Bio-Based Active Packaging: Carrageenan Film with Olive Leaf Extract for Lamb Meat Preservation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.1.1. Olive Leaves
2.1.2. Chemicals
2.1.3. Bacterial Isolates
2.2. Preparation of the Plant Extract
2.3. Extract Characterization
2.4. Carrageenan Biodegradable Films
2.4.1. Film Preparation
2.4.2. Film Properties
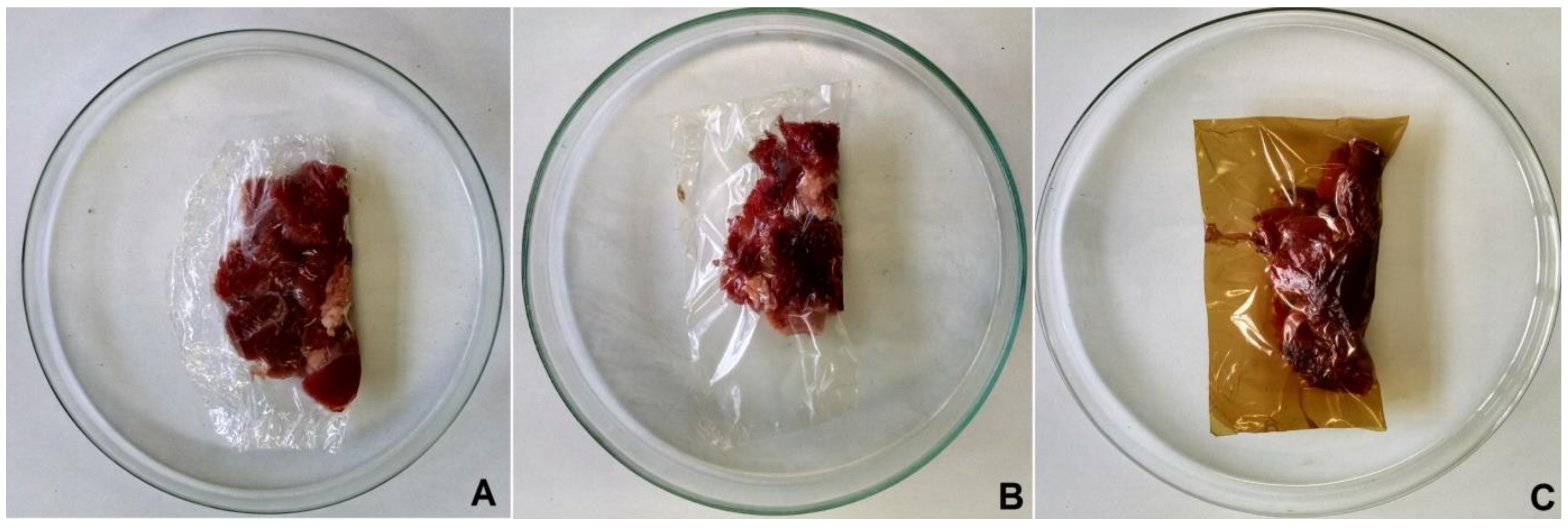
2.5. Storage Study: Inhibition of Psychrophiles in Lamb Meat
2.6. Statistical Analysis
3. Results and Discussion
3.1. Olive Leaf Extract
3.2. Film Evaluation
3.3. Storage Study
4. Conclusions
5. Patents
Author Contributions
Funding
Conflicts of Interest
References
- Berk, Z. Food packaging. In Food Process Engineering and Technology; Academic Press: Cambridge, MA, USA, 2018; pp. 625–641. [Google Scholar]
- Yildirim, S.; Röcker, B.; Pettersen, M.K.; Nilsen-Nygaard, J.; Ayhan, Z.; Rutkaite, R.; Radusin, T.; Suminska, P.; Marcos, B.; Coma, V. Active Packaging Applications for Food. Compr. Rev. Food Sci. Food Saf. 2018, 17, 165–199. [Google Scholar] [CrossRef] [Green Version]
- Ahvenainen, R. (Ed.) Novel Food Packaging Techniques; Woodhead Publishing: Cambridge, UK, 2003. [Google Scholar]
- Robertson, G.L. Food Packaging: Principles and Practice, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2013. [Google Scholar]
- Andrade-Mahecha, M.M.; Tapia-Blácido, D.R.; Menegalli, F.C. Development and optimization of biodegradable films based on achira flour. Carbohydr. Polym. 2012, 88, 449–458. [Google Scholar] [CrossRef]
- Domínguez, R.; Barba, F.J.; Gómez, B.; Putnik, P.; Kovačević, D.B.; Pateiro, M.; Santos, E.M.; Lorenzo, J.M. Active packaging films with natural antioxidants to be used in meat industry: A review. Food Res. Int. 2018, 113, 93–101. [Google Scholar] [CrossRef] [PubMed]
- Jalal, H.; Salhuddin, M.; Para, P.A.; Rajasthan, S.; Pal, M.A. Bio-based Packaging Materials for Preservation of Processed Meat Products. In Recent Research Trends in Veterinary Sciences and Animal Husbandry; AkiNik Publications: New Delhi, India, 2018; pp. 31–48. [Google Scholar]
- Cha, D.S.; Chinnan, M.S. Biopolymer-based antimicrobial packaging: A review. Crit. Rev. Food Sci. Nutr. 2004, 44, 223–237. [Google Scholar] [CrossRef] [PubMed]
- Paolucci, M.; Fasulo, G.; Volpe, M.G. Employment of marine polysaccharides to manufacture functional biocomposites for aquaculture feeding applications. Mar. Drugs 2015, 13, 2680–2693. [Google Scholar] [CrossRef] [Green Version]
- Tavassoli-Kafrani, E.; Shekarchizadeh, H.; Masoudpour-Behabadi, M. Development of edible films and coatings from alginates and carrageenans. Carbohydr. Polym. 2016, 137, 360–374. [Google Scholar] [CrossRef] [PubMed]
- Webber, V.; de Carvalho, S.M.; Ogliari, P.J.; Hayashi, L.; Barreto, P.L.M. Optimization of the extraction of carrageenan from Kappaphycus alvarezii using response surface methodology. Food Sci. Technol. 2012, 32, 812–818. [Google Scholar] [CrossRef] [Green Version]
- Sedayu, B.B.; Cran, M.J.; Bigger, S.W. Reinforcement of refined and semi-refined carrageenan film with nanocellulose. Polymers 2020, 12, 1145. [Google Scholar] [CrossRef]
- Balqis, A.I.; Khaizura, M.N.; Russly, A.R.; Hanani, Z.N. Effects of plasticizers on the physicochemical properties of kappa-carrageenan films extracted from Eucheuma cottonii. Int. J. Biol. Macromol. 2017, 103, 721–732. [Google Scholar] [CrossRef]
- Giacometti, J.; Žauhar, G.; Žuvić, M. Optimization of ultrasonic-assisted extraction of major phenolic compounds from olive leaves (Olea europaea L.) using response surface methodology. Foods 2018, 7, 149. [Google Scholar] [CrossRef] [Green Version]
- El Sedef, S.; Karakaya, S. Olive tree (Olea europaea) leaves: Potential beneficial effects on human health. Nutr. Rev. 2009, 67, 632–638. [Google Scholar] [CrossRef] [PubMed]
- Kiritsakis, K.; Goula, A.M.; Adamopoulos, K.G. Valorization of Olive Leaves: Spray Drying of Olive Leaf Extract. Waste Biomass Valoriz. 2017, 9, 619–633. [Google Scholar] [CrossRef]
- Stamatopoulos, K.; Chatzilazarou, A.; Katsoyannos, E. Optimization of Multistage Extraction of Olive Leaves for Recovery of Phenolic Compounds at Moderated Temperatures and Short Extraction Times. Foods 2014, 3, 66–81. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, O.H.; Lee, B.Y. Antioxidant and antimicrobial activities of individual and combined phenolics in Olea europaea leaf extract. Bioresour. Technol. 2010, 101, 3751–3754. [Google Scholar] [CrossRef]
- Liu, Y.; McKeever, L.C.; Malik, N.S.A. Assessment of the antimicrobial activity of olive leaf extract against foodborne bacterial pathogens. Front. Microbiol. 2017, 8, 113. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Papoti, V.T.; Papageorgiou, M.; Dervisi, K.; Alexopoulos, E.; Apostolidis, K.; Petridis, D. Screening olive leaves from unexploited traditional Greek cultivars for their phenolic antioxidant dynamic. Foods 2018, 7, 197. [Google Scholar] [CrossRef] [Green Version]
- Abaza, L.; Youssef, N.B.; Manai, H.; Mahjoub, F.H.; Methenni, K.; Zarrouk, M. Chétoui olive leaf extracts: Influence of the solvent type on phenolics and antioxidant activities. Grasas Aceites 2011, 62, 96–104. [Google Scholar] [CrossRef] [Green Version]
- Albertos, I.; Avena-Bustillos, R.J.; Martín-Diana, A.B.; Du, W.; Rico, D.; Mchugh, T.H. Antimicrobial Olive Leaf Gelatin fi lms for enhancing the quality of cold-smoked Salmon. Food Packag. Shelf Life 2017, 13, 49–55. [Google Scholar] [CrossRef]
- Da Rosa, G.S.; Vanga, S.K.; Gariepy, Y.; Raghavan, V. Development of Biodegradable Films with Improved Antioxidant Properties Based on the Addition of Carrageenan Containing Olive Leaf Extract for Food Packaging Applications. J. Polym. Environ. 2019, 28, 123–130. [Google Scholar] [CrossRef]
- Bermúdez-Oria, A.; Rodríguez-Gutiérrez, G.; Vioque, B.; Rubio-Senent, F.; Fernández-Bolaños, J. Physical and functional properties of pectin-fish gelatin films containing the olive phenols hydroxytyrosol and 3,4-dihydroxyphenylglycol. Carbohydr. Polym. 2017, 178, 368–377. [Google Scholar] [CrossRef]
- Da Rosa, G.S.; de Moraes, C.C.; Alves, R.C.; Ribeiro, P.B.; Martiny, T.R. Biofilmes Antimicrobianos para Proteção de Alimentos; Instituto Nacional da Propriedade Industrial: Brasilia, Brazil, 2019. [Google Scholar]
- Da Rosa, G.S.; de Moraes, C.C.; Ribeiro, P.B.; de Silva, B.Z.; de Pereira, C.M.P.; Pacheco, B.S.; Freitas, V.O.; Martiny, T.R. Filme Bioativo Antimicrobiano À Base de Carragenana e Extrato de Folhas de Oliveira; Instituto Nacional da Propriedade Industrial: Brasil, Brazil, 2020. [Google Scholar]
- Quintavalla, S.; Vicini, L. Antimicrobial food packaing in meat industry. Meat Sci. 2002, 62, 373–380. [Google Scholar] [CrossRef]
- Bellés, M.; Alonso, V.; Roncalés, P.; Beltrán, J.A. A review of fresh lamb chilling and preservation. Small Rumin. Res. 2016, 146, 41–47. [Google Scholar] [CrossRef]
- Camo, J.; Beltrán, J.A.; Roncalés, P. Extension of the display life of lamb with an antioxidant active packaging. Meat Sci. 2008, 80, 1086–1091. [Google Scholar] [CrossRef]
- Kuorwel, K.K.; Cran, M.J.; Sonneveld, K.; Miltz, J.; Bigger, S.W. Essential Oils and Their Principal Constituents as Antimicrobial Agents for Synthetic Packaging Films. J. Food Sci. 2011, 76, 164–177. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Joerger, R.D. Antimicrobial films for food applications: A quantitative analysis of their effectiveness. Packag. Technol. Sci. 2007, 20, 231–273. [Google Scholar] [CrossRef]
- Da Rosa, G.S.; Vanga, S.K.; Gariepy, Y.; Raghavan, V. Comparison of microwave, ultrasonic and conventional techniques for extraction of bioactive compounds from olive leaves (Olea europaea L.). Innov. Food Sci. Emerg. Technol. 2019, 58, 102234. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, J.A. Colorimetry of total phenolics with phosphomolybdic phosphotungstic acid reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar]
- Martiny, T.R.; Pacheco, B.S.; Pereira, C.M.P.; Mansilla, A.; Astorga–España, M.S.; Dotto, G.L.; Moraes, C.C.; Rosa, G.S. A novel biodegradable film based on κ-carrageenan activated with olive leaves extract. Food Sci. Nutr. 2020, 8, 3147–3156. [Google Scholar] [CrossRef] [PubMed]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Balouiri, M.; Sadiki, M.; Ibnsouda, S.K. Methods for in vitro evaluating antimicrobial activity: A review. J. Pharm. Anal. 2016, 6, 71–79. [Google Scholar] [CrossRef] [Green Version]
- CLSI. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically. In Proceedings of the Approved Standard, 10th ed.; CLSI document M07-A10; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2015; pp. 1–87. [Google Scholar]
- ASTM. Standard E96/E96M Standard Test Methods for Water Vapor Transmission of Material. In Proceedings of the ASTM International; ASTM International: West Conshohocken, PA, USA, 2012. [Google Scholar]
- ASTM. Standard D882-09 Standard Test Method for Tensile Properties of Thin Plastic Sheeting. In Proceedings of the ASTM International; ASTM International: West Conshohocken, PA, USA, 2012. [Google Scholar]
- Gontard, N.; Guilbert, S. Bio-packaging: Technology and properties of edible and/or biodegradable material of agricultural origin. In Food Packaging and Preservation; Springer: Boston, MA, USA, 1994; pp. 159–181. [Google Scholar]
- Martin, P.G. Manuals of Food Quality Control; FAO: Rome, Italy, 1997; ISBN 925102412X.
- APHA. Compendium of Methods for the Microbiological Examination of Foods, 2nd ed.; Speck, M.L., Ed.; American Public Health Association: Washington, DC, USA, 1984. [Google Scholar]
- Sahin, S.; Samli, R.; Birteks, Z.; Tan, A.S.; Barba, F.J.; Chemat, F.; Cravotto, G.; Lorenzo, J.M. Solvent-free microwave-assisted extraction of polyphenols from olive tree leaves: Antioxidant and antimicrobial properties. Molecules 2017, 22, 1056. [Google Scholar] [CrossRef] [Green Version]
- Moudache, M.; Colon, M.; Nerín, C.; Zaidi, F. Phenolic content and antioxidant activity of olive by-products and antioxidant film containing olive leaf extract. Food Chem. 2016, 212, 521–527. [Google Scholar] [CrossRef]
- Japón-Luján, R.; Luque-Rodríguez, J.M.; de Luque, M.D.C. Multivariate optimisation of the microwave-assisted extraction of oleuropein and related biophenols from olive leaves. Anal. Bioanal. Chem. 2006, 385, 753–759. [Google Scholar] [CrossRef]
- Khemakhem, I.; Gargouri, O.D.; Dhouib, A.; Ayadi, M.A.; Bouaziz, M. Oleuropein rich extract from olive leaves by combining microfiltration, ultrafiltration and nanofiltration. Sep. Purif. Technol. 2017, 172, 310–317. [Google Scholar] [CrossRef]
- Jemai, H.; Bouaziz, M.; Fki, I.; El, A.; Sayadi, S. Hypolipidimic and antioxidant activities of oleuropein and its hydrolysis derivative-rich extracts from Chemlali olive leaves. Chem. Biol. Interact. 2008, 176, 88–98. [Google Scholar] [CrossRef] [PubMed]
- Ansari, M.; Kazemipour, M.; Fathi, S. Development of a Simple Green Extraction Procedure and HPLC Method for Determination of Oleuropein in Olive Leaf Extract Applied to a Multi-Source Comparative Study. J. Iran. Chem. Soc. 2011, 8, 38–47. [Google Scholar] [CrossRef]
- Pereira, A.P.; Ferreira, I.C.F.R.; Marcelino, F.; Valentão, P.; Andrade, P.B.; Seabra, R.; Estevinho, L.; Bento, A.; Pereira, J.A. Phenolic Compounds and Antimicrobial Activity of Olive (Olea europaea L. Cv. Cobrançosa) Leaves. Molecules 2007, 12, 1153–1162. [Google Scholar] [CrossRef]
- Rhim, J.W. Physical-Mechanical Properties of Agar/k-Carrageenan Blend Film and Derived Clay Nanocomposite Film. J. Food Sci. 2012, 77. [Google Scholar] [CrossRef] [PubMed]
- Du, W.X.; Olsen, C.W.; Avena-Bustillos, R.J.; McHugh, T.H.; Levin, C.E.; Friedman, M. Effects of allspice, cinnamon, and clove bud essential oils in edible apple films on physical properties and antimicrobial activities. J. Food Sci. 2009, 74. [Google Scholar] [CrossRef]
- McHugh, T.H.; Avenabustillos, R.; Krochta, J.M. Hydrophilic Edible Films—Modified Procedure for Water-Vapor Permeability and Explanation of Thickness Effects. J Food Sci 1993, 58, 899–903. [Google Scholar] [CrossRef]
- Turco, R.; Ortega-Toro, R.; Tesser, R.; Mallardo, S.; Collazo-Bigliardi, S.; Boix, A.C.; Malinconico, M.; Rippa, M.; Di Serio, M.; Santagata, G. Poly (lactic acid)/thermoplastic starch films: Effect of cardoon seed epoxidized oil on their chemicophysical, mechanical, and barrier properties. Coatings 2019, 9, 574. [Google Scholar] [CrossRef] [Green Version]
- Rojas, I.M. Efecto del Tipo y Contenido de Aceites Esenciales Sobre las Propiedades Mecánicas y Barrera de Películas Comestibles Basadas en Zeína. Master’s Thesis, Public University of Navarra, Navarra, Spain, 2010. [Google Scholar]
- Ferracin, R.J.; Filho, A.R.; Marconcini, J.M.; de Diniz, J.H.O. Avaliação das propriedades mecânicas de material polimérico utilizado na confecção de caixas de medição de energia elétrica. Polímeros 2009, 19, 166–176. [Google Scholar] [CrossRef] [Green Version]
- De Campo, C.; Costa, T.M.H.; de Rios, A.O.; Flôres, S.H. Effect of incorporation of nutraceutical capsule waste of safflower oil in the mechanical characteristics of corn starch films. Food Sci. Technol. 2016, 36, 33–36. [Google Scholar] [CrossRef] [Green Version]
- Endres, H.-J.; Siebert-Raths, A. End-of-Life Options for Biopolymers. In Engineering Biopolymers; Hanser Publishers: Munich, Germany, 2011. [Google Scholar]
- Paula, G.A.; Benevides, N.M.B.; Cunha, A.P.; de Oliveira, A.V.; Pinto, A.M.B.; Morais, J.P.S.; Azeredo, H.M.C. Development and characterization of edible films from mixtures ofκ-carrageenan, Ι-carrageenan, and alginate. Food Hydrocoll. 2015, 47, 140–145. [Google Scholar] [CrossRef]
- Pereda, M.; Amica, G.; Marcovich, N.E. Development and characterization of edible chitosan/olive oil emulsion films. Carbohydr. Polym. 2012, 87, 1318–1325. [Google Scholar] [CrossRef]
- Zactiti, É.M. Desenvolvimento e Caracterização de Filmes Biodegradáveis de Alginato de Cálcio Sem e Com Sorbato de Potássio. Ph.D. Thesis, Universidade Estadual de Campinas, Campinas, Brazil, 2004. [Google Scholar]
- Shojaee-Aliabadi, S.; Hosseini, H.; Mohammadifar, M.A.; Mohammadi, A.; Ghasemlou, M.; Hosseini, S.M.; Khaksar, R. Characterization of κ-carrageenan films incorporated plant essential oils with improved antimicrobial activity. Carbohydr. Polym. 2014, 101, 582–591. [Google Scholar] [CrossRef]
- Al-Sheddy, I.A.; Fung, D.Y.C.; Kastner, C.L. Microbiology of fresh and restructured lamb meat: A review. Crit. Rev. Microbiol. 1995, 21, 31–52. [Google Scholar] [CrossRef]
- EFSA BIOHAZ Panel (EFSA Panel on Biological Hazards). Scientific opinion on the growth of spoilage bacteria during storage and transport of meat. EFSA J. 2016, 14, 38. [Google Scholar] [CrossRef]
- Karabagias, I.; Badeka, A.; Kontominas, M.G. Shelf life extension of lamb meat using thyme or oregano essential oils and modified atmosphere packaging. Meat Sci. 2011, 88, 109–116. [Google Scholar] [CrossRef]
- Jancikova, S.; Dordevic, D.; Jamroz, E.; Behalova, H.; Tremlova, B. Chemical and physical characteristics of edible films, based on κ- And ι-carrageenans with the addition of lapacho tea extract. Foods 2020, 9, 357. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saleh, E.; Morshdy, A.E.; El-Manakhly, E.; Al-rashed, S.; Hetta, H.F.; Jeandet, P.; Yahia, R.; Batiha, G.E.; Ali, E. Effects of Olive Leaf Extracts as Natural Preservative on Retailed Poultry Meat Quality. Foods 2020, 9, 1017. [Google Scholar] [CrossRef] [PubMed]

| Physical Properties | CAR-C | CAR-OLE |
|---|---|---|
| Thickness (mm) | 0.032 ± 0.004 a | 0.048 ± 0.004 b |
| WVP (g∙m−1∙s−1∙Pa−1) | 6.61 × 10−11 ± 1.6 × 10−12 a | 7.43 × 10−11 ± 9.1 × 10−13 b |
| Elongation at break (%) | 29.21 ± 0.12 a | 36.58 ± 1.70 b |
| Tensile strength (MPa) | 11.83 ± 0.23 a | 8.51 ± 0.09 b |
| Elastic modulus (MPa) | 40.50 ± 0.97 a | 23.34 ± 1.33 b |
| Solubility (%) | 82.60 ± 3.47 a | 76.60 ± 0.33 a |
| Optical Properties | CAR-C | CAR-OLE |
| L* | 94.49 ± 0.21 a | 71.41 ± 0.92 b |
| a* | −0.145 ± 0.03 a | 7.78 ± 0.43 b |
| b* | 3.32 ± 0.31 a | 63.26 ± 1.59 b |
| ΔE | - | 64.72 |
| Samples Packed Using Different Films | Initial (CFU∙g−1) | 2 Days of Storage (CFU∙g−1) |
|---|---|---|
| Fresh lamb meat | (1.10 ± 5.66) × 105 | - |
| CAR-C | - | (27.6 ± 8.66 a) × 105 |
| CAR-OLE | - | (5.51 ± 7.62 b) × 105 |
| PVC film | - | (23.9 ± 7.78 c) × 105 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martiny, T.R.; Raghavan, V.; Moraes, C.C.d.; Rosa, G.S.d.; Dotto, G.L. Bio-Based Active Packaging: Carrageenan Film with Olive Leaf Extract for Lamb Meat Preservation. Foods 2020, 9, 1759. https://doi.org/10.3390/foods9121759
Martiny TR, Raghavan V, Moraes CCd, Rosa GSd, Dotto GL. Bio-Based Active Packaging: Carrageenan Film with Olive Leaf Extract for Lamb Meat Preservation. Foods. 2020; 9(12):1759. https://doi.org/10.3390/foods9121759
Chicago/Turabian StyleMartiny, Thamiris Renata, Vijaya Raghavan, Caroline Costa de Moraes, Gabriela Silveira da Rosa, and Guilherme Luiz Dotto. 2020. "Bio-Based Active Packaging: Carrageenan Film with Olive Leaf Extract for Lamb Meat Preservation" Foods 9, no. 12: 1759. https://doi.org/10.3390/foods9121759







