [CrossRef]
- Fowler, S.M.; Schmidt, H.; Van de Ven, R.; Wynn, P.; Hopkins, D.L. Predicting tenderness of fresh ovine semimembranosus using Raman spectroscopy. Meat Sci. 2014, 97, 597–601. [Google Scholar] [CrossRef] [PubMed]
- Fowler, S.M.; Schmidt, H.; van de Ven, R.; Wynn, P.; Hopkins, D.L. Predicting meat quality traits of ovine m. semimembranosus, both fresh and following freezing and thawing, using a hand held Raman spectroscopic device. Meat Sci. 2015, 108, 138–144. [Google Scholar] [CrossRef] [PubMed]
- Fowler, S.M.; Schmidt, H.; van de Ven, R.; Hopkins, D.L. Preliminary investigation of the use of Raman spectroscopy to predict meat and eating quality traits of beef loins. Meat Sci. 2018, 138, 53–58. [Google Scholar] [CrossRef] [PubMed]
- Bauer, A.; Scheier, R.; Eberle, T.; Schmidt, H. Assessment of tenderness of aged bovine gluteus medius muscles using Raman spectroscopy. Meat Sci. 2016, 115, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Brereton, R.G.; Lloyd, G.R. Support Vector Machines for classification and regression. Analyst 2010, 135, 230–267. [Google Scholar] [CrossRef] [PubMed]
- Scheier, R.; Schmidt, H. Measurement of the pH value in pork meat early postmortem by Raman spectroscopy. Appl. Phys. B Lasers Opt. 2013, 111, 289–297. [Google Scholar] [CrossRef]
- Scheier, R.; Bauer, A.; Schmidt, H. Early Postmortem Prediction of Meat Quality Traits of Porcine Semimembranosus Muscles Using a Portable Raman System. Food Bioprocess Technol. 2014, 7, 2732–2741. [Google Scholar] [CrossRef]
- Scheier, R.; Scheeder, M.; Schmidt, H. Prediction of pork quality at the slaughter line using a portable Raman device. Meat Sci. 2015, 103, 96–103. [Google Scholar] [CrossRef]
- Nache, M.; Hinrichs, J.; Scheier, R.; Schmidt, H.; Hitzmann, B. Prediction of the pH as indicator of porcine meat quality using Raman spectroscopy and metaheuristics. Chemom. Intell. Lab. Syst. 2016, 154, 45–51. [Google Scholar] [CrossRef]
- Schmidt, H.; Sowoidnich, K.; Kronfeldt, H.D. A prototype hand-held raman sensor for the in situ characterization of meat quality. App. Spectrosc. 2010, 64, 888–894. [Google Scholar] [CrossRef]
- Sowoidnich, K.; Schmidt, H.; Kronfeldt, H.D.; Schwägele, F. A portable 671 nm Raman sensor system for rapid meat spoilage identification. Vib. Spectrosc. 2012, 62, 70–76. [Google Scholar] [CrossRef]
- Liu, X.; Schmidt, H.; Mörlein, D. Feasibility of boar taint classification using a portable Raman device. Meat Sci. 2016, 116, 133–139. [Google Scholar] [CrossRef] [PubMed]
- Fowler, S.M.; Ponnampalam, E.N.; Schmidt, H.; Wynn, P.; Hopkins, D.L. Prediction of intramuscular fat content and major fatty acid groups of lamb M. longissimus lumborum using Raman spectroscopy. Meat Sci. 2015, 110, 70–75. [Google Scholar] [CrossRef] [PubMed]
- Dutson, T.R. The Measurement of pH in Muscle and its Importance to Meat Quality. In Reciprocal Meat Conference Proceeding; American Meat Science Association: Chicago, IL, USA, 1983; Volume 36, pp. 92–97. [Google Scholar]
- Williams, P.C. Implementation of Near-Infrared Technology. In Near-Infrared Technology in the Agricultural and Food Industries, 2nd ed.; Williams, P., Norris, K., Eds.; The American Association of Cereal Chemists: St. Paul, MN, USA, 2001; Chapter 8; pp. 145–169. [Google Scholar]
- Allegrini, F.; Olivieri, A.C. A new and efficient variable selection algorithm based on ant colony optimization. Applications to near infrared spectroscopy/partial least-squares analysis. Anal. Chim. Acta 2011, 699, 18–25. [Google Scholar] [CrossRef] [PubMed]
- De Jong, S. SIMPLS: An alternative approach squares regression to partial least. Chemom. Intell. Lab. Syst. 1993, 18, 2–263. [Google Scholar] [CrossRef]
- Bonneau, M.; Le Denmat, M.; Vaudelet, J.C.; Veloso Nunes, J.R.; Mortensen, A.B.; Mortensen, H.P. Contributions of fat androstenone and skatole to boar taint: I. Sensory attributes of fat and pork meat. Livest. Prod. Sci. 1992, 32, 63–80. [Google Scholar] [CrossRef]
- Sørensen, K.M.; Engelsen, S.B. Measurement of boar taint in porcine fat using a high-throughput gas chromatography-mass spectrometry protocol. J. Agric. Food Chem. 2014, 62, 9420–9427. [Google Scholar] [CrossRef]
- Erdmann, B.; Erdmann, B. Verfahren und Vorrichtung zum Erkennen und Aussortieren von GeruchsauffäLligen Geschlachteten Ebern in Einer Schlachtlinie. Germany Patent DE102014117572A1, 2 June 2016. [Google Scholar]
- Sørensen, K.M.; Westley, C.; Goodacre, R.; Engelsen, S.B. Simultaneous quantification of the boar-taint compounds skatole and androstenone by surface-enhanced Raman scattering (SERS) and multivariate data analysis. Anal. Bioanal. Chem. 2015, 407, 7787–7795. [Google Scholar] [CrossRef]
- Ponnampalam, E.N.; Butler, K.L.; Pearce, K.M.; Mortimer, S.I.; Pethick, D.W.; Ball, A.J.; Hopkins, D.L. Sources of variation of health claimable long chain omega-3 fatty acids in meat from Australian lamb slaughtered at similar weights. Meat Sci. 2014, 96, 1095–1103. [Google Scholar] [CrossRef]
- Ropodi, A.I.; Panagou, E.Z.; Nychas, G.J.E. Data mining derived from food analyses using non-invasive/ non-destructive analytical techniques; determination of food authenticity, quality & safety in tandem with computer science disciplines. Trends Food Sci. Technol. 2016, 50, 11–25. [Google Scholar]
| Target Figure | Method | Meat Type | Authors |
|---|---|---|---|
| shear force | PLS-R | lamb | Schmidt et al. (2013) [48] |
| lamb | Fowler et al. (2014a) [49] | ||
| lamb | Fowler et al. (2014b) [50] | ||
| lamb | Fowler et al. (2015b) [51] | ||
| beef | Bauer et al. (2016) [53] | ||
| beef | Fowler et al. (2018) [52] | ||
| PLS-DA | beef | Bauer et al. (2016) [53] | |
| tenderness & juiciness | PLS-R | beef | Fowler et al. (2018) [52] |
| pH | PLS-R | pork | Scheier & Schmidt (2013) [55] |
| pork | Scheier et al. (2014) [56] | ||
| pork | Scheier et al. (2015) [57] | ||
| lamb | Fowler et al. (2015b) [51] | ||
| SIMPLS-R | pork | Nache et al. (2016) [58] | |
| MLR | pork | Schmidt et al. (2013) [48] | |
| peak intensity ratio | pork | Schmidt et al. (2013) [48] | |
| L* | PLS-R | pork | Scheier et al. (2014) [56] |
| pork | Scheier et al. (2015) [57] | ||
| lamb | Fowler et al. (2015b) [51] | ||
| beef | Fowler et al. (2018) [52] | ||
| drip loss | PLS-R | pork | Scheier et al. (2014) [56] |
| pork | Scheier et al. (2015) [57] | ||
| lamb | Fowler et al. (2015b) [51] | ||
| beef | Fowler et al. (2018) [52] | ||
| meat spoilage | PCA | pork | Schmidt et al. (2010) [59] |
| pork | Sowoidnich et al. (2012) [60] | ||
| boar taint | PLS-DA | pork | Liu et al. (2016) [61] |
| IMF &major FA groups | PLS-R | lamb | Fowler et al. (2015a) [62] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Beganović, A.; Hawthorne, L.M.; Bach, K.; Huck, C.W. Critical Review on the Utilization of Handheld and Portable Raman Spectrometry in Meat Science. Foods 2019, 8, 49. https://doi.org/10.3390/foods8020049
Beganović A, Hawthorne LM, Bach K, Huck CW. Critical Review on the Utilization of Handheld and Portable Raman Spectrometry in Meat Science. Foods. 2019; 8(2):49. https://doi.org/10.3390/foods8020049
Chicago/Turabian StyleBeganović, Anel, Luzia Maria Hawthorne, Katrin Bach, and Christian W. Huck. 2019. "Critical Review on the Utilization of Handheld and Portable Raman Spectrometry in Meat Science" Foods 8, no. 2: 49. https://doi.org/10.3390/foods8020049






