Effect of Different Packaging Methods on Protein Oxidation and Degradation of Grouper (Epinephelus coioides) During Refrigerated Storage
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sampling
2.2. Extraction of Myofibrillar Protein
2.3. Carbonyl Content
2.4. Surface Hydrophobicity
2.5. Total Sulfhydryl and Disulfide Bond Content
2.6. Ca2+ ATPase Activity
2.7. Trichloroacetic Acid-Soluble Peptide Content
2.8. Myofibril Fragmentation Index
2.9. Free Amino Acid Content
2.10. FTIR Measure
2.11. SDS-PAGE
2.12. Statistical Analysis
3. Results and Discussion
3.1. Changes in Total Sulfhydryl and Disulfide Bonds
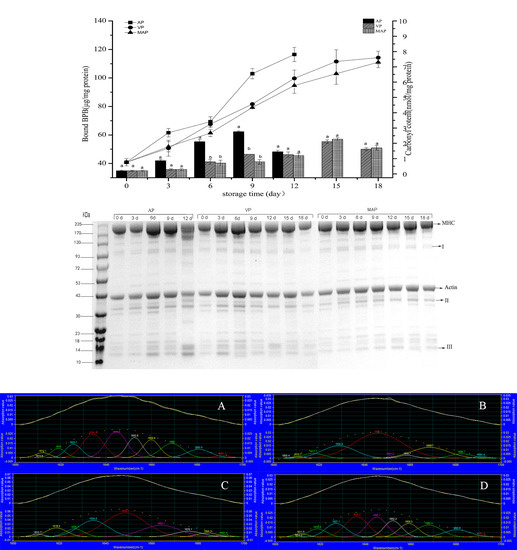
3.2. Changes in Carbonyl Content and Hydrophobicity
3.3. Changes in Ca2+ ATPase Activity
3.4. Trichloroacetic Acid-Soluble Peptide Analysis
3.5. Myofibril Fragmentation Index Analysis
3.6. Free Amino Acid Analysis
3.7. FTIR Analysis
3.8. SDS-PAGE Analysis
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Kirtil, E.; Kilercioglu, M.; Oztop, M.H. Modified atmosphere packaging of foods. In Reference Module in Food Science; Elsevier: Amsterdam, The Netherlands, 2016. [Google Scholar]
- Guimarães, C.F.M.; Mársico, E.T.; Monteiro, M.L.G.; Lemos, M.; Mano, S.B.; Junior, C.A.C. The chemical quality of frozen vietnamese pangasius hypophthalmus fillets. Food Sci. Nutr. 2016, 4, 398–408. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Liu, S.; Tian, C.; Yan, G.; Wang, D. An overview of current status of cold chain in china. Int. J. Refrig. 2018, 88, 483–495. [Google Scholar] [CrossRef]
- Estévez, M. Protein carbonyls in meat systems: A review. Meat Sci. 2011, 89, 259–279. [Google Scholar] [CrossRef] [PubMed]
- Turgut, S.S.; Işıkçı, F.; Soyer, A. Antioxidant activity of pomegranate peel extract on lipid and protein oxidation in beef meatballs during frozen storage. Meat Sci. 2017, 129, 111–119. [Google Scholar] [CrossRef] [PubMed]
- Ge, L.; Xu, Y.; Xia, W.; Jiang, Q.; Jiang, X. Differential role of endogenous cathepsin and microorganism in texture softening of ice-stored grass carp (ctenopharyngodon idella) fillets. J. Sci. Food Agric. 2016, 96, 3233–3239. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.; Liu, X.; Zhang, Y.; Wang, H.; Luo, Y. Effects of chilling and partial freezing on rigor mortis changes of bighead carp (aristichthys nobilis) fillets: Cathepsin activity, protein degradation and microstructure of myofibrils. J. Food Sci. 2016, 80, C2725–C2731. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Xiao, S.; Ahn, D.U. Protein oxidation: Basic principles and implications for meat quality. Crit. Rev. Food Sci. Nutr. 2013, 53, 1191–1201. [Google Scholar] [CrossRef]
- Van Haute, S.; Raes, K.; Devlieghere, F.; Sampers, I. Combined use of cinnamon essential oil and map/vacuum packaging to increase the microbial and sensorial shelf life of lean pork and salmon. Food Packag. Shelf Life 2017, 12, 51–58. [Google Scholar] [CrossRef]
- dos Santos, P.R.; Donado-Pestana, C.M.; Delgado, E.F.; Tanaka, F.O.; Contreras-Castillo, C.J. Tenderness and oxidative stability of nellore bulls steaks packaged under vacuum or modified atmosphere during storage at 2 °C. Food Packag. Shelf Life 2015, 4, 10–18. [Google Scholar] [CrossRef]
- Łopacka, J.; Półtorak, A.; Wierzbicka, A. Effect of map, vacuum skin-pack and combined packaging methods on physicochemical properties of beef steaks stored up to 12days. Meat Sci. 2016, 119, 147–153. [Google Scholar] [CrossRef]
- Aguilera Barraza, F.A.; León, R.A.Q.; Álvarez, P.X.L. Kinetics of protein and textural changes in atlantic salmon under frozen storage. Food Chem. 2015, 182, 120–127. [Google Scholar] [CrossRef] [PubMed]
- Pan, S.; Wu, S. Effect of chitooligosaccharides on the denaturation of weever myofibrillar protein during frozen storage. Int. J. Biol. Macromol. 2014, 65, 549–552. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Jing, D.; Yu, D.; Xia, W.; Jiang, Q.; Xu, Y.; Yu, P. Differential roles of ice crystal, endogenous proteolytic activities and oxidation in softening of obscure pufferfish (takifugu obscurus) fillets during frozen storage. Food Chem. 2019, 278, 452–459. [Google Scholar] [CrossRef] [PubMed]
- Davies, K.J.A. Degradation of oxidized proteins by the 20 s proteasome. Biochimie 2001, 83, 301–310. [Google Scholar] [CrossRef]
- Berardo, A.; Claeys, E.; Vossen, E.; Leroy, F.; De Smet, S. Protein oxidation affects proteolysis in a meat model system. Meat Sci. 2015, 106, 78–84. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, M.; Nakamura, S.; Horimoto, Y.; An, H.; Tsuchiya, T.; Nakai, S. Raman spectroscopic study of changes in fish actomyosin during setting. J. Agric. Food Chem. 1999, 47, 3309. [Google Scholar] [CrossRef] [PubMed]
- Abbey, L.D.; Glover-Amengor, M.; Atikpo, M.O.; Howell, N.K. Proximate and biochemical characterization of burrito (bachydeuterus auritus) and flying gurnard (dactylopterus volitans). Food Sci. Nutr. 2017, 5, 369–373. [Google Scholar] [CrossRef] [PubMed]
- Oliver, C.N.; Ahn, B.W.; Moerman, E.J.; Goldstein, S.; Stadtman, E.R. Age-related changes in oxidized proteins. J. Biol. Chem. 1987, 262, 5488. [Google Scholar]
- Chelh, I.; Gatellier, P.; Santé-Lhoutellier, V. Technical note: A simplified procedure for myofibril hydrophobicity determination. Meat Sci. 2006, 74, 681–683. [Google Scholar] [CrossRef]
- Benjakul, S.; Visessanguan, W.C.; Tanaka, M. Comparative study on physicochemical changes of muscle proteins from some tropical fish during frozen storage. Food Res. Int. 2003, 36, 787–795. [Google Scholar] [CrossRef]
- Thannhauser, T.W.; Konishi, Y.; Scheraga, H.A. Analysis for disulfide bonds in peptides and proteins. Methods Enzymol. 1987, 143, 115. [Google Scholar] [PubMed]
- Benjakul, S.; Seymour, T.A.; Morrissey, M.T.; Haejung, A.N. Physicochemical changes in pacific whiting muscle proteins during iced storage. J. Food Sci. 2010, 62, 729–733. [Google Scholar] [CrossRef]
- Thanonkaew, A.; Benjakul, S.; Visessanguan, W.; Decker, E.A. The effect of metal ions on lipid oxidation, colour and physicochemical properties of cuttlefish (sepia pharaonis) subjected to multiple freeze–thaw cycles. Food Chem. 2006, 95, 591–599. [Google Scholar] [CrossRef]
- Sriket, C.; Benjakul, S.; Visessanguan, W.; Hara, K.; Yoshida, A. Retardation of post-mortem changes of freshwater prawn (macrobrachium rosenbergii) stored in ice by legume seed extracts. Food Chem. 2012, 135, 571–579. [Google Scholar] [CrossRef] [PubMed]
- Culler, R.D.; Parrish, F.C., Jr.; Smith, G.C.; Cross, H.R. Relationship of myofibril fragmentation index to certain chemical, physical and sensory characteristics of bovine longissimus muscle. J. Food Sci. 2010, 43, 1177–1180. [Google Scholar] [CrossRef]
- Yu, D.; Xu, Y.; Regenstein, J.M.; Xia, W.; Yang, F.; Jiang, Q.; Wang, B. The effects of edible chitosan-based coatings on flavor quality of raw grass carp (ctenopharyngodon idellus) fillets during refrigerated storage. Food Chem. 2018, 242, 412–420. [Google Scholar] [CrossRef] [PubMed]
- Verrez-Bagnis, V.; Ladrat, C.; Morzel, M.; Noël, J.; Fleurence, J. Protein changes in post mortem sea bass (dicentrarchus labrax) muscle monitored by one-and two-dimensional gel electrophoresis. Electrophoresis 2015, 22, 1539–1544. [Google Scholar] [CrossRef]
- Biswas, S.; Chida, A.S.; Rahman, I. Redox modifications of protein-thiols: Emerging roles in cell signaling. Biochem. Pharmacol. 2006, 71, 551–564. [Google Scholar] [CrossRef]
- Soyer, A.; Özalp, B.; Dalmış, Ü.; Bilgin, V. Effects of freezing temperature and duration of frozen storage on lipid and protein oxidation in chicken meat. Food Chem. 2010, 120, 1025–1030. [Google Scholar] [CrossRef]
- Nikoo, M.; Benjakul, S.; Rahmanifarah, K. Hydrolysates from marine sources as cryoprotective substances in seafoods and seafood products. Trends Food Sci. Technol. 2016, 57, 40–51. [Google Scholar] [CrossRef]
- Zhang, L.; Gui, P.; Zhang, Y.; Lin, J.; Li, Q.; Hong, H.; Luo, Y. Assessment of structural, textural, and gelation properties of myofibrillar protein of silver carp (hypophthalmichthys molitrix) modified by stunning and oxidative stress. LWT-Food Sci. Technol. 2019, 102, 142–149. [Google Scholar] [CrossRef]
- Yarnpakdee, S.; Benjakul, S.; Visessanguan, W.; Kijroongrojana, K. Thermal properties and heat-induced aggregation of natural actomyosin extracted from goatfish (mulloidichthys martinicus) muscle as influenced by iced storage. Food Hydrocoll. 2009, 23, 1779–1784. [Google Scholar] [CrossRef]
- He, Y.; Huang, H.; Li, L.; Yang, X.; Hao, S.; Chen, S.; Deng, J. The effects of modified atmosphere packaging and enzyme inhibitors on protein oxidation of tilapia muscle during iced storage. LWT-Food Sci. Technol. 2018, 87, 186–193. [Google Scholar] [CrossRef]
- Ko, W.-C.; Shi, H.-Z.; Chang, C.-K.; Huang, Y.-H.; Chen, Y.-A.; Hsieh, C.-W. Effect of adjustable parallel high voltage on biochemical indicators and actomyosin Ca2+-atpase from tilapia (Orechromis niloticus). LWT-Food Sci. Technol. 2016, 69, 417–423. [Google Scholar] [CrossRef]
- Zhang, B.; Hao, G.-J.; Cao, H.-J.; Tang, H.; Zhang, Y.-Y.; Deng, S.-G. The cryoprotectant effect of xylooligosaccharides on denaturation of peeled shrimp (litopenaeus vannamei) protein during frozen storage. Food Hydrocoll. 2018, 77, 228–237. [Google Scholar] [CrossRef]
- Yu, D.; Regenstein, J.M.; Zang, J.; Xia, W.; Xu, Y.; Jiang, Q.; Yang, F. Inhibitory effects of chitosan-based coatings on endogenous enzyme activities, proteolytic degradation and texture softening of grass carp (ctenopharyngodon idellus) fillets stored at 4 °C. Food Chem. 2018, 262, 1. [Google Scholar] [CrossRef] [PubMed]
- Rawdkuen, S.; Jongjareonrak, A.; Phatcharat, S.; Benjakul, S. Assessment of protein changes in farmed giant catfish (pangasianodon gigas) muscles during refrigerated storage. Int. J. Food Sci. Technol. 2010, 45, 985–994. [Google Scholar] [CrossRef]
- Taylor, R.G.; Geesink, G.H.; Thompson, V.F.; Koohmaraie, M.; Goll, D.E. Is z-disk degradation responsible for postmortem tenderization? J. Anim. Sci. 1995, 73, 1351. [Google Scholar] [CrossRef] [PubMed]
- Shi, C.; Cui, J.; Na, Q.; Luo, Y.; Han, L.; Hang, W. Effect of ginger extract and vinegar on atp metabolites, imp-related enzyme activity, reducing sugars and phosphorylated sugars in silver carp during postslaughter storage. Int. J. Food Sci. Technol. 2017, 52, 413–423. [Google Scholar] [CrossRef]
- Ruiz-Capillas, C.; Moral, A. Changes in free amino acids during chilled storage of hake (merluccius merluccius L.) in controlled atmospheres and their use as a quality control index. Eur. Food Res. Technol. 2001, 212, 302–307. [Google Scholar] [CrossRef]
- Hanne, C.B.; Achim, K.; Ulrike, B.C.; Ragni, O.; Andersen, H.J. Heat-induced changes in myofibrillar protein structures and myowater of two pork qualities. A combined ft-ir spectroscopy and low-field nmr relaxometry study. J. Agric. Food Chem. 2006, 54, 1740–1746. [Google Scholar]
- Carton, I.; Bocker, U.; Ofstad, R. Monitoring secondary structural changes in salted and smoked salmon muscle myofiber proteins by ft-ir microspectroscopy. J. Agric. Food Chem. 2009, 57, 3563–3570. [Google Scholar] [CrossRef] [PubMed]
- Tu, Z.C.; Huang, T.; Wang, H.; Sha, X.M.; Shi, Y.; Huang, X.Q.; Man, Z.Z.; Li, D.J. Physico-chemical properties of gelatin from bighead carp (hypophthalmichthys nobilis) scales by ultrasound-assisted extraction. J. Food Sci. Technol. 2015, 52, 2166–2174. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Y.L.; Blanchard, S.P.; Tooru, O.; Yuanyuan, M. Hydroxyl radical and ferryl-generating systems promote gel network formation of myofibrillar protein. J. Food Sci. 2010, 75, C215–C221. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.; Zhang, L.; Li, Q.; Luo, Y. Comparison of gel properties and biochemical characteristics of myofibrillar protein from bighead carp (aristichthys nobilis) affected by frozen storage and a hydroxyl radical-generation oxidizing system. Food Chem. 2017, 223, 96–103. [Google Scholar] [CrossRef] [PubMed]
- Cayuela, J.M.; Gil, M.D.; Bañón, S.; Garrido, M.D. Effect of vacuum and modified atmosphere packaging on the quality of pork loin. Eur. Food Res. Technol. 2004, 219, 316–320. [Google Scholar] [CrossRef]
- Lametsch, R.; Lonergan, S.; Huff-Lonergan, E. Disulfide bond within mu-calpain active site inhibits activity and autolysis. Biochim. Et Biophys. Acta Proteins Proteom. 2008, 1784, 1215–1221. [Google Scholar] [CrossRef]









| FAA | Day 0 | AP Day 6 | AP Day 12 | VP Day 6 | VP Day 12 | MAP Day 6 | MAP Day 12 |
|---|---|---|---|---|---|---|---|
| Asp | 5.69 ± 0.13 a | 1.93 ± 0.12 c | 1.09 ± 0.06 c | 3.15 ± 0.12 b | 1.71 ± 0.12 c | 1.49 ± 0.05 c | 3.32 ± 0.10 b |
| Thr | 14.24 ± 0.48 b | 20.57 ± 1.55 a | 13.41 ± 1.10 b,c | 20.84 ± 1.42 a | 12.71 ± 0.14 c | 12.65 ± 0.23 c | 13.09 ± 0.15 b,c |
| Ser | 20.24 ± 0.60 b | 25.79 ± 0.16 a | 13.78 ± 0.25 b,c | 21.87 ± 1.57 a | 12.25 ± 0.07 c | 12.19 ± 0.11 c | 14.01 ± 0.12 b,c |
| Glu | 8.71 ± 0.30 d | 27.20 ± 0.09 a,b | 18.59 ± 0.31 c | 18.34 ± 1.3 c | 22.06 ± 0.1 b,c | 30.98 ± 0.10 a | 22.73 ± 0.01 b,c |
| Gly | 91.14 ± 2.98 b | 153.15 ± 1.21 a | 78.74 ± 0.81 c | 114.93 ± 7.94 a,b | 88.33 ± 0.1 b,c | 84.20 ± 0.85 b,c | 108.33 ± 1.75 a,b |
| Ala | 23.54 ± 0.78 b,c | 43.80 ± 0.40 a | 25.55 ± 0.25 b,c | 27.65 ± 1.89 b,c | 22.88 ± 0.04 b,c | 30.04 ± 0.84 b | 28.55 ± 0.49 c |
| Cys | 1.23 ± 0.03 a | 0.95 ± 0.11 a,b | 0.48 ± 0.07 c | 0.78 ± 0.09 a,b | 0.24 ± 0.20 d | 1.56 ± 0.09 a | 0.48 ± 0.05 c |
| Val | 3.69 ± 0.14 c | 4.99 ± 0.12 b,c | 3.90 ± 0.09 c | 5.52 ± 0.38 b | 2.91 ± 0.06 c | 7.81 ± 0.04 a | 5.77 ± 0.12 b |
| Met | 1.70 ± 0.19 d | 2.18 ± 0.20 c | 1.90 ± 0.13 d | 2.87 ± 0.11 b,c | 1.16 ± 0.10 d | 6.25 ± 0.11 a | 3.25 ± 0.08 b |
| Ile | 2.64 ± 0.15 c | 3.43 ± 0.03 b,c | 2.74 ± 0.11 c | 3.89 ± 0.41 b | 2.03 ± 0.13 c | 5.47 ± 0.82 a | 3.85 ± 0.03 b |
| Leu | 4.18 ± 0.03 c | 5.54 ± 0.09 b | 4.44 ± 0.09 c | 6.28 ± 0.49 b | 3.12 ± 0.05 c | 9.05 ± 0.03 a | 6.39 ± 0.12 b |
| Tyr | 1.61 ± 0.28 c | 2.64 ± 0.26 b,c | 1.55 ± 0.49 c | 2.64 ± 0.37 b,c | 1.62 ± 0.12 c | 6.05 ± 0.85 a | 2.93 ± 0.02 b,c |
| Phe | 4.15 ± 0.39 d | 5.34 ± 0.21 c,d | 5.98 ± 0.14 c | 6.57 ± 0.85 b,c | 5.91 ± 0.39 c | 9.54 ± 0.94 a | 7.01 ± 0.42 b,c |
| Lys | 33.66 ± 1.04 b | 39.20 ± 0.29 a,b | 30.85 ± 0.33 c | 38.60 ± 2.87 a,b | 36.00 ± 0.20 b | 43.79 ± 0.27 a | 28.30 ± 0.46 c |
| His | 3.39 ± 0.25 c | 5.27 ± 0.18 a | 3.09 ± 0.91 c | 4.74 ± 0.38 b,c | 2.16 ± 0.10 c,d | 4.61 ± 0.46 b,c | 3.82 ± 0.34 b,c |
| Arg | 4.57 ± 0.12 c | 9.38 ± 0.48 a | 7.61 ± 1.85 a,b | 8.81 ± 0.49 a | 5.82 ± 0.36 b,c | 7.76 ± 0.37 b,c | 5.24 ± 0.14 a,b |
| Pro | 7.55 ± 0.26 d | 10.67 ± 0.19 b | 8.76 ± 0.15 c,d | 17.42 ± 1.30 a | 9.28 ± 0.07 b,c | 18.78 ± 0.07 a | 8.04 ± 0.37 c,d |
| total | 231.91 ± 6.67 c | 362.03 ± 4.03 a | 219.49 ± 3.25 c | 304.89 ± 21.5 b | 230.19 ± 0.51 c | 292.23 ± 6.03 b,c | 265.09 ± 3.92 b,c |
| Group | Indicator of Protein Oxidation | Indicator of Protein Degradation | Pearson Correlation Coefficient |
|---|---|---|---|
| AP | Carbonyl content | TCA-soluble peptide content | 0.683 |
| AP | Total sulfhydryl content | TCA-soluble peptide content | −0.981 ** |
| AP | Carbonyl content | Myofibril fragmentation index | 0.956 * |
| AP | Total sulfhydryl content | Myofibril fragmentation index | −0.961 * |
| VP | Carbonyl content | TCA-soluble peptide content | 0.941 * |
| VP | Total sulfhydryl content | TCA-soluble peptide content | −0.975 * |
| VP | Carbonyl content | Myofibril fragmentation index | 0.940 * |
| VP | Total sulfhydryl content | Myofibril fragmentation index | −0.975 ** |
| MAP | Carbonyl content | TCA-soluble peptide content | 0.793 |
| MAP | Total sulfhydryl content | TCA-soluble peptide content | −0.982 ** |
| MAP | Carbonyl content | Myofibril fragmentation index | 0.934 * |
| MAP | Total sulfhydryl content | Myofibril fragmentation index | −0.929 * |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, X.; Huang, W.; Xie, J. Effect of Different Packaging Methods on Protein Oxidation and Degradation of Grouper (Epinephelus coioides) During Refrigerated Storage. Foods 2019, 8, 325. https://doi.org/10.3390/foods8080325
Zhang X, Huang W, Xie J. Effect of Different Packaging Methods on Protein Oxidation and Degradation of Grouper (Epinephelus coioides) During Refrigerated Storage. Foods. 2019; 8(8):325. https://doi.org/10.3390/foods8080325
Chicago/Turabian StyleZhang, Xicai, Wenbo Huang, and Jing Xie. 2019. "Effect of Different Packaging Methods on Protein Oxidation and Degradation of Grouper (Epinephelus coioides) During Refrigerated Storage" Foods 8, no. 8: 325. https://doi.org/10.3390/foods8080325
APA StyleZhang, X., Huang, W., & Xie, J. (2019). Effect of Different Packaging Methods on Protein Oxidation and Degradation of Grouper (Epinephelus coioides) During Refrigerated Storage. Foods, 8(8), 325. https://doi.org/10.3390/foods8080325