Electronic Nose for Monitoring Odor Changes of Lactobacillus Species during Milk Fermentation and Rapid Selection of Probiotic Candidates
Abstract
:1. Introduction
2. Materials and Methods
2.1. Analyzed Bacteria Strains
2.2. Preparation of the Fermented Milk Product
2.2.1. Preparation of Reconstituted Milk
2.2.2. Preparation of Strain Suspension (Activation of Freeze-Dried Bacteria)
2.2.3. Preparation of the Milk Suspension
2.3. Determination of the Cell Count at the Different Fermentation Time Points
2.4. Analysis of pH
2.5. Analysis of the Aroma Composition of the Milk Suspension during the Fermentation Process Using the E-Nose
2.6. Data Analysis
2.6.1. Microbial Assessment
2.6.2. E-Nose Data Construction and Analysis
3. Results and Discussion
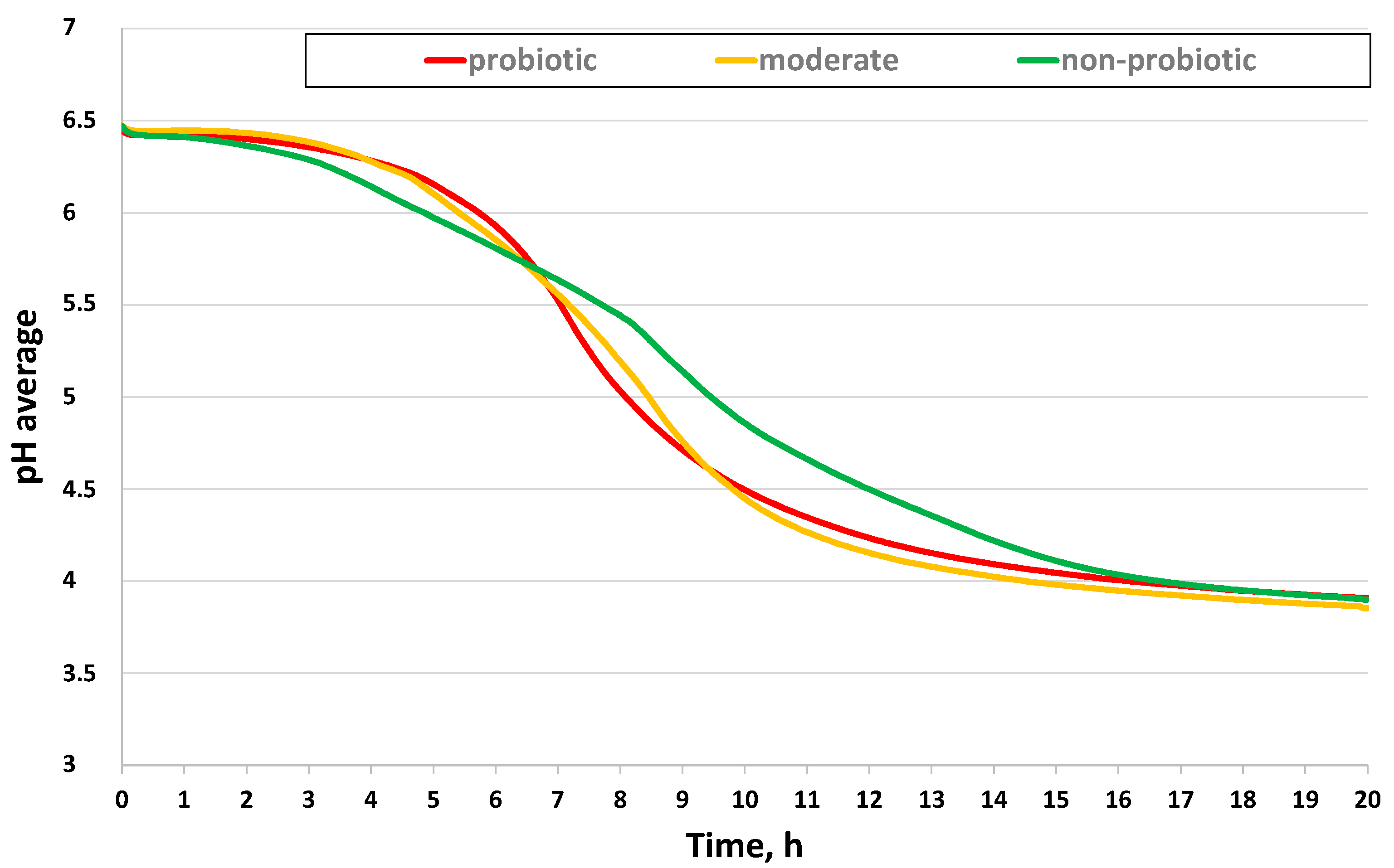
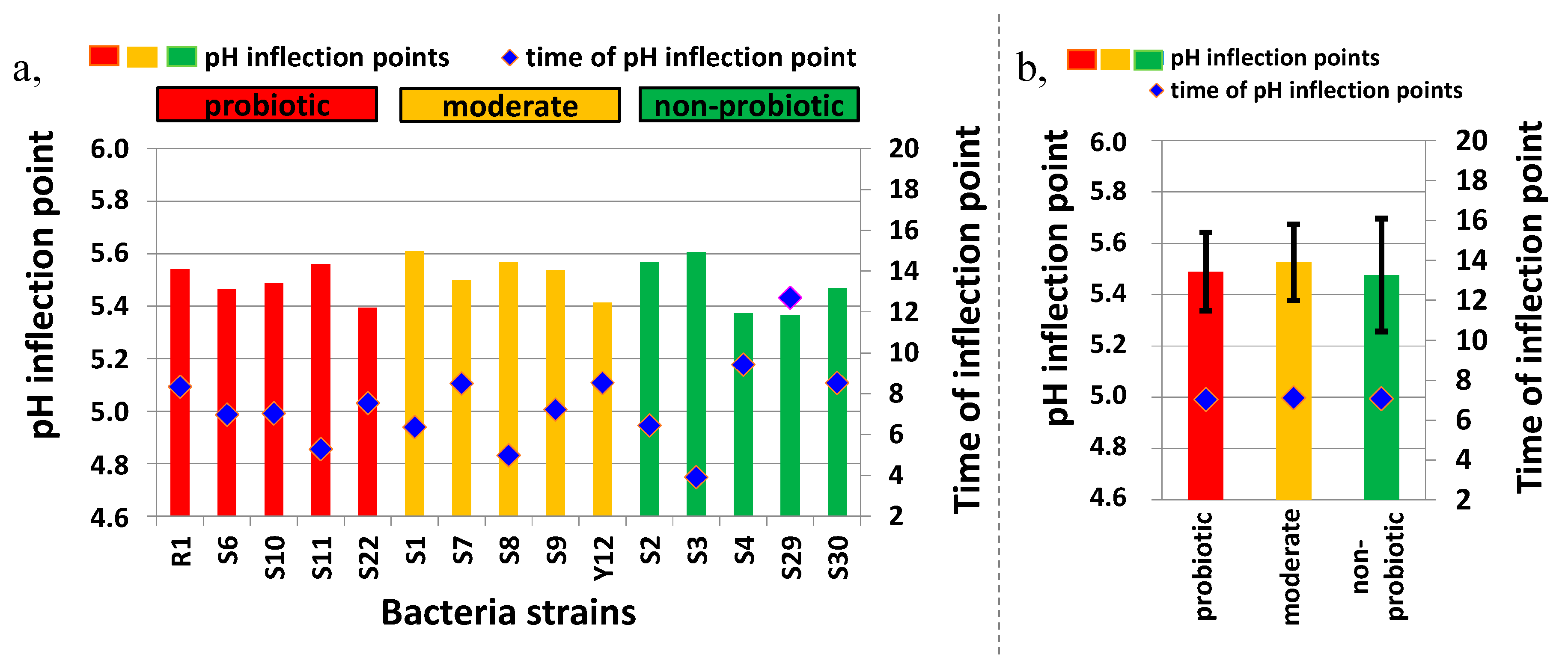
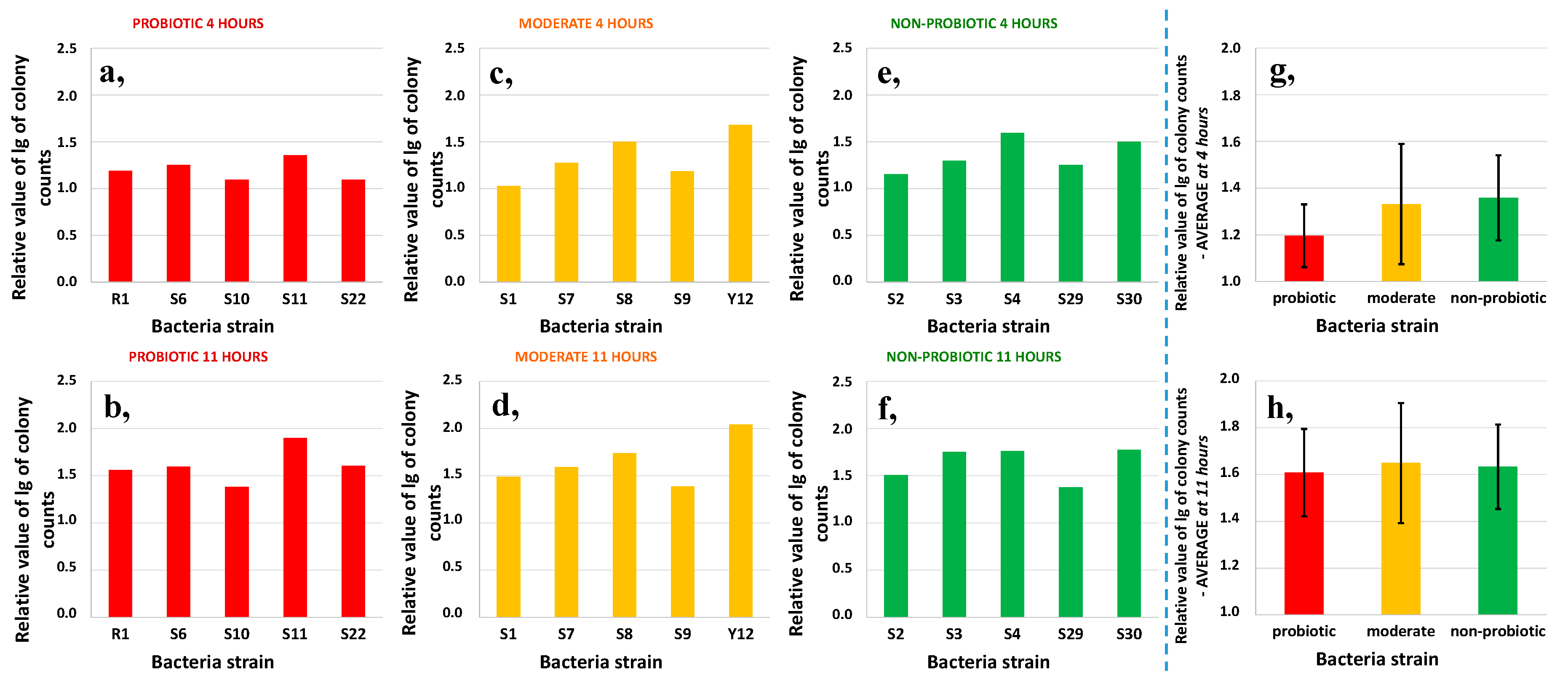
3.1. Growth Characterization of Bacteria Strains in Reconstituted Milk
3.2. Discrimination of Probiotic Strains Based on Their Aroma Composition Using E-Nose
3.3. Discrimination of Inoculated Milk Samples Fermented for Different Times Based on Their Aroma Composition Using the E-Nose
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Tamime, A.Y.; Robinson, R.K. Traditional and recent developments in yoghurt production and related products. In Tamime and Robinson’s Yoghurt; Woodhead Publishing: Cambridge, UK, 2007; pp. 348–467. ISBN 978-1-84569-213-1. [Google Scholar]
- Teixeira, P. Lactobacillus: Lactobacillus Delbrueckii Ssp. Bulgaricus, 2nd ed.; Elsevier: London, UK, 2014; Volume 2, ISBN 9780123847331. [Google Scholar]
- Fehér, O.; Boros, P.; Podruzsik, S. Analysis of Food Consuming Attitudes in the Context of Functional Food Products and Food Supplements. Int. J. Sales Retail. Mark. 2012, 1, 3–8. [Google Scholar]
- Slavchev, A.; Kovacs, Z.; Koshiba, H.; Bazar, G.; Pollner, B.; Krastanov, A.; Tsenkova, R. Monitoring of water spectral patterns of lactobacilli development as a tool for rapid selection of probiotic candidates. J. Near Infrared Spectrosc. 2017, 25, 423–431. [Google Scholar] [CrossRef]
- Liu, W.; Yu, J.; Sun, Z.; Song, Y.; Wang, X.; Wang, H.; Wuren, T.; Zha, M.; Menghe, B.; Heping, Z. Relationships between functional genes in Lactobacillus delbrueckii ssp. bulgaricus isolates and phenotypic characteristics associated with fermentation time and flavor production in yogurt elucidated using multilocus sequence typing. J. Dairy Sci. 2015, 99, 89–103. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gutiérrez-Méndez, N.; Vallejo-Cordoba, B.; González-Córdova, A.F.; Nevárez-Moorillón, G.V.; Rivera-Chavira, B. Evaluation of Aroma Generation of Lactococcus lactis with an Electronic Nose and Sensory Analysis. J. Dairy Sci. 2008, 91, 49–57. [Google Scholar] [CrossRef] [Green Version]
- Darnay, L.; Len, A.; Koncz, Á.; Friedrich, L.; Rosta, L. Small angle neutron scattering study of nanostructural changes in microbial transglutaminase-treated low-fat yogurt during fermentation. Food Sci. Biotechnol. 2015, 24, 2125–2128. [Google Scholar] [CrossRef]
- Darnay, L.; Koncz, Á.; Gelencsér, É.; Pásztor-Huszár, K.; Friedrich, L. Utjecaj dodatka mikrobne transglutaminaze na teksturalna svojstva jogurta. Mljekarstvo 2016, 66, 225–230. [Google Scholar] [CrossRef] [Green Version]
- Slavchev, A.; Kovacs, Z.; Koshiba, H.; Nagai, A.; Bázár, G.; Krastanov, A.; Kubota, Y.; Tsenkova, R. Monitoring of water spectral pattern reveals differences in probiotics growth when used for rapid bacteria selection. PLoS ONE 2015, 10, e0130698. [Google Scholar] [CrossRef] [Green Version]
- Kovács, Z.; Dalmadi, I.; Lukács, L.; Sipos, L.; Szántai-Kőhegyi, K.; Kókai, Z.; Fekete, A. Geographical origin identification of pure Sri Lanka tea infusions with electronic nose, electronic tongue and sensory profile analysis. J. Chemom. 2010, 24, 121–130. [Google Scholar] [CrossRef]
- Zaukuu, J.L.Z.; Bazar, G.; Gillay, Z.; Kovacs, Z. Emerging trends of advanced sensor based instruments for meat, poultry and fish quality—A review. Crit. Rev. Food Sci. Nutr. 2019. [Google Scholar] [CrossRef] [PubMed]
- Peris, M.; Escuder-Gilabert, L. On-line monitoring of food fermentation processes using electronic noses and electronic tongues: A review. Anal. Chim. Acta 2013, 804, 29–36. [Google Scholar] [CrossRef]
- Tóth, T.; Mwau, P.J.; Bázár, G.; Andrássy-Baka, G.; Hingyi, H.; Csavajda, É.; Varga, L. Effect of feed supplementation based on extruded linseed meal and fish oil on composition and sensory properties of raw milk and ultra-high temperature treated milk. Int. Dairy J. 2019, 99, 104552. [Google Scholar] [CrossRef]
- Marilley, L.; Ampuero, S.; Zesiger, T.; Casey, M.G. Screening of aroma-producing lactic acid bacteria with an electronic nose. Int. Dairy J. 2004, 14, 849–856. [Google Scholar] [CrossRef]
- Kalit, M.T.; Marković, K.; Kalit, S.; Vahčić, N.; Havranek, J. Application of electronic nose and electronic tongue in the dairy industry. Mljekarstvo 2014, 64, 228–244. [Google Scholar] [CrossRef] [Green Version]
- Navrátil, M.; Cimander, C.; Mandenius, C.-F. On-line Multisensor Monitoring of Yogurt and Filmjölk Fermentations on Production Scale. J. Agric. Food Chem. 2004, 52, 415–420. [Google Scholar] [CrossRef]
- Prescott, S.C.; Breed, R.S. The Determination of the Number of Body Cells in Milk by a Direct Method. Am. J. Public Hygiene 1910, 20, 663–664. [Google Scholar] [CrossRef] [PubMed]
- Zenkevich, I.G. Kovats Retention Index System. In Encyclopedia of Chromatography; Cazes, J., Ed.; Marcel Dekker Inc.: New York, NY, USA, 2002. [Google Scholar]
- Alpha MOS. Heracles Manual; Alpha MOS: Toulouse, France, 2018; pp. 1–163. Available online: https://www.alpha-mos.com/heracles-smell-analysis (accessed on 24 October 2020).
- Torrestiana, B.S.; Brito de la Fuente, E.; Lacroix, C.; Choplin, L. Modelling the acidifying activity profile of Lactobacillus bulgaricus cultures. Appl. Microbiol. Biotechnol. 1994, 42, 192–196. [Google Scholar] [CrossRef]
- Reiczigel, J.; Harnos, A.; Solymosi, N. Biostatistics for Non Statistical Experts (Biostatisztika Nem Statisztikusoknak); Pars Kft: Budapest, Hungary, 2014. [Google Scholar]
- Zheng, X.; Ge, Z.; Lin, K.; Zhang, D.; Chen, Y.; Xiao, J.; Wang, B.; Shi, X. Dynamic changes in bacterial microbiota succession and flavour development during milk fermentation of Kazak artisanal cheese. Int. Dairy J. 2020, 113, 104878. [Google Scholar] [CrossRef]
- Dan, T.; Wang, D.; Jin, R.L.; Zhang, H.P.; Zhou, T.T.; Sun, T.S. Characterization of volatile compounds in fermented milk using solid-phase microextraction methods coupled with gas chromatography-mass spectrometry. J. Dairy Sci. 2017, 100, 2488–2500. [Google Scholar] [CrossRef] [Green Version]
- Dan, T.; Wang, D.; Wu, S.; Jin, R.; Ren, W.; Sun, T. Profiles of Volatile Flavor Compounds in Milk Fermented with Different Proportional Combinations of Lactobacillus delbrueckii subsp. bulgaricus and Streptococcus thermophilus. Molecules 2017, 22, 1633. [Google Scholar]
- Milesi, M.M.; Wolf, I.V.; Bergamini, C.V.; Hynes, E.R. Two strains of nonstarter lactobacilli increased the production of flavor compounds in soft cheeses. J. Dairy Sci. 2010, 93, 5020–5031. [Google Scholar] [CrossRef] [Green Version]
- Sfakianakis, P.; Tzia, C. Flavour profiling by gas chromatography–mass spectrometry and sensory analysis of yoghurt derived from ultrasonicated and homogenised milk. Int. Dairy J. 2017, 75, 120–128. [Google Scholar] [CrossRef]
- Moonga, H.B.; Schoustra, S.E.; Linnemann, A.R.; van den Heuvel, J.; Shindano, J.; Smid, E.J. Influence of fermentation temperature on microbial community composition and physicochemical properties of mabisi, a traditionally fermented milk. LWT 2020, 136, 110350. [Google Scholar] [CrossRef]
- Pan, D.D.; Wu, Z.; Peng, T.; Zeng, X.Q.; Li, H. Volatile organic compounds profile during milk fermentation by Lactobacillus pentosus and correlations between volatiles flavor and carbohydrate metabolism. J. Dairy Sci. 2014, 97, 624–631. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muelas, R.; Martí de Olives, A.; Romero, G.; Díaz, J.R.; Sayas-Barberá, M.E.; Sendra, E. Evaluation of individual lactic acid bacteria for the fermentation of goat milk: Quality parameters. LWT 2018, 98, 506–514. [Google Scholar] [CrossRef]
- Mugampoza, D.; Gkatzionis, K.; Linforth, R.S.T.; Dodd, C.E.R. Acid production, growth kinetics and aroma profiles of Lactobacillus flora from Stilton cheese. Food Chem. 2019, 287, 222–231. [Google Scholar] [CrossRef]
- University of Alberta FooDB Version 1.0. Available online: http://foodb.ca/ (accessed on 24 October 2020).



| Identified Compound by E-Nose | Expressed Sensory Characteristic * | Products and References |
|---|---|---|
| 1-hexen-3-one | metallic | |
| 1-propanol, 2-methyl- | alcoholic, bitter, chemical, glue, leek, licorice, winey | milk [27] |
| 2,4 -heptadienal | fatty aroma | milk [23,24] |
| 2-octanol | fatty, oily aroma | cheese [22] |
| 2-Propionylpyrrole | popcorn, roast aroma | |
| 3-hexanone | ethereal, fresh, fruity, grape | |
| 5-ethyldihydro-2(3h)-furanone | coumarin, sweet, tonka broad bean aroma | |
| acetic acid | acidic, pungent, sour, vinegar aroma | milk [23,24,27,28,29] cheese [28,30] |
| benzoic acid, 4-(isopropyl) oxy-, methyl ester, benzoic acid, hex-3-yl ester | [31] | |
| decane | alkane, fruity, fusel, sweet aroma) | |
| epoxy-2-nonenal | metallic aroma | |
| heptan-2-ol | acrid, fruity, pungent, roquefort cheese aroma | milk [24,31] cheese [22,28] |
| linalyl formate (citrus, coriander, herbaceous aroma) | citrus, coriander, herbaceous aroma | |
| methyl propanoate | fruity, rum, ethereal aroma | |
| n-butanol | cheese, medicinal aroma | |
| nicotinic acid, pentyl ester | [31] | |
| pentan-2-ol | fruity, green, sweet, pungent | cheese [25] |
| propionylpyrroline | fishy, roast aroma | |
| propyl propanoate | apple, chemical, pineapple | |
| pyridine, 2-pentyl | fatty, tallowy aroma |
| Column 1 (MXT-5) | Column 2 (MXT-1701) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 0th hour | 0th hour | ||||||||
| Total accuracy | Probiotic | Moderate | Non-Probiotic | Total accuracy | Probiotic | Moderate | Non-Probiotic | ||
| Recognition 74.44% | Probiotic | 75 | 15 | 11.67 | Recognition 82.78% | Probiotic | 85 | 15 | 6.67 |
| Moderate | 16.67 | 78.33 | 18.33 | Moderate | 13.33 | 81.67 | 11.67 | ||
| Non-Probiotic | 8.33 | 6.67 | 70 | Non-Probiotic | 1.67 | 3.33 | 81.67 | ||
| Cross validated 26.66% | Probiotic | Moderate | Non-Probiotic | Cross validated 48.89% | Probiotic | Moderate | Non-Probiotic | ||
| Probiotic | 40 | 40 | 20 | Probiotic | 60 | 46.67 | 13.33 | ||
| Moderate | 40 | 0 | 40 | Moderate | 33.33 | 26.67 | 26.67 | ||
| Non-Probiotic | 20 | 60 | 40 | Non-Probiotic | 6.67 | 26.67 | 60 | ||
| 4th hour | 4th hour | ||||||||
| Total accuracy | Probiotic | Moderate | Non-Probiotic | Total accuracy | Probiotic | Moderate | Non-Probiotic | ||
| Recognition 89.44% | Probiotic | 88.33 | 5 | 5 | Recognition 92.22% | Probiotic | 85 | 0 | 5 |
| Moderate | 10 | 95 | 10 | Moderate | 5 | 100 | 3.33 | ||
| Non-Probiotic | 1.67 | 0 | 85 | Non-Probiotic | 10 | 0 | 91.67 | ||
| Cross validated 60.00% | Probiotic | Moderate | Non-Probiotic | Cross validated 66.67% | Probiotic | Moderate | Non-Probiotic | ||
| Probiotic | 73.33 | 33.33 | 13.33 | Probiotic | 60 | 20 | 13.33 | ||
| Moderate | 13.33 | 53.33 | 33.33 | Moderate | 6.67 | 73.33 | 20 | ||
| Non-Probiotic | 13.33 | 13.33 | 53.33 | Non-Probiotic | 33.33 | 6.67 | 66.67 | ||
| 11th hour | 11th hour | ||||||||
| Total accuracy | Probiotic | Moderate | Non-Probiotic | Total accuracy | Probiotic | Moderate | Non-Probiotic | ||
| Recognition 81.67% | Probiotic | 78.33 | 13.33 | 1.67 | Recognition 81.67% | Probiotic | 78.33 | 13.33 | 0 |
| Moderate | 21.67 | 73.33 | 5 | Moderate | 21.67 | 71.67 | 5 | ||
| Non-Probiotic | 0 | 13.33 | 93.33 | Non-Probiotic | 0 | 15 | 95 | ||
| Cross validated 55.55% | Probiotic | Moderate | Non-Probiotic | Cross validated 59.99% | Probiotic | Moderate | Non-Probiotic | ||
| Probiotic | 60 | 46.67 | 6.67 | Probiotic | 73.33 | 46.67 | 6.67 | ||
| Moderate | 40 | 20 | 6.67 | Moderate | 26.67 | 20 | 6.67 | ||
| Non-Probiotic | 0 | 33.33 | 86.67 | Non-Probiotic | 0 | 33.33 | 86.67 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kovacs, Z.; Bodor, Z.; Zinia Zaukuu, J.-L.; Kaszab, T.; Bazar, G.; Tóth, T.; Mohácsi-Farkas, C. Electronic Nose for Monitoring Odor Changes of Lactobacillus Species during Milk Fermentation and Rapid Selection of Probiotic Candidates. Foods 2020, 9, 1539. https://doi.org/10.3390/foods9111539
Kovacs Z, Bodor Z, Zinia Zaukuu J-L, Kaszab T, Bazar G, Tóth T, Mohácsi-Farkas C. Electronic Nose for Monitoring Odor Changes of Lactobacillus Species during Milk Fermentation and Rapid Selection of Probiotic Candidates. Foods. 2020; 9(11):1539. https://doi.org/10.3390/foods9111539
Chicago/Turabian StyleKovacs, Zoltan, Zsanett Bodor, John-Lewis Zinia Zaukuu, Timea Kaszab, George Bazar, Tamás Tóth, and Csilla Mohácsi-Farkas. 2020. "Electronic Nose for Monitoring Odor Changes of Lactobacillus Species during Milk Fermentation and Rapid Selection of Probiotic Candidates" Foods 9, no. 11: 1539. https://doi.org/10.3390/foods9111539








