Quality Control of Fresh-Cut Apples after Coating Application
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
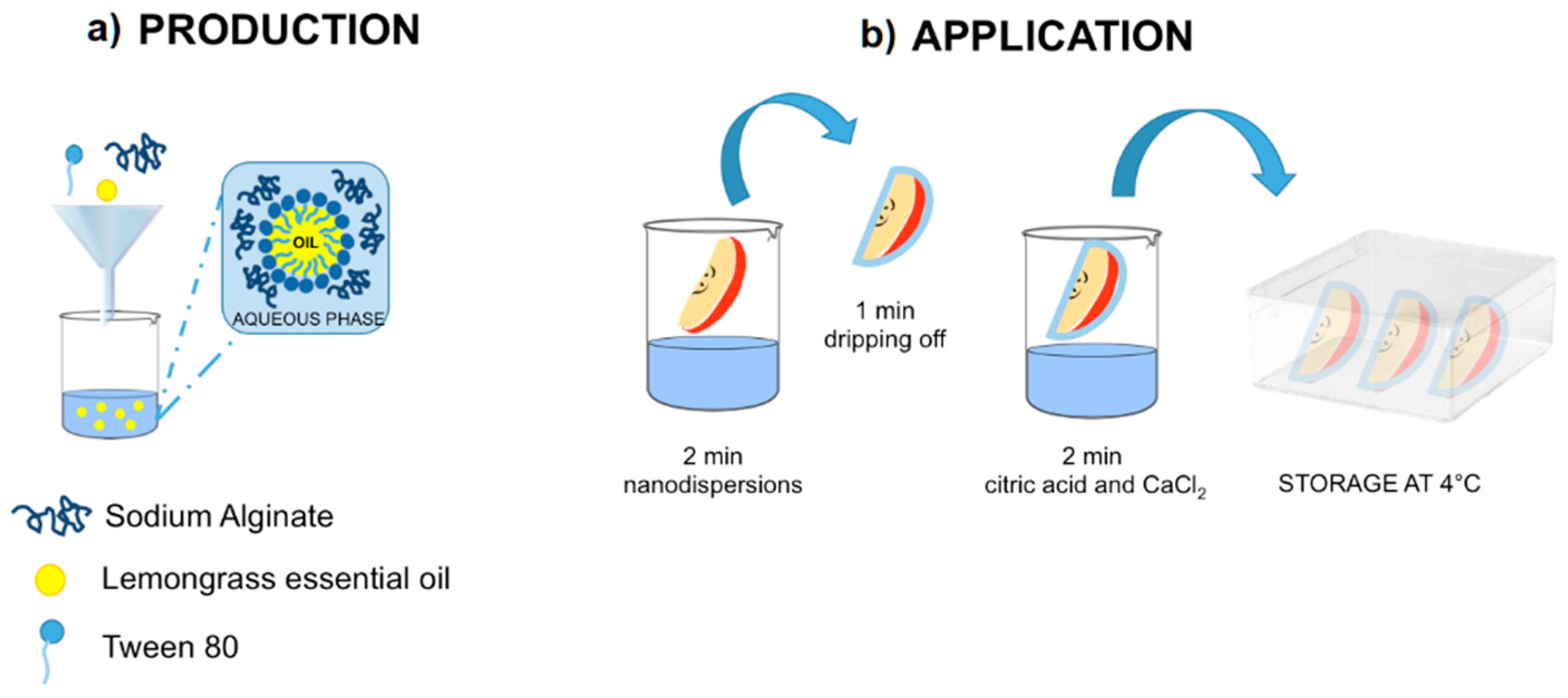
2.2. Preparation of Coating Nanoformulation
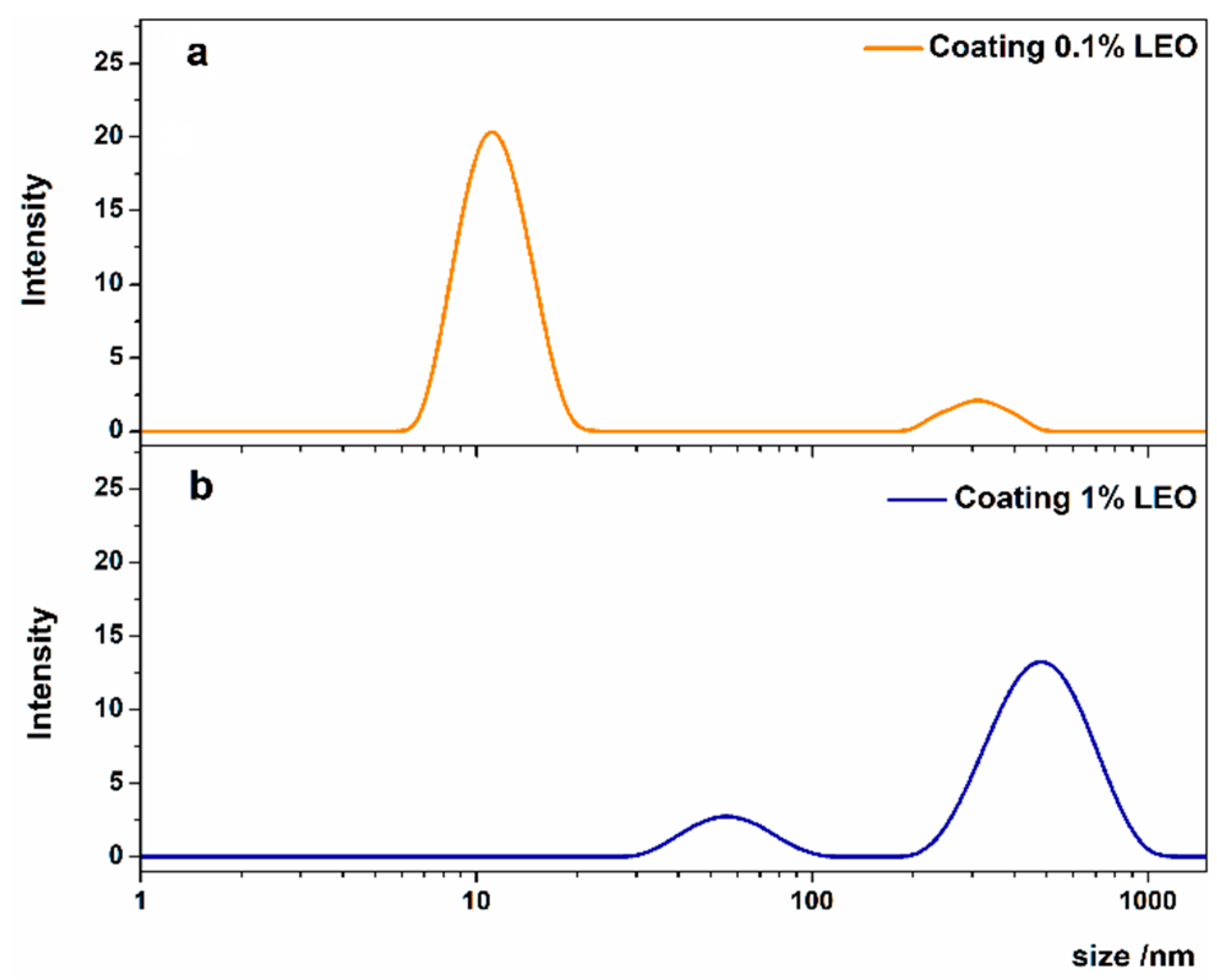
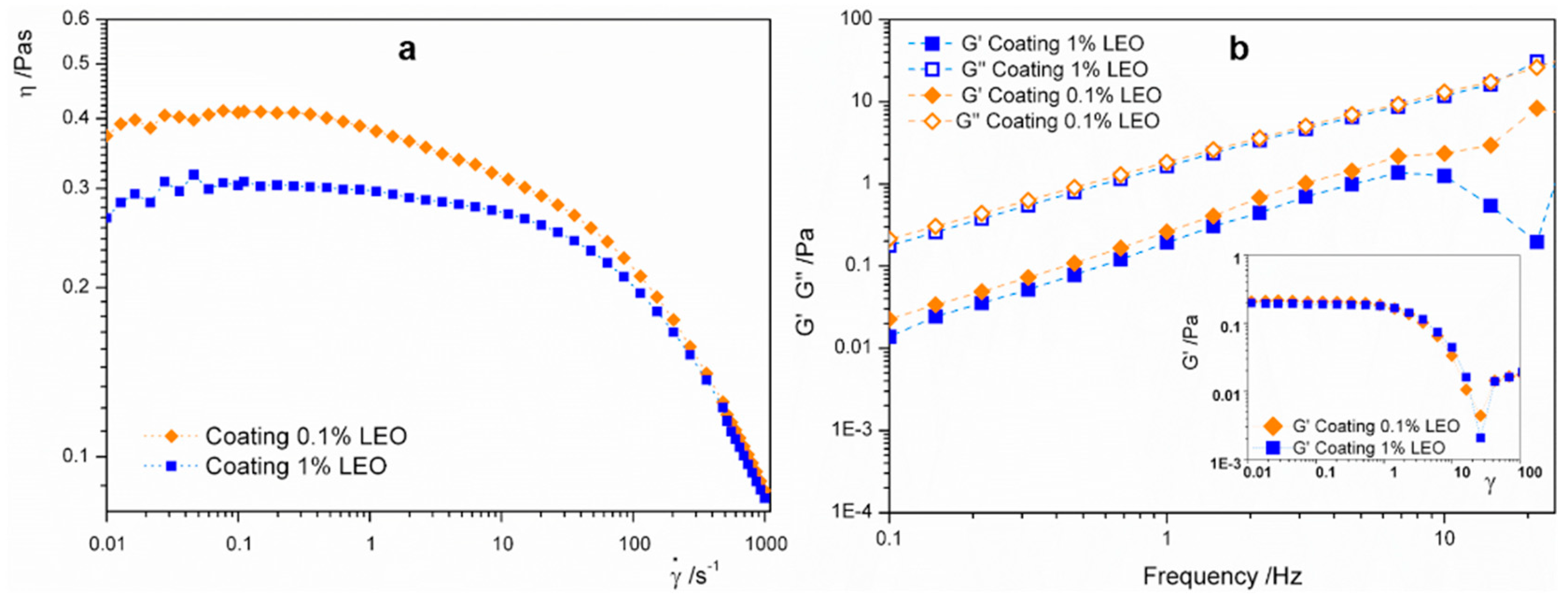
2.3. Nanoformulations Characterization
2.4. Coating Application
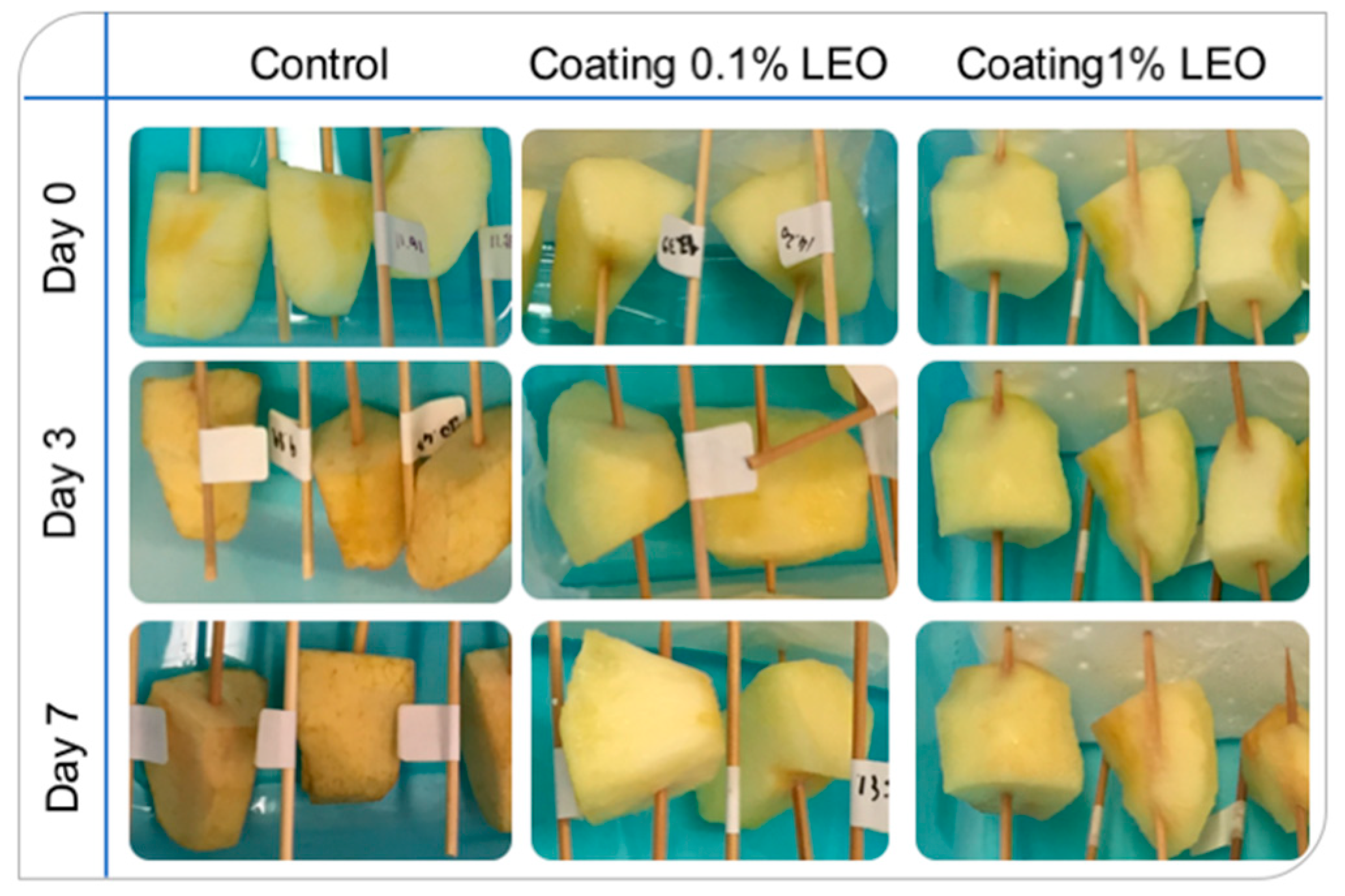
2.5. Fresh-Cut Fruit Evaluation
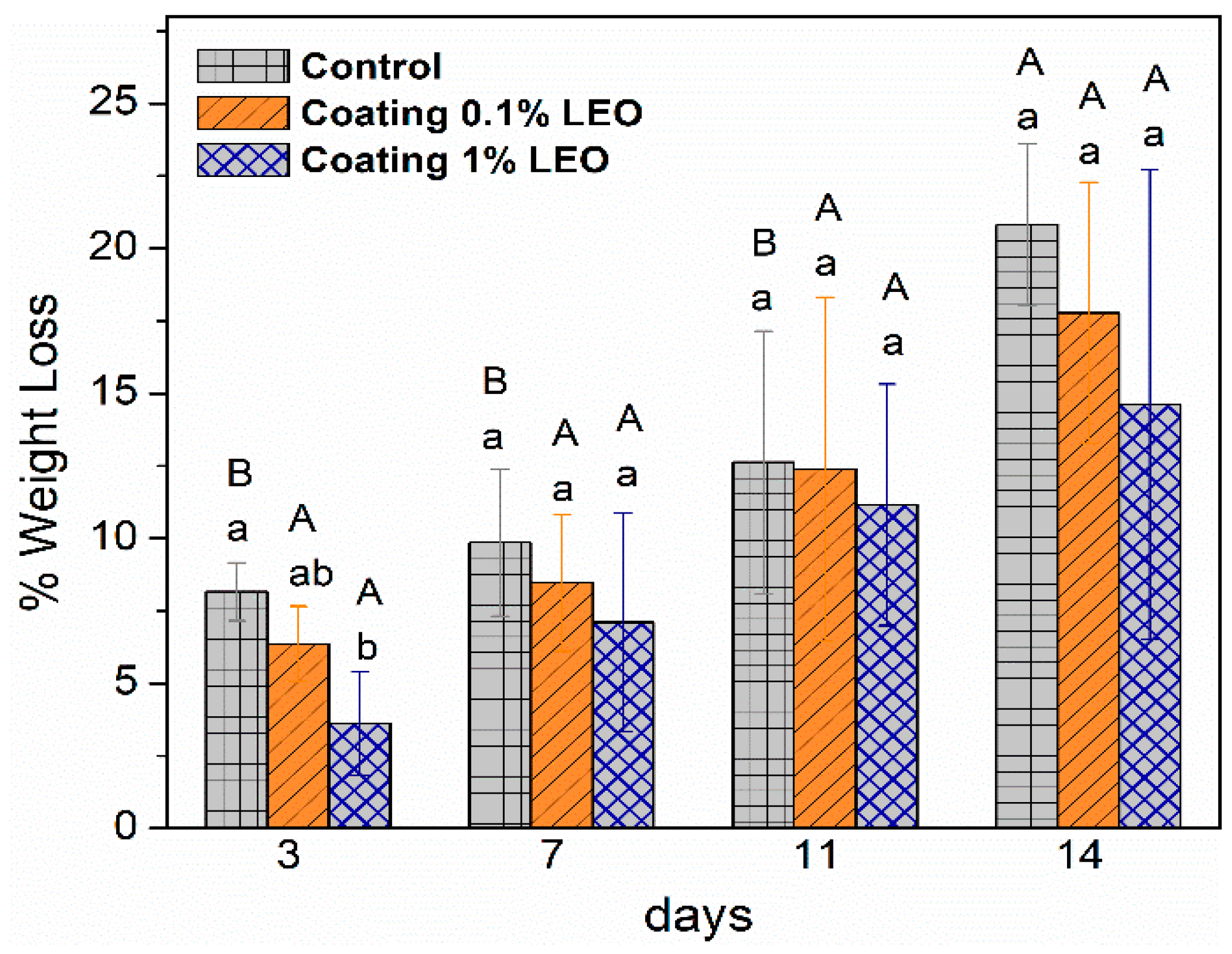
2.5.1. Weight Loss
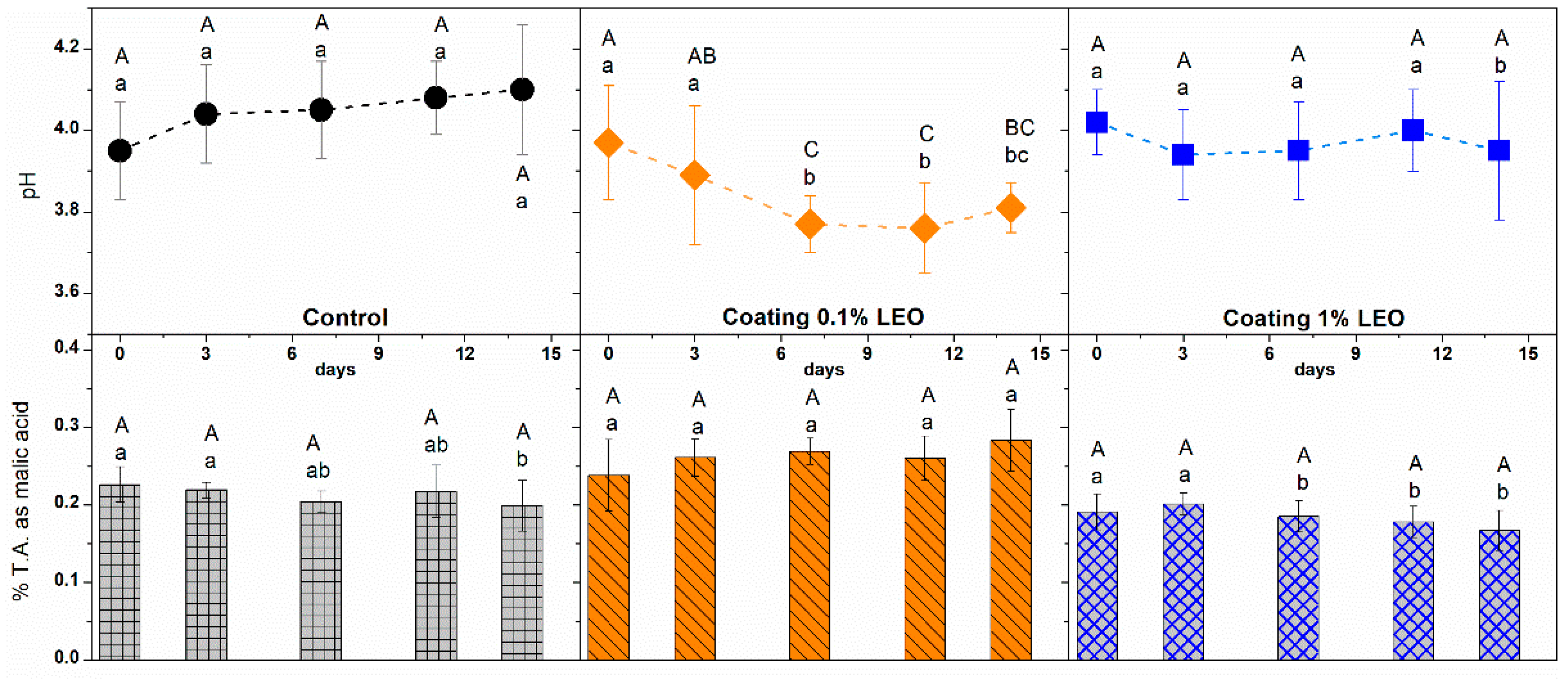
2.5.2. Titratable Acidity and pH
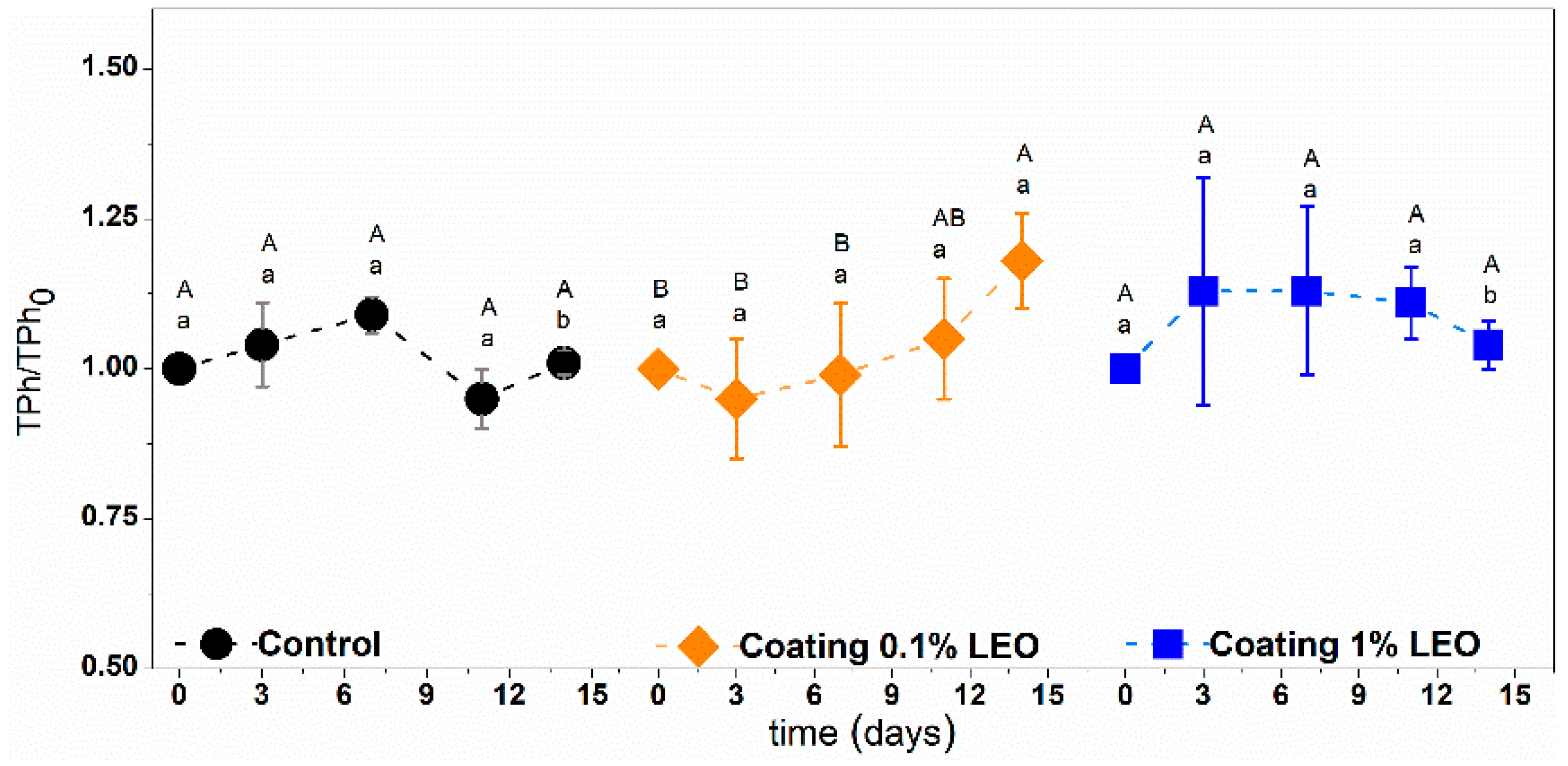
2.5.3. Total Phenol Content
2.6. Statistical Analysis
3. Results and Discussion
3.1. Characterization of Coating Formulations
3.2. Fresh-Cut Fruit Evaluation
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Yildiz, F.; Wiley, R.C. Minimally Processed Refrigerated Fruits and Vegetables; Springer: Berlin, Germany, 2017. [Google Scholar]
- Raudino, M.; Selvolini, G.; Montis, C.; Baglioni, M.; Bonini, M.; Berti, D.; Baglioni, P. Polymer films removed from solid surfaces by nanostructured fluids: Microscopic mechanism and implications for the conservation of cultural heritage. ACS Appl. Mater. Interfaces 2015, 7, 6244–6253. [Google Scholar] [CrossRef]
- Baldwin, E.A.; Nisperos-Carriedo, M.; Shaw, P.E.; Burns, J.K. Effect of coatings and prolonged storage conditions on fresh orange flavor volatiles, degrees Brix, and ascorbic acid levels. J. Agric. Food Chem. 1995, 43, 1321–1331. [Google Scholar] [CrossRef]
- McClements, D.J.; Rao, J. Food-grade nanoemulsions: Formulation, fabrication, properties, performance, biological fate, and potential toxicity. Crit. Rev. Food Sci. Nutr. 2011, 51, 285–330. [Google Scholar] [CrossRef] [PubMed]
- Salvia-Trujillo, L.; Soliva-Fortuny, R.; Rojas-Graü, M.A.; McClements, D.J.; Martín-Belloso, O. Edible nanoemulsions as carriers of active ingredients: A review. Annu. Rev. Food Sci. Technol. 2017, 8, 439–466. [Google Scholar] [CrossRef]
- Cuomo, F.; Cofelice, M.; Lopez, F. Rheological characterization of hydrogels from alginate-based nanodispersion. Polymers 2019, 11, 259. [Google Scholar] [CrossRef] [PubMed]
- Saha, D.; Bhattacharya, S. Hydrocolloids as thickening and gelling agents in food: A critical review. J. Food Sci. Technol. 2010, 47, 587–597. [Google Scholar] [CrossRef] [PubMed]
- Burt, S. Essential oils: Their antibacterial properties and potential applications in foods—A review. Int. J. Food Microbiol. 2004, 94, 223–253. [Google Scholar] [CrossRef]
- Rojas-Graü, M.A.; Soliva-Fortuny, R.; Martín-Belloso, O. Edible coatings to incorporate active ingredients to fresh-cut fruits: A review. Trends Food Sci. Technol. 2009, 20, 438–447. [Google Scholar] [CrossRef]
- Raybaudi-Massilia, R.M.; Rojas-Graü, M.A.; Mosqueda-Melgar, J.; Martín-Belloso, O. Comparative study on essential oils incorporated into an alginate-based edible coating to assure the safety and quality of fresh-cut Fuji apples. J. Food Prot. 2008, 71, 1150–1161. [Google Scholar] [CrossRef] [PubMed]
- Rojas-Graü, M.A.; Raybaudi-Massilia, R.M.; Soliva-Fortuny, R.C.; Avena-Bustillos, R.J.; McHugh, T.H.; Martín-Belloso, O. Apple puree-alginate edible coating as carrier of antimicrobial agents to prolong shelf-life of fresh-cut apples. Postharvest Biol. Technol. 2007, 45, 254–264. [Google Scholar] [CrossRef]
- Cofelice, M.; Lopez, F.; Cuomo, F. Rheological Properties of Alginate–Essential Oil Nanodispersions Colloids Interfaces. Colloids Interfaces 2018, 2, 48. [Google Scholar] [CrossRef]
- Heydari, R.; Bavandi, S.; Javadian, S.R. Effect of sodium alginate coating enriched with horsemint (Mentha longifolia) essential oil on the quality of bighead carp fillets during storage at 4 °C. Food Sci. Nutr. 2015, 3, 188–194. [Google Scholar] [CrossRef]
- Raeisi, M.; Tabaraei, A.; Hashemi, M.; Behnampour, N. Effect of sodium alginate coating incorporated with nisin, Cinnamomum zeylanicum, and rosemary essential oils on microbial quality of chicken meat and fate of Listeria monocytogenes during refrigeration. Int. J. Food Microbiol. 2016, 238, 139–145. [Google Scholar] [CrossRef]
- Vital, A.C.P.; Guerrero, A.; de Oliveira Monteschio, J.; Valero, M.V.; Carvalho, C.B.; de Abreu Filho, B.A.; Madrona, G.S.; do Prado, I.N. Effect of edible and active coating (with rosemary and oregano essential oils) on beef characteristics and consumer acceptability. PLoS ONE 2016, 11, e0160535. [Google Scholar] [CrossRef]
- Raybaudi-Massilia, R.M.; Mosqueda-Melgar, J.; Martín-Belloso, O. Edible alginate-based coating as carrier of antimicrobials to improve shelf-life and safety of fresh-cut melon. Int. J. Food Microbiol. 2008, 121, 313–327. [Google Scholar] [CrossRef]
- Azarakhsh, N.; Osman, A.; Ghazali, H.M.; Tan, C.P.; Adzahan, N.M. Lemongrass essential oil incorporated into alginate-based edible coating for shelf-life extension and quality retention of fresh-cut pineapple. Postharvest Biol. Technol. 2014, 88, 1–7. [Google Scholar] [CrossRef]
- Salvia-Trujillo, L.; Rojas-Graü, M.A.; Soliva-Fortuny, R.; Martín-Belloso, O. Use of antimicrobial nanoemulsions as edible coatings: Impact on safety and quality attributes of fresh-cut Fuji apples. Postharvest Biol. Technol. 2015, 105, 8–16. [Google Scholar] [CrossRef]
- Salvia-Trujillo, L.; Rojas-Graü, A.; Soliva-Fortuny, R.; Martín-Belloso, O. Physicochemical characterization of lemongrass essential oil–alginate nanoemulsions: Effect of ultrasound processing parameters. Food Bioprocess Technol. 2013, 6, 2439–2446. [Google Scholar] [CrossRef]
- Sánchez-Ortega, I.; García-Almendárez, B.E.; Santos-López, E.M.; Reyes-González, L.R.; Regalado, C. Characterization and antimicrobial effect of starch-based edible coating suspensions. Food Hydrocoll. 2016, 52, 906–913. [Google Scholar] [CrossRef]
- Guttoff, M.; Saberi, A.H.; McClements, D.J. Formation of vitamin D nanoemulsion-based delivery systems by spontaneous emulsification: Factors affecting particle size and stability. Food Chem. 2015, 171, 117–122. [Google Scholar] [CrossRef]
- Perugini, L.; Cinelli, G.; Cofelice, M.; Ceglie, A.; Lopez, F.; Cuomo, F. Effect of the coexistence of sodium caseinate and Tween 20 as stabilizers of food emulsions at acidic pH. Colloids Surf. B Biointerfaces 2018, 168, 163–168. [Google Scholar] [CrossRef] [PubMed]
- Michel-Sanchez, E. Impact of Particle Morphology on the Rheology of PCC-Based Coatings; Georgia Institute of Technology: Atlanta, GA, USA, 2005. [Google Scholar]
- Bonilla, J.; Atarés, L.; Vargas, M.; Chiralt, A. Effect of essential oils and homogenization conditions on properties of chitosan-based films. Food Hydrocoll. 2012, 26, 9–16. [Google Scholar] [CrossRef]
- Ferreira, M.S.L.; Fai, A.E.C.; Andrade, C.T.; Picciani, P.H.; Azero, E.G.; Gonçalves, É.C.B.A. Edible films and coatings based on biodegradable residues applied to acerolas (Malpighia punicifolia L.). J. Sci. Food Agric. 2016, 96, 1634–1642. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Qian, L.; Shu, T.; Tong, Z. Rheology characterization of sol–gel transition in aqueous alginate solutions induced by calcium cations through in situ release. Polymer 2003, 44, 407–412. [Google Scholar] [CrossRef]
- Liu, X.; Ren, J.; Zhu, Y.; Han, W.; Xuan, H.; Ge, L. The preservation effect of ascorbic acid and calcium chloride modified chitosan coating on fresh-cut apples at room temperature. Colloids Surf. A Physicochem. Eng. Asp. 2016, 502, 102–106. [Google Scholar] [CrossRef] [Green Version]
- Olivas, G.I.; Barbosa-Cánovas, G.V. Edible coatings for fresh-cut fruits. Crit. Rev. Food Sci. Nutr. 2005, 45, 657–670. [Google Scholar] [CrossRef] [PubMed]
- Olivas, G.I.; Mattinson, D.S.; Barbosa-Cánovas, G.V. Alginate coatings for preservation of minimally processed ‘Gala’ apples. Postharvest Biol. Technol. 2007, 45, 89–96. [Google Scholar] [CrossRef]
- Guerreiro, A.C.; Gago, C.M.L.; Faleiro, M.L.; Miguel, M.G.C.; Antunes, M.D.C. The effect of edible coatings on the nutritional quality of ‘Bravo de Esmolfe’ fresh-cut apple through shelf-life. LWT Food Sci. Technol. 2017, 75, 210–219. [Google Scholar] [CrossRef]
- Serrano, M.; Martínez-Romero, D.; Guillén, F.; Valverde, J.M.; Zapata, P.J.; Castillo, S.; Valero, D. The addition of essential oils to MAP as a tool to maintain the overall quality of fruits. Trends Food Sci. Technol. 2008, 19, 464–471. [Google Scholar] [CrossRef]
- Vargas, M.; Pastor, C.; Chiralt, A.; McClements, D.J.; González-Martínez, C. Recent advances in edible coatings for fresh and minimally processed fruits. Crit. Rev. Food Sci. Nutr. 2008, 48, 496–511. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.; Khan, M.Z.H.; Sarkar, M.A.R.; Absar, N.; Sarkar, S.K. Changes in acidity, TSS, and sugar content at different storage periods of the postharvest mango (Mangifera indica L.) influenced by Bavistin DF. Int. J. Food Sci. 2013, 2013. [Google Scholar] [CrossRef]
- Rocha, A.; Morais, A. Shelf life of minimally processed apple (cv. Jonagored) determined by colour changes. Food Control 2003, 14, 13–20. [Google Scholar] [CrossRef]
- Soares, J.M.; Fonseca, G.G. Effect of L-ascorbic acid and sodium metabisulfite in the inhibition of the enzymatic browning of minimally processed apple. Int. J. Agric. Res. 2008, 3, 196–201. [Google Scholar]
- Song, H.Y.; Jo, W.S.; Song, N.B.; Min, S.C.; Song, K.B. Quality change of apple slices coated with Aloe vera gel during storage. J. Food Sci. 2013, 78, C817–C822. [Google Scholar] [CrossRef] [PubMed]
- Zambrano-Zaragoza, M.L.; Mercado-Silva, E.; Del Real, L.A.; Gutiérrez-Cortez, E.; Cornejo-Villegas, M.A.; Quintanar-Guerrero, D. The effect of nano-coatings with α-tocopherol and xanthan gum on shelf-life and browning index of fresh-cut “Red Delicious” apples. Innov. Food Sci. Emerg. Technol. 2014, 22, 188–196. [Google Scholar] [CrossRef]
- Soares, M.C.; Taciana Ribeiro, É.; Kuskoski, E.M.; Valdemiro Gonzaga, L.; Lima, A.; Mancini Filho, J.; Fett, R. Composition of phenolic acids content in apple (Malus sp) pomace. Semin. Ciências Agrárias 2008, 29. [Google Scholar] [CrossRef]
- Heras-Ramírez, M.E.; Quintero-Ramos, A.; Camacho-Dávila, A.A.; Barnard, J.; Talamás-Abbud, R.; Torres-Muñoz, J.V.; Salas-Muñoz, E. Effect of blanching and drying temperature on polyphenolic compound stability and antioxidant capacity of apple pomace. Food Bioprocess Technol. 2012, 5, 2201–2210. [Google Scholar] [CrossRef]
- Arias, E.; Oria, R.; López-Buesa, P. Determination of acceptability and shelf life of fresh-cut pear by digital image analysis. J. Food Meas. Charact. 2018, 12, 2916–2926. [Google Scholar] [CrossRef]
- Rana, S.S.; Pradhan, R.C.; Mishra, S. Image analysis to quantify the browning in fresh cut tender jackfruit slices. Food Chem. 2019, 278, 185–189. [Google Scholar] [CrossRef] [PubMed]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cofelice, M.; Lopez, F.; Cuomo, F. Quality Control of Fresh-Cut Apples after Coating Application. Foods 2019, 8, 189. https://doi.org/10.3390/foods8060189
Cofelice M, Lopez F, Cuomo F. Quality Control of Fresh-Cut Apples after Coating Application. Foods. 2019; 8(6):189. https://doi.org/10.3390/foods8060189
Chicago/Turabian StyleCofelice, Martina, Francesco Lopez, and Francesca Cuomo. 2019. "Quality Control of Fresh-Cut Apples after Coating Application" Foods 8, no. 6: 189. https://doi.org/10.3390/foods8060189




