Influence of Different Wood Chip Extracts Species on Color Changes and Anthocyanin Content in Synthetic Wine Solutions
Abstract
1. Introduction
2. Materials and Methods
2.1. Wood Chip Samples
2.2. Wood Chip Extracts Preparation
2.3. Anthocyanin Grape Skin Extracts Preparation
2.4. Red Synthetic Wine Solutions
2.5. Total Phenol and Anthocyanin Content
2.6. Individual Anthocyanins Analysis
2.7. Chromatic Parameters Evaluation
2.8. Statistical Analysis
3. Results and Discussion
3.1. Effect on Chromatic Characteristics of Red Synthetic Wine Solutions
3.2. Effect on Total Anthocyanin and Phenolic Levels
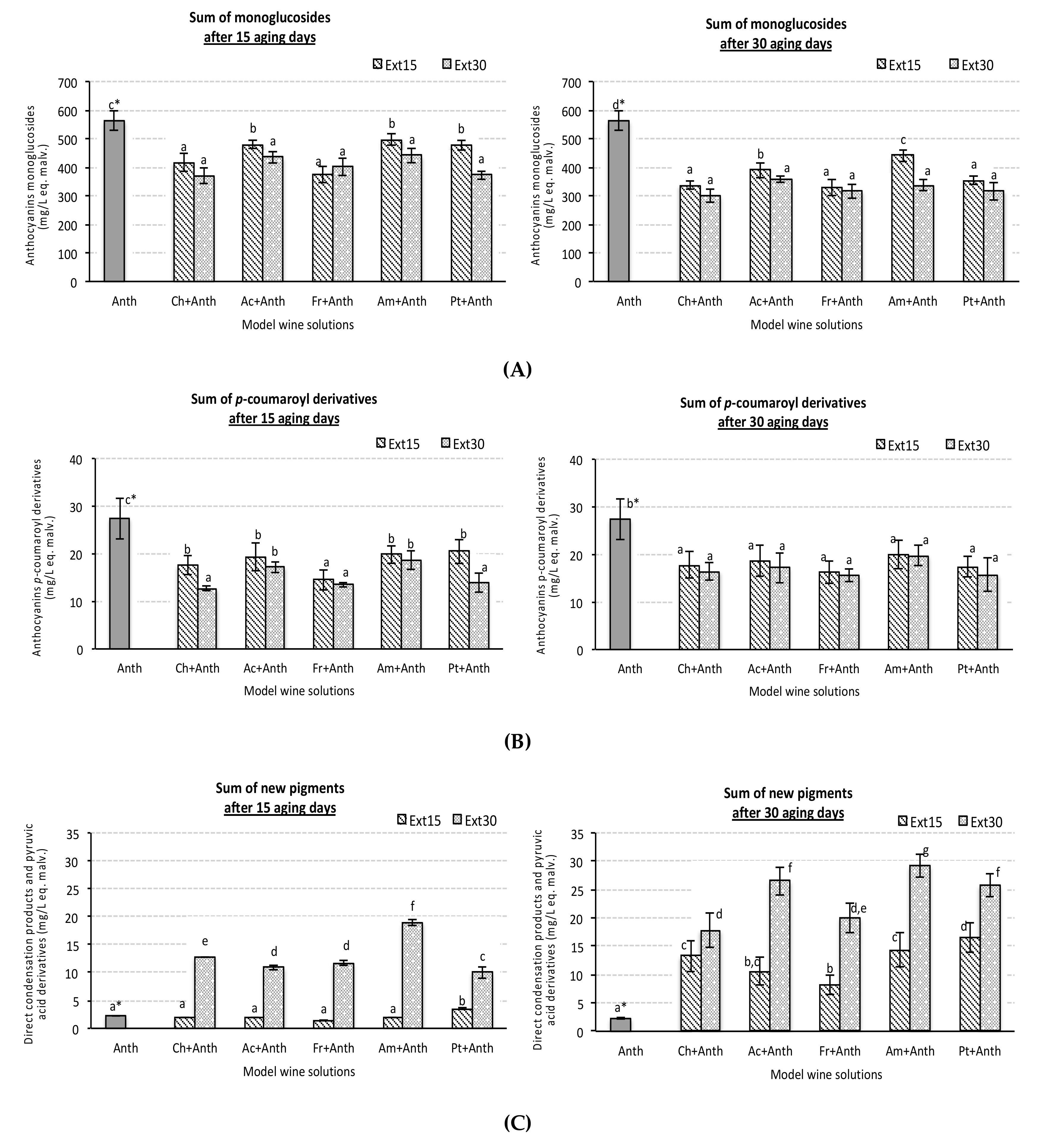
3.3. Effect on Individual Anthocyanin Levels
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Revilla, E.; López, J.F.; Ryan, J.-M. Anthocyanin pattern of Tempranillo wines during ageing in oak barrels and storage in stainless-steel tanks. Eur. Food Res. Technol. 2005, 220, 592–596. [Google Scholar] [CrossRef]
- Monagas, M.; Bartolomé, B. Anthocyanins and anthocyanin-derived compounds. In Wine Chemistry and Biochemistry, 1st ed.; Victoria Moreno-Arribas, M., Carmen Polo, M., Eds.; Springer Science and Business Media: New York, NY, USA, 2009; pp. 439–462. [Google Scholar]
- Wrolstad, R.E.; Durst, R.W.; Lee, J. Tracking color and pigment changes in anthocyanin products. Trends Food Sci. Technol. 2005, 16, 423–428. [Google Scholar] [CrossRef]
- Tseng, K.-C.; Chang, H.-M.; Wu, J.S.-B. Degradation kinetics of anthocyanin in ethanolic solutions. J. Food Process. Preserv. 2006, 30, 503–514. [Google Scholar] [CrossRef]
- Morata, A.; Gómez-Cordovés, M.C.; Suberviola, J.; Bartolomé, B.; Colomo, B.; Suárez, J.A. Adsorption of anthocyanins by yeast cell walls during the fermentation of red wines. J. Agric. Food Chem. 2003, 51, 4084–4088. [Google Scholar] [CrossRef] [PubMed]
- Pomar, M.; Gonzalez-Mendoza, L.A. Changes in composition and sensory quality of red wine aged in American and French oak barrels. Int. J. Vine Wine Sci. 2001, 35, 41–48. [Google Scholar] [CrossRef]
- De Coninck, G.; Jordão, A.M.; Ricardo-da-Silva, J.M.; Laureano, O. Evolution of phenolic composition and sensory properties in red wine aged in contact with Portuguese and French oak wood chips. Int. J. Vine Wine Sci. 2006, 40, 25–34. [Google Scholar] [CrossRef]
- Gonçalves, F.J.; Jordão, A.M. Changes in antioxidant activity and proanthocyanidin fraction of red wine aged in contact with Portuguese (Quercus pyrenaica Willd.) and American (Quercus alba L.) oak wood chips. Ital. J. Food Sci. 2009, 21, 51–64. [Google Scholar]
- Barrera-García, V.D.; Gougeon, R.D.; Majo, D.D.; Aguirre, C.; Voilley, A.; Chassagne, D. Different sorption behaviors for wine polyphenols in contact with oak wood. J. Agric. Food Chem. 2007, 55, 7021–7027. [Google Scholar] [CrossRef]
- Del Álamo Sanza, M.; Domínguez, I.N. Wine aging in bottle from artificial systems (staves and chips) and oak woods: Anthocyanin composition. Anal. Chim. Acta 2006, 563, 255–263. [Google Scholar] [CrossRef]
- Gambuti, A.; Capuano, R.; Lisanti, M.T.; Strollo, D.; Moio, L. Effect of aging in new oak, one-year-used oak, chestnut barrels and bottle on color, phenolics and gustative profile of three monovarietal red wines. Eur. Food Res. Technol. 2010, 231, 455–465. [Google Scholar] [CrossRef]
- Cejudo-Bastante, M.J.; Hermosín-Gutiérrez, I.; Pérez-Coello, M.S. Micro-oxygenation and oak chip treatments of red wines: Effects on colour-related phenolics, volatile composition and sensory characteristics. Part II: Merlot wines. Food Chem. 2011, 124, 738–748. [Google Scholar] [CrossRef]
- Gortzi, O.; Metaxa, X.; Mantanis, G.; Lalas, S. Effect of artificial ageing using different wood chips on the antioxidant activity, resveratrol and catechin concentration, sensory properties and colour of two Greek red wines. Food Chem. 2013, 141, 2887–2895. [Google Scholar] [CrossRef] [PubMed]
- Venturi, F.; Andrich, G.; Samartin, C.; Taglieri, I.; Serni, E.; Zinnai, A. Winemaking of Sangiovese grapes with and without the addition of different oenological tannins in order to increase the colour intensity of Chianti wine. Agrochimica 2015, 59, 261–271. [Google Scholar]
- Revilla, I.; González -Sanjosé, M.L.; Gómez-Cordovés, M.C. Chromatic modifications of aged red wines depending on aging barrel type. Food Sci. Technol. Int. 1999, 5, 177–181. [Google Scholar] [CrossRef]
- Es-Safi, N.E.; Le Guernevé, C.; Fulcrand, H.; Cheynier, V.; Moutounet, M. Xanthylium salts formation involved in wine colour changes. Int. J. Food Sci. Technol. 2000, 35, 63–74. [Google Scholar] [CrossRef]
- Jordão, A.M.; Ricardo-da-Silva, J.M.; Laureano, O. Effect of oak wood constituents and oxygen on the evolution of malvidin-3-glucoside and (+)-catechin in model wine. Am. J. Enol. Vitic. 2006, 57, 377–381. [Google Scholar]
- Jordão, A.M.; Ricardo-da-Silva, J.M.; Laureano, O.; Mullen, W.; Crozier, A. Effect of ellagitannins, ellagic acid and some volatile compounds from oak wood on the (+)-catechin, procyanidin B1 and malvidin-3-glucoside content of model wine solutions. Aust. J. Grape Wine Res. 2008, 14, 260–270. [Google Scholar] [CrossRef][Green Version]
- De Freitas, V.; Sousa, C.; González-Paramás, A.M.; Santos-Buelga, C.; Mateus, N. Synthesis of new catechin-pyrylium derived pigment. Tetrahedron Lett. 2004, 45, 9349–9352. [Google Scholar] [CrossRef]
- Pissarra, J.; Lourenço, S.; González-Paramás, A.M.; Mateus, N.; Santos-Buelga, C.; Silva, A.M.S.; De Freitas, V. Isolation and structural characterization of new anthocyanin-alkyl-catechin pigments. Food Chem. 2005, 90, 81–87. [Google Scholar] [CrossRef]
- Sousa, C.; Mateus, N.; Perez-Alonso, J.; Santos-Buelga, C.; De Freitas, V. Preliminary study of oaklins, a new class of brick-red catechinpyrylium pigments from the reaction between catechin and wood aldehydes. J. Agric. Food Chem. 2005, 53, 9249–9256. [Google Scholar] [CrossRef]
- Lefeuvre, D.; Jourdes, M.; Quideau, S.; Saucier, C.; Glories, Y. Hemissyntheses of anthocyano-ellagitannins. In Proceedings of the XXII International Conference on Polyphenols, Helsinki, Finland, 25–28 August 2004; pp. 657–658. [Google Scholar]
- Kozlovic, G.; Jeromel, A.; Maslov, L.; Pollnitz, A.; Orlic, S. Use of acacia barrique barrels- Influence on the quality of Malvazika from Istria wines. Food Chem. 2010, 120, 698–702. [Google Scholar] [CrossRef]
- Chinnici, F.; Natali, N.; Sonni, F.; Bellachioma, A.; Riponi, C. Comparative changes in color features and pigment composition of red wines aged in oak and cherry wood casks. J. Agric. Food Chem. 2011, 59, 6575–6682. [Google Scholar] [CrossRef] [PubMed]
- Sanz, M.; Fernández de Simón, B.; Esteruelas, E.; Muñoz, A.M.; Cadahía, E.; Hernández, T.; Estrella, I.; Martinez, J. Polyphenols in red wine aged in acacia (Robinia pseudoacacia) and oak (Quercus petraea) wood barrels. Anal. Chim. Acta 2012, 732, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Fernández de Simon, B.; Sanz, M.; Cadahía, E.; Martínez, J.; Esteruelas, E.; Muñoz, A.M. Polyphenolic compounds as chemical markers of wine ageing in contact with cherry, chestnut, false acacia, ash and oak wood. Food Chem. 2014, 143, 66–76. [Google Scholar] [CrossRef] [PubMed]
- Chinnici, F.; Natali, N.; Bellachioma, A.; Versari, A.; Riponi, C. Changes in phenolic composition of red wines aged in cherry wood. LWT—Food Sci. Technol. 2015, 60, 977–984. [Google Scholar] [CrossRef]
- Délia, L.; Jordão, A.M.; Ricardo-da-Silva, J.M. Influence of different wood chips species (oak, acacia and cherry) used in a short period of aging on the quality of ’Encruzado’ white wines. Mitt. Klosterneuburg 2017, 67, 84–96. [Google Scholar]
- Tavares, M.; Jordão, A.M.; Ricardo-da-Silva, J.M. Impact of cherry, acacia and oak chips on red wine phenolic parameters and sensory profile. OENO One 2017, 51, 329–342. [Google Scholar] [CrossRef]
- Gonzaléz-Sanjosé, M.L.; DiStefano, R. Fattori che condizionano la stabilità degli antociani in soluzione. Riv. Viticoltura e Enologia 1990, 43, 63–68. [Google Scholar]
- Singleton, V.L.; Rossi, J.A. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar]
- Paronetto, L. Polifenoli e Tecnica Enological; Edagricole: Milano, Italy, 1977. [Google Scholar]
- Pérez-Magariño, S.; Ortega-Heras, M.; Cano-Mozo, E.; González-SanJosé, M.L. The influence of oak wood chips, micro-oxygenation treatment, and grape variety on colour, and anthocyanin and phenolic composition of red wines. J. Food Comp. Anal. 2009, 22, 204–211. [Google Scholar] [CrossRef]
- Pérez-Magariño, S.; González-SanJosé, M.L. Evolution of flavanols, anthocyanins, and their derivatives during the aging of red wines elaborated from grapes harvested at different stages of ripening. J. Agric. Food Chem. 2004, 52, 1181–1189. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Magariño, S.; Sánchez-Iglesias, M.; Ortega-Heras, M.; González-SanJosé, M.L. Colour stabilization of red wines by microoxygenation treatment before malolactic fermentation. Food Chem. 2007, 101, 881–893. [Google Scholar] [CrossRef]
- Glories, Y. La couleur des vins rouges. 2e partie: Mesure, origine et interprétation. Connaiss. Vigne Vin. 1984, 18, 253–271. [Google Scholar] [CrossRef]
- Organisation International de la Vigne et du Vin (OIV). Organisation International de la Vigne et du Vin (OIV). International Oenological Codex. Organisation International de la Vigne et du Vin (OIV): Paris, France, 2012. [Google Scholar]
- Martínez, J.A.; Melgosa, M.; Pérez, M.M.; Hita, E.; Negueruela, A.I. Visual and instrumental color evaluation in red wines. Food Sci. Technol. Int. 2001, 7, 439–444. [Google Scholar] [CrossRef]
- De Rosso, M.; Panighel, A.; Dalla Vedova, A.; Stella, L.; Flamini, R. Changes in chemical composition of a red wine aged in acacia, cherry, chestnut, mulberry, and oak wood barrels. J. Agric. Food Chem. 2009, 57, 1915–1920. [Google Scholar] [CrossRef] [PubMed]
- Del Álamo Sanza, M.; Domínguez, I.N.; Merino, S.G. Influence of different aging systems and oak woods on aged wine color and anthocyanin composition. Eur. Food Res. Technol. 2004, 219, 124–132. [Google Scholar] [CrossRef]
- Pérez-Magariño, S.; González-Sanjosé, M.L. Application of absorbance values used in wineries for estimating CIELAB parameters in red wines. Food Chem. 2003, 81, 301–306. [Google Scholar] [CrossRef]
- Jordão, A.M.; Ricardo-da-Silva, J.M.; Laureano, O. Extraction and evolution of some ellagic tannins and ellagic acid of oak wood chips (Quercus pyrenaica) in model wine solutions: Effect of time, pH, temperature and alcoholic content. South Afr. J. Enol. Vitic. 2005, 26, 25–31. [Google Scholar]
- Fernández de Simón, B.; Cadahía, E.; Muiño, I.; Del Álamo-Sanza, M.; Nevares, I. Volatile composition of toasted oak chips and staves and of red wine aged with them. Am. J. Enol. Vitic. 2010, 61, 157–165. [Google Scholar]
- De Rosso, M.; Cancian, D.; Panighel, A.; Dalla Vedova, A.; Flamini, R. Chemical compounds released from five different woods used to make barrels for aging wines and spirits: Volatile compounds and polyphenols. Wood Sci. Technol. 2009, 43, 375–385. [Google Scholar] [CrossRef]
- Soares, B.; Garcia, R.; Costa, A.M.; Cabrita, M.J. Phenolic compounds realesed from oak, cherry, chestnut and robinia chips into a synthetic wine: Influence of toasting level. Ciênc. Tec. Vitivinic. 2012, 27, 17–26. [Google Scholar]
- Liazid, A.; Barbero, G.F.; Azaroual, L.; Palma, M.; Barroso, C.G. Stability of anthocyanins from red grape skins under pressurized liquid extraction and ultrasound-assisted extraction conditions. Molecules 2014, 19, 21034–21043. [Google Scholar] [CrossRef] [PubMed]
- Rinaldo, A.M.; Cavallini, E.; Jia, Y.; Moss, S.M.A.; McDavid, D.A.J.; Hooper, L.C.; Robinson, S.P.; Tornielli, G.B.; Zenoni, S.; Ford, C.M.; et al. A grapevine anthocyanin acyltransferase, transcriptionally regulated by VvMYBA, can produce most acylated anthocyanins present in grape skins. Plant Physiol. 2015, 169, 1897–1916. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.L.; Yu, Y.Q.; Chen, Z.J.; Wen, G.S.; Wei, F.G.; Zhen, Q.; Wang, C.D.; Xiao, X.L. Stability-increasing effects of anthocyanin glycosyl acylation. Food Chem. 2017, 214, 119–128. [Google Scholar] [CrossRef] [PubMed]
- Smart, R. Pinot Noir: The ultimate viticultural challenge? Aust. Grapegrower Winemaker 1992, 340, 77–85. [Google Scholar]
- Jordão, A.M.; Ricardo-da-Silva, J.M.; Laureano, O. Ellagitannins from Portuguese oak wood (Quercus pyrenaica Willd.) used in cooperage: Influence of geographical origin, coarseness of the grain and toasting level. Hollforschung 2007, 61, 155–160. [Google Scholar] [CrossRef]
- Saucier, C.; Jourdes, M.; Glories, Y.; Quideau, S. Extraction, detection, and quantification of flavano-ellagitannins and ethylvescalagin in a Bordeaux red wine aged in oak barrels. J. Agric. Food Chem. 2006, 54, 7349–7354. [Google Scholar] [CrossRef] [PubMed]
- Quideau, S.; Jourdes, M.; Lefeuvre, D.; Montaudon, D.; Saucier, C.; Glories, Y.; Pardon, P.; Pourquier, P. The chemistry of wine polyphenolic C-glycosidic ellagitannins targeting human topoisomerase II. Chem. Eur. J. 2005, 11, 6503–6513. [Google Scholar] [CrossRef]


| Synthetic Wine Solutions | Sample Code |
|---|---|
| Control wine solution with anthocyanins | Anth |
| Control wine solution with anthocyanins after 15 aging days | Anth15 |
| Control wine solution with anthocyanins after 30 aging days | Anth30 |
| Cherry wood chip extract 1 + anthocyanin grape skin extract | Ch+Anth |
| Acacia wood chip extract 1 + anthocyanin grape skin extract | Ac+Anth |
| French wood chip extract 1 + anthocyanin grape skin extract | Fr+Anth |
| American wood chip extract 1 + anthocyanin grape skin extract | Am+Anth |
| Portuguese wood chip extract 1 + anthocyanin grape skin extract | Pt+Anth |
| CIELAB Coordinates | Experimental Synthetic Wine Solutions | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Anth | Ch + Anth | Ac + Anth | Fr + Anth | Am + Anth | Pt + Anth | ||||||||||||||||
| 15 Aging Days | 30 Aging Days | 15 Aging Days | 30 Aging Days | 15 Aging Days | 30 Aging Days | 15 Aging Days | 30 Aging Days | 15 Aging Days | 30 Aging Days | ||||||||||||
| ext15 | ext30 | ext15 | ext30 | ext15 | ext30 | ext15 | ext30 | ext15 | ext30 | ext15 | ext30 | ext15 | ext30 | ext15 | ext30 | ext15 | ext30 | ext15 | ext30 | ||
| L* | 53.4 a ± 3.2 | 49.1 a ± 3.6 | 66.6 b ± 1.5 | 82.4 c ± 3.9 | 73.8 d ± 3.6 | 45.3 a ± 3.6 | 64.66 b ± 2.9 | 76.3 d ± 1.9 | 65.1 b ± 1.2 | 49.3 a ± 4.5 | 66.2b ± 3.3 | 79.2 c ± 2.7 | 70.8 d ± 5.3 | 46.4 a ± 4.2 | 62.6 b ± 2.0 | 71.9 d ± 9.6 | 69.3 d ± 1.7 | 45.7 a ± 1.8 | 63.9 b ± 1.7 | 79.03 c ± 1.4 | 67.1 d ± 1.3 |
| a* | 45.5 c ± 2.8 | 42.0 c ± 2.7 | 23.6 a ± 1.4 | 25.4 a ± 3.1 | 23.5 a ± 2.8 | 45.4c ± 2.8 | 30.2 b ± 2.1 | 32.8 b ± 2.9 | 30.2 b ± 0.8 | 41.9 c ± 3.4 | 23.4a ± 2.4 | 27.0 a ± 3.4 | 23.3 a ± 3.2 | 44.2 c ± 3.2 | 32.0 b ± 1.5 | 29.5 b ± 3.2 | 27.1 b ± 1.3 | 44.7 b ± 1.4 | 28.6 b ± 1.2 | 29.4 b ± 1.6 | 30.2 b ± 0.9 |
| b* | −5.7 b ± 0.8 | −12.2 a ± 1.7 | −1.6 c ± 0.3 | −2.1 b ± 0.1 | −0.19 d ± 0.1 | −14.1 a ± 1.7 | −1.5 c ± 0.2 | −3.4 b ± 0.6 | −0.3 d ± 0.1 | −11.6 a ± 1.7 | −0.8 d ± 0.2 | −2.6 b ± 0.9 | 0.7 d ± 0.2 | −12.7 a ± 2.3 | −2.0 b ± 0.4 | −2.5 b ± 1.0 | 0.6 d ± 0.1 | −13.7 a ± 0.8 | −1.7 c ± 0.2 | −2.3 b ± 0.5 | −0.3 d ± 0.1 |
| ΔE* | - | 8.5 a ± 0.9 | 21.4 b ± 1.3 | 35.4 c ± 2.6 | 30.5 c ± 2.1 | 21.3 b ± 1.9 | 11.6 a ± 2.0 | 26.2 b ± 3.1 | 20.8 b ± 1.1 | 8.0 a ± 1.2 | 21.2b ± 0.5 | 31.c ± 3.4 | 28.9 c ± 1.4 | 9.9 a ± 1.0 | 16.7 d ± 2.0 | 25.7 b ± 2.1 | 25.0 b ± 1.1 | 11.1 a ± 0.6 | 18.3 d ± 0.9 | 30.4 c ± 2.9 | 22.4 b ± 1.2 |
| Synthetic Wine Solutions (Wood Chip Species) | Extraction Time | |
|---|---|---|
| 15 Days (ext15) | 30 Days (ext30) | |
| Cherry | 39.91 A,a ± 0.96 | 46.82 A,b ± 1.31 |
| Acacia | 40.11 A,a ± 2.26 | 51.23 B,b ± 0.99 |
| French | 42.98 A,a ± 1.31 | 61.39 C,b ± 1.70 |
| American | 59.85 B,a ± 1.70 | 61.58 C,a ± 1.40 |
| Portuguese | 56.40 B,a ± 1.31 | 65.03 D,b ± 0.99 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jordão, A.M.; Lozano, V.; González-SanJosé, M.L. Influence of Different Wood Chip Extracts Species on Color Changes and Anthocyanin Content in Synthetic Wine Solutions. Foods 2019, 8, 254. https://doi.org/10.3390/foods8070254
Jordão AM, Lozano V, González-SanJosé ML. Influence of Different Wood Chip Extracts Species on Color Changes and Anthocyanin Content in Synthetic Wine Solutions. Foods. 2019; 8(7):254. https://doi.org/10.3390/foods8070254
Chicago/Turabian StyleJordão, António M., Virginia Lozano, and María L. González-SanJosé. 2019. "Influence of Different Wood Chip Extracts Species on Color Changes and Anthocyanin Content in Synthetic Wine Solutions" Foods 8, no. 7: 254. https://doi.org/10.3390/foods8070254
APA StyleJordão, A. M., Lozano, V., & González-SanJosé, M. L. (2019). Influence of Different Wood Chip Extracts Species on Color Changes and Anthocyanin Content in Synthetic Wine Solutions. Foods, 8(7), 254. https://doi.org/10.3390/foods8070254






