Improving Stability and Accessibility of Quercetin in Olive Oil-in-Soy Protein Isolate/Pectin Stabilized O/W Emulsion
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Emulsion Preparation
2.3. Microstructure of Emulsion
2.4. Zeta-Potential
2.5. Rheology Analysis
2.6. Freeze-Thaw Protocol
2.7. Creaming Index
2.8. FT-IR Analysis
2.9. Measurement of Quercetin Encapsulation Efficiency
2.10. In Vitro Gastrointestinal Digestion
2.11. Statistical Analysis
3. Results and Discussion
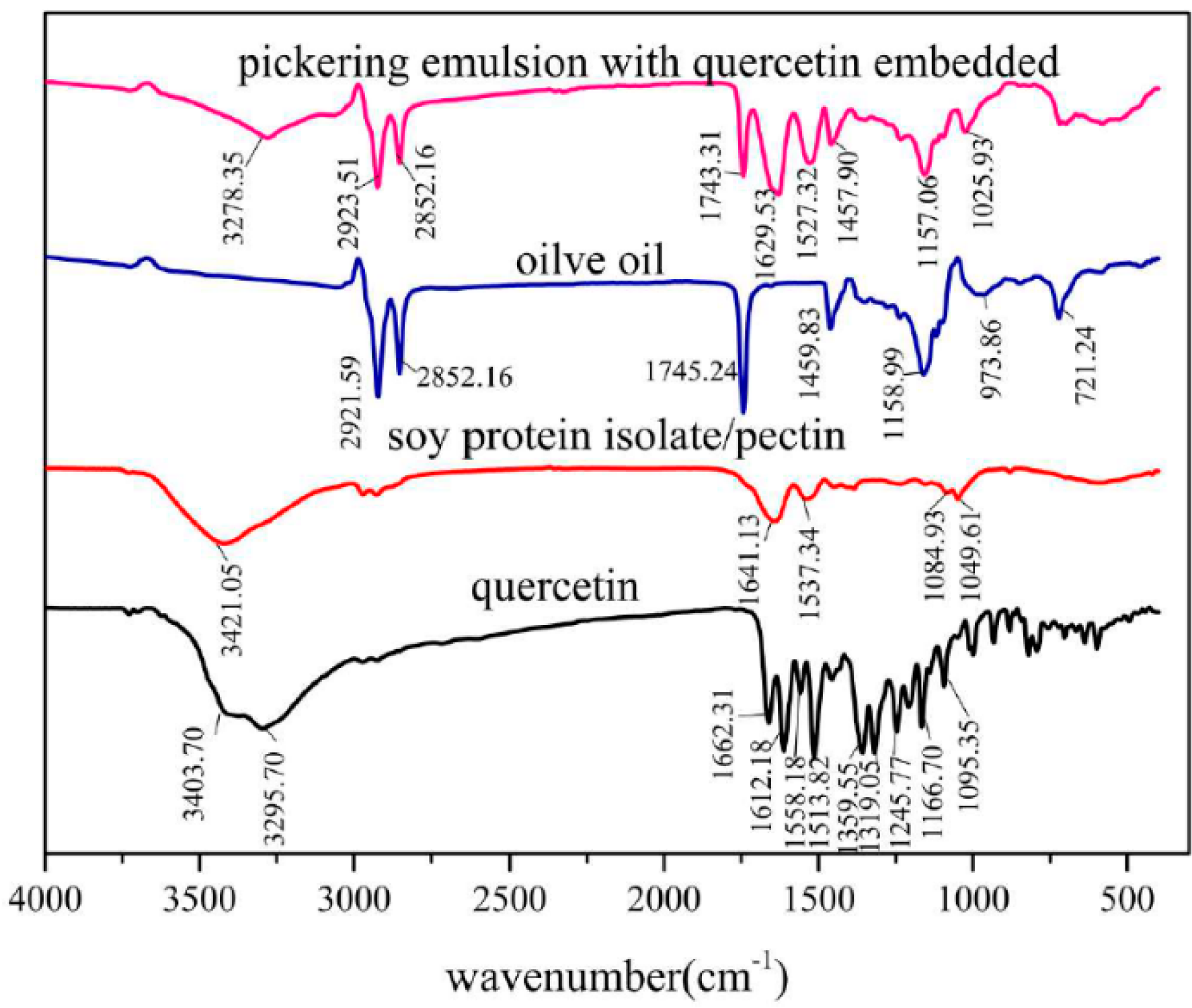
3.1. FT-IR Spectrometer Analysis of SPI/Pectin Complex and Olive oil-SPI/Pectin Emulsion
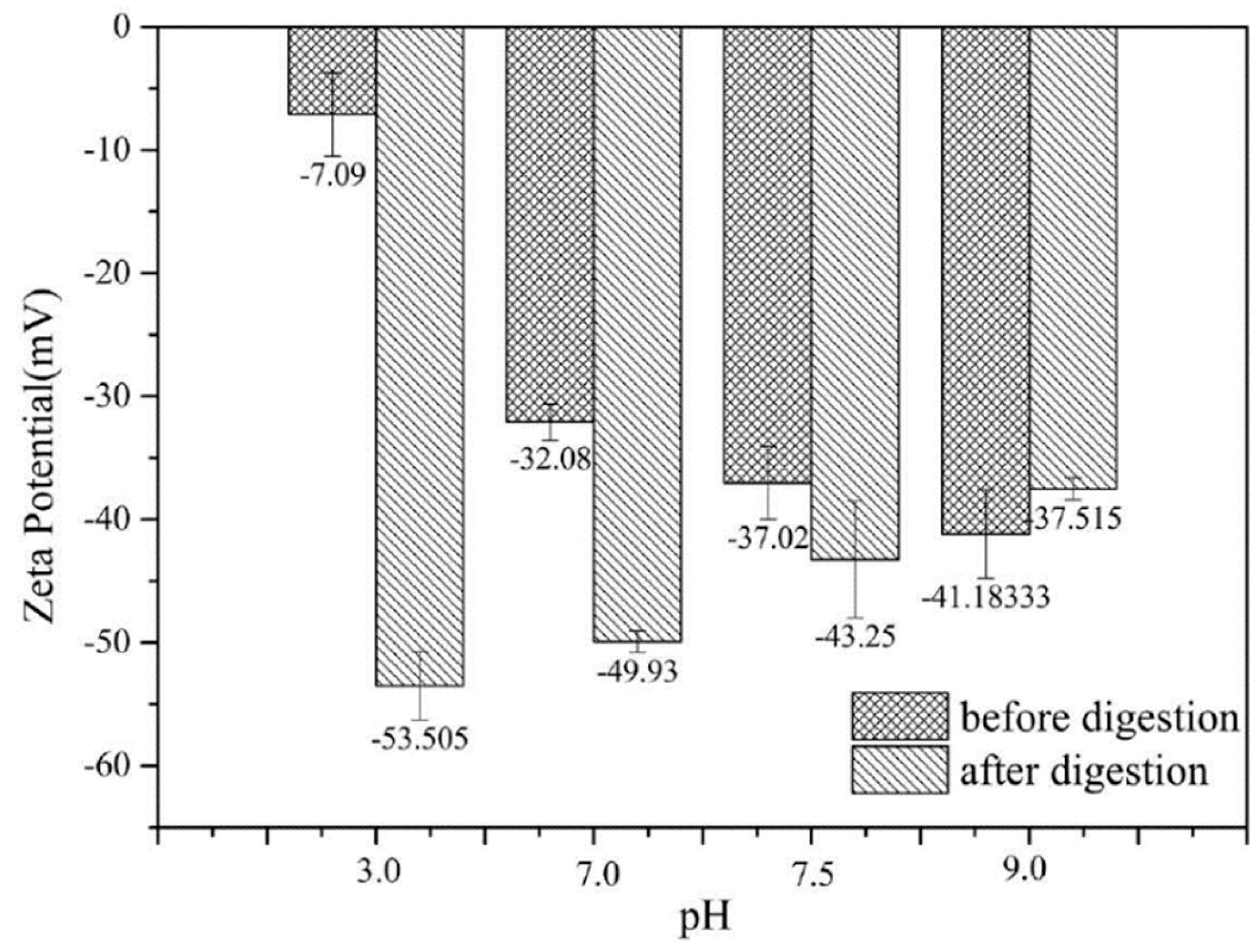
3.2. Zeta-Potential of SPI/Pectin Complex and Olive Oil-SPI/Pectin Emulsion.
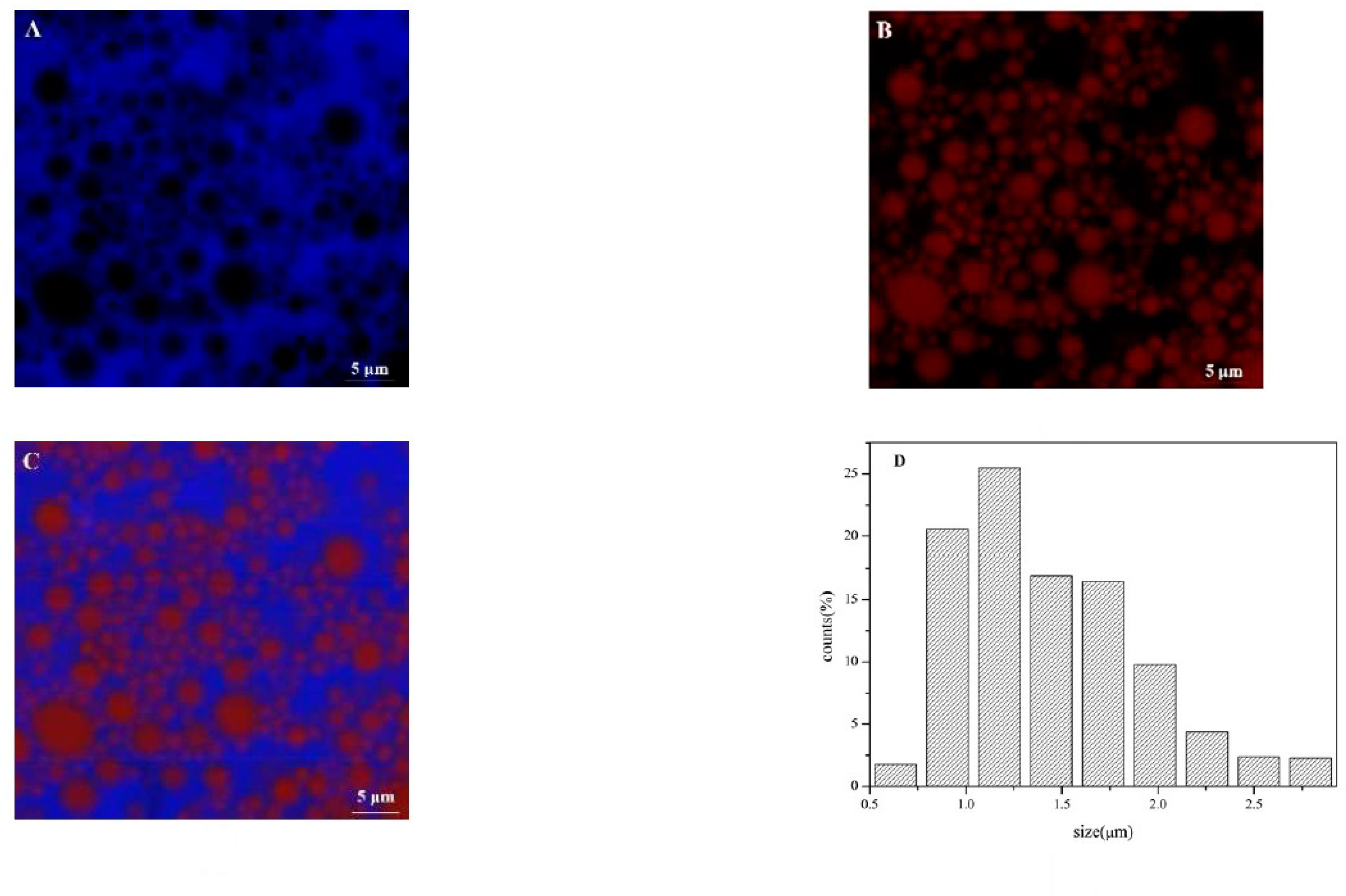
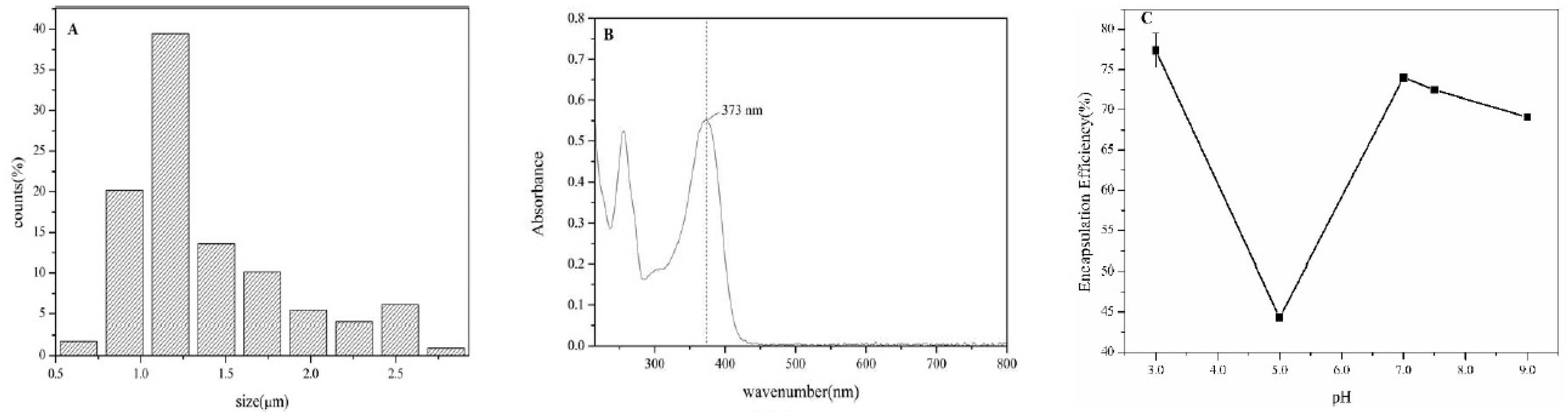
3.3. Microstructure and Particle Size Distribution of Olive oil-SPI/Pectin Emulsion
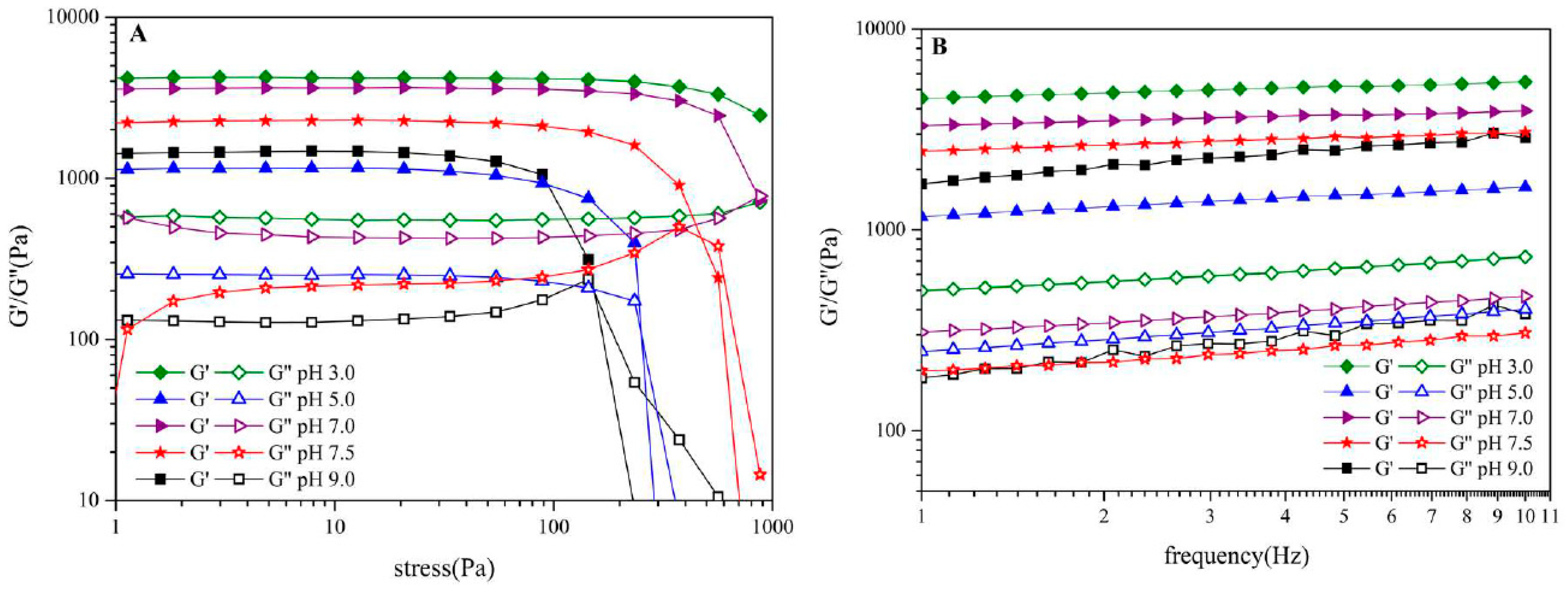
3.4. Rheological Behavior of Oil-SPI/Pectin Emulsion
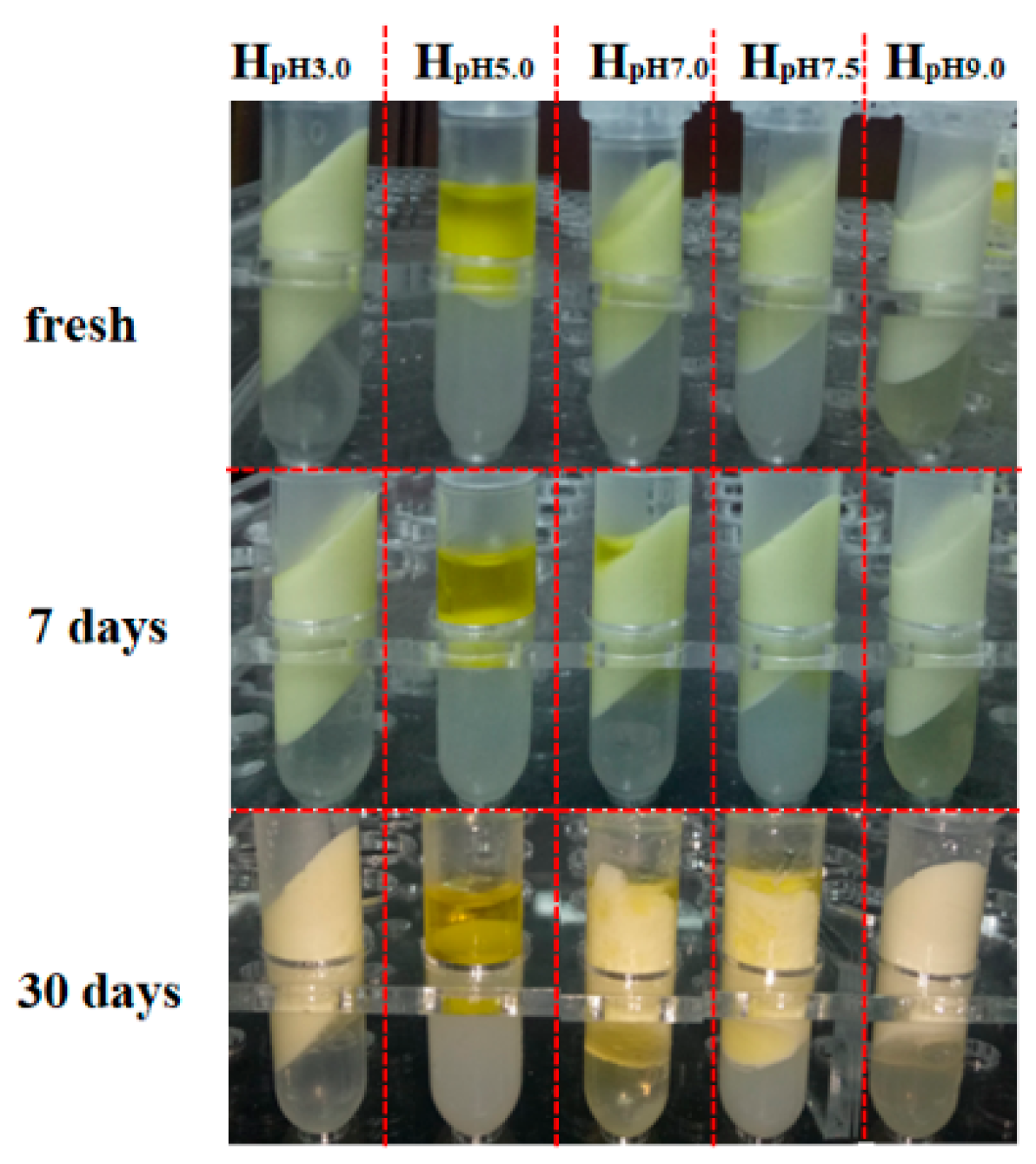
3.5. Storage Stability and Freeze-Thaw Stability of Olive Oil-SPI/Pectin Emulsion
3.6. FT-IR Spectrometer Analysis of Olive Oil-SPI/Pectin Emulsion with Embedded Quercetin
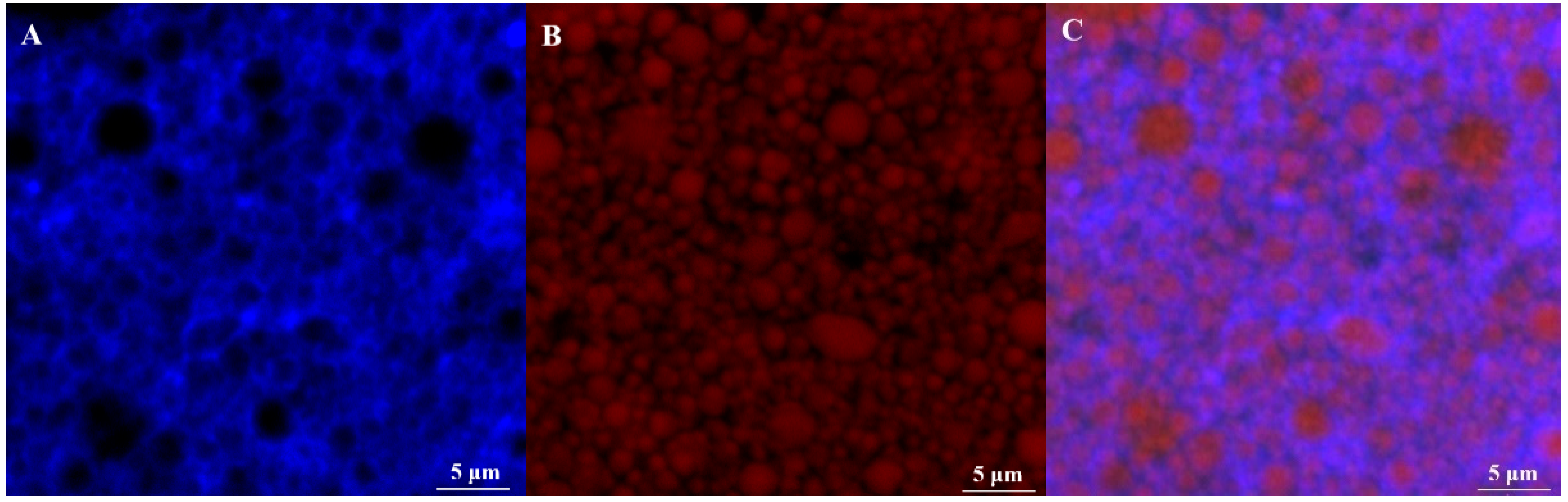
3.7. Microstructure and Encapsulation Efficiency of Olive Oil-SPI/Pectin Emulsions with Embedded Quercetin
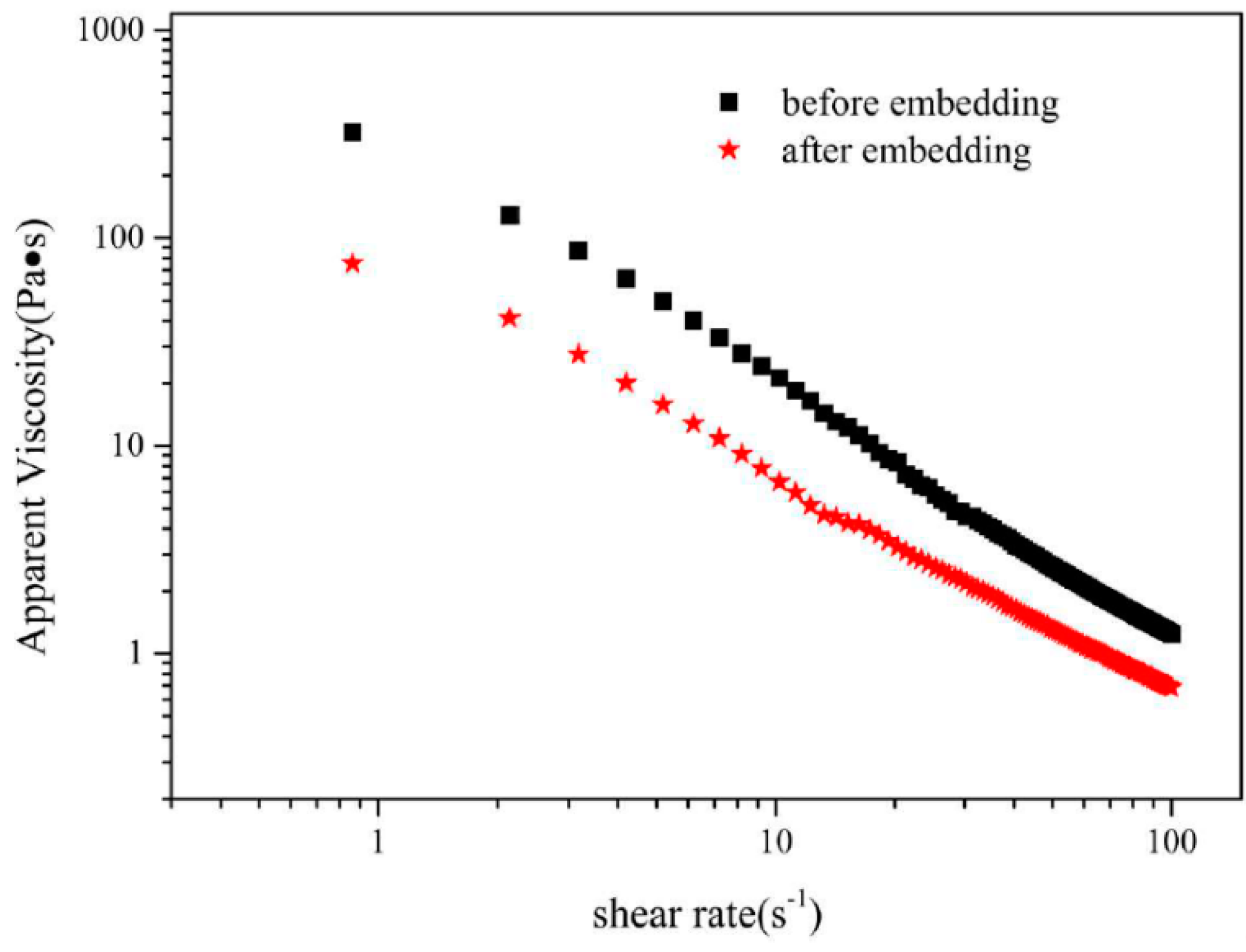
3.8. Rheological Behavior of Olive Oil-SPI/Pectin Emulsion with Embedded Quercetin
3.9. Bioavailability Analysis of Quercetin Stabilized by Olive Oil-SPI/Pectin Emulsion
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Hollman, P.C.; van Trijp, J.M.; Buysman, M.N.; van der Gaag, M.S.; Mengelers, M.J.; de Vries, J.H.; Katan, M.B. Relative bioavailability of the antioxidant flavonoid quercetin from various foods in man. FEBS Lett. 1997, 418, 152–156. [Google Scholar] [CrossRef] [Green Version]
- Erlund, I. Review of the flavonoids quercetin, hesperetin, and naringenin. Dietary sources, bioactivities, bioavailability, and epidemiology. Nutr. Res. 2004, 24, 851–874. [Google Scholar] [CrossRef]
- Murakami, A.; Ashida, H.; Terao, J. Multitargeted cancer prevention by quercetin. Cancer Lett. 2008, 269, 315–325. [Google Scholar] [CrossRef] [PubMed]
- Wijaya, W.; Harfieyanto, R.C.; Dewettinck, K.; Patel, A.R.; Van der Meeren, P. Whey protein isolate-low methoxyl pectin nanocomplexes improve physicochemical and stability properties of quercetin in a model fat-free beverage. Food Funct. 2019, 10, 986–996. [Google Scholar] [CrossRef]
- Fang, R.; Hao, R.F.; Wu, X.; Li, Q.; Leng, X.J.; Jing, H. Bovine serum albumin nanoparticle promotes the stability of quercetin in simulated intestinal fluid. J. Agric. Food Chem. 2011, 59, 6292–6298. [Google Scholar] [CrossRef]
- Wang, Y.F.; Wang, X.Y. Binding, stability, and antioxidant activity of quercetin with soy protein isolate particles. Food Chem. 2015, 188, 24–29. [Google Scholar] [CrossRef]
- Farrell, T.L.; Gomez-Juaristi, M.; Poquet, L.; Redeuil, K.; Nagy, K.; Renouf, M.; Williamson, G. Absorption of dimethoxycinnamic acid derivatives in vitro and pharmacokinetic profile in human plasma following coffee consumption. Mol. Nutr. Food Res. 2012, 56, 1413–1423. [Google Scholar] [CrossRef] [Green Version]
- Pandey, S.K.; Patel, D.K.; Thakur, R.; Mishra, D.P.; Maiti, P.; Haldar, C. Anti-cancer evaluation of quercetin embedded PLA nanoparticles synthesized by emulsified nanoprecipitation. Int. J. Biol. Macromol. 2015, 75, 521–529. [Google Scholar] [CrossRef]
- Chen, X.; McClements, D.J.; Zhu, Y.Q.; Chen, Y.; Zou, L.Q.; Liu, W.; Cheng, C.; Fu, D.W.; Liu, C.M. Enhancement of the solubility, stability and bioaccessibility of quercetin using protein-based excipient emulsions. Food Res. Int. 2018, 114, 30–37. [Google Scholar] [CrossRef]
- Jullian, C.; Moyano, L.; Yanez, C.; Olea-Azar, C. Complexation of quercetin with three kinds of cyclodextrins: An antioxidant study. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2007, 67, 230–234. [Google Scholar] [CrossRef]
- Aytac, Z.; Kusku, S.I.; Durgun, E.; Uyar, T. Quercetin/beta-cyclodextrin inclusion complex embedded nanofibres: Slow release and high solubility. Food Chem. 2016, 197, 864–871. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.B.; Wang, X.Y. Spectrometric study on the interaction of sodium cholate aggregates with quercetin. Colloids Surf. A Physicochem. Eng. Asp. 2015, 481, 31–37. [Google Scholar] [CrossRef]
- Aveyard, R.; Binks, B.P.; Clint, J.H. Emulsions stabilised solely by colloidal particles. Adv. Colloid Interface Sci. 2003, 100–102, 503–546. [Google Scholar] [CrossRef]
- Chevalier, Y.; Bolzinger, M.-A. Emulsions stabilized with solid nanoparticles: Pickering emulsions. Colloids Surf. A Physicochem. Eng. Asp. 2013, 439, 23–34. [Google Scholar] [CrossRef]
- Yukuyama, M.N.; Ghisleni, D.D.M.; Pinto, T.J.A.; Bou-Chacra, N.A. Nanoemulsion: Process selection and application in cosmetics—A review. Int. J. Cosmet. Sci. 2016, 38, 13–24. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.J.; Hu, Y.Q.; Yin, S.W.; Yang, X.Q.; Lai, F.R.; Wang, S.Q. Fabrication and characterization of antioxidant pickering emulsions stabilized by zein/chitosan complex particles (ZCPs). J. Agric. Food Chem. 2015, 63, 2514–2524. [Google Scholar] [CrossRef]
- Chen, J.S.; Dickinson, E.; Langton, M.; Hermansson, A.-M. Mechanical Properties and Microstructure of Heat-set Whey Protein Emulsion Gels: Effect of Emulsifiers. LWT Food Sci. Technol. 2000, 33, 299–307. [Google Scholar] [CrossRef]
- Caporaso, N.; Genovese, A.; Burke, R.; Barry-Ryan, C.; Sacchi, R. Effect of olive mill wastewater phenolic extract, whey protein isolate and xanthan gum on the behaviour of olive O/W emulsions using response surface methodology. Food Hydrocoll. 2016, 61, 66–76. [Google Scholar] [CrossRef] [Green Version]
- Chen, L.; Chen, J.S.; Yu, L.; Wu, K.G. Improved emulsifying capabilities of hydrolysates of soy protein isolate pretreated with high pressure microfluidization. LWT Food Sci. Technol. 2016, 69, 1–8. [Google Scholar] [CrossRef]
- Pham, M.; Mintz, E.A.; Nguyen, T.H. Deposition kinetics of bacteriophage MS2 to natural organic matter: Role of divalent cations. J. Colloid Interface Sci. 2009, 338, 1–9. [Google Scholar] [CrossRef]
- Lo, W.M.Y.; Farnworth, E.R.; Li-Chan, E.C.Y. Angiotensin I-Converting Enzyme Inhibitory Activity of Soy Protein Digests in a Dynamic Model System Simulating the Upper Gastrointestinal Tract. J. Food Sci. 2006, 71, 231–237. [Google Scholar] [CrossRef]
- Li, Z.F.; Ming, T.; Wang, J.F.; Ngai, T. High internal phase emulsions stabilized solely by microgel particles. Angew. Chem. Int. Ed. Engl. 2009, 48, 8490–8493. [Google Scholar] [CrossRef] [PubMed]
- Hu, M.; McClements, D.J.; Decker, E.A. Lipid Oxidation in Corn Oil-in-Water Emulsions Stabilized by Casein, Whey Protein Isolate, and Soy Protein Isolate. J. Agric. Food Chem. 2003, 51, 1696–1700. [Google Scholar] [CrossRef] [PubMed]
- Molina, E.; Papadopoulou, D.A.; Ledward, D.A. Emulsifying properties of high pressure treated soy protein isolate and 7S and 11S globulins. Food Hydrocoll. 2001, 15, 263–269. [Google Scholar] [CrossRef]
- Chove, B.E.; Grandison, A.S.; Lewis, M.J. Emulsifying properties of soy protein isolate fractions obtained by isoelectric precipitation. J. Sci. Food Agric. 2001, 81, 759–763. [Google Scholar] [CrossRef]
- Diftis, N.; Kiosseoglou, V. Competitive adsorption between a dry-heated soy protein–dextran mixture and surface-active materials in oil-in-water emulsions. Food Hydrocoll. 2004, 18, 639–646. [Google Scholar] [CrossRef]
- Tang, C.H. Emulsifying properties of soy proteins: A critical review with emphasis on the role of conformational flexibility. Crit. Rev. Food Sci. Nutr. 2017, 57, 2636–2679. [Google Scholar] [CrossRef]
- Roudsari, M.; Nakamura, A.; Smith, A.; Corredig, M. Stabilizing Behavior of Soy Soluble Polysaccharide or High Methoxyl Pectin in Soy Protein Isolate Emulsions at Low pH. J. Agric. Food Chem. 2006, 54, 1434–1441. [Google Scholar] [CrossRef]
- Cerda, J.J.; Robbins, F.L.; Burgin, C.W.; Baumgartner, T.G.; Rice, R.W. The Effects of Grapefruit Pectin on Patients at Risk for Coronary Heart Disease Without Altering Diet or Lifestyle. Clin. Cardiol. 1988, 11, 589–594. [Google Scholar] [CrossRef]
- Smistad, G.; Bøyum, S.; Alund, S.J.; Samuelsen, A.B.C.; Hiorth, M. The potential of pectin as a stabilizer for liposomal drug delivery systems. Carbohydr. Polym. 2012, 90, 1337–1344. [Google Scholar] [CrossRef]
- Huang, P.H.; Lu, H.T.; Wang, Y.T.; Wu, M.C. Antioxidant activity and emulsion-stabilizing effect of pectic enzyme treated pectin in soy protein isolate-stabilized oil/water emulsion. J. Agric. Food Chem. 2011, 59, 9623–9628. [Google Scholar] [CrossRef] [PubMed]
- Ngouémazong, E.D.; Christiaens, S.; Shpigelman, A.; Van Loey, A.; Hendrickx, M. The Emulsifying and Emulsion-Stabilizing Properties of Pectin: A Review. Compr. Rev. Food Sci. Food Saf. 2015, 14, 705–718. [Google Scholar] [CrossRef]
- Khalid, N.; Kobayashi, I.; Neves, M.A.; Uemura, K.; Nakajima, M.; Nabetani, H. Microchannel emulsification study on formulation and stability characterization of monodisperse oil-in-water emulsions encapsulating quercetin. Food Chem. 2016, 212, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Paglarini, C.S.; Martini, S.; Pollonio, M.A.R. Physical properties of emulsion gels formulated with sonicated soy protein isolate. Int. J. Food Sci. Technol. 2019, 54, 451–459. [Google Scholar] [CrossRef]
- Wang, Q.; Xie, Y.; Johnson, D.R.; Li, Y.; He, Z.; Li, H. Ultrasonic-pretreated lipase-catalyzed synthesis of medium–long–medium lipids using different fatty acids as sn-2 acyl-site donors. Food Sci. Nutr. 2019, 7, 2361–2373. [Google Scholar] [CrossRef] [Green Version]
- Taha, A.; Ahmed, E.; Hu, T.; Xu, X.Y.; Pan, S.Y.; Hu, H. Effects of different ionic strengths on the physicochemical properties of plant and animal proteins-stabilized emulsions fabricated using ultrasound emulsification. Ultrason. Sonochem. 2019, 58, 104627. [Google Scholar] [CrossRef]
- Taha, A.; Hu, T.; Zhang, Z.; Bakry, A.M.; Khalifa, I.; Pan, S.Y.; Hu, H. Effect of different oils and ultrasound emulsification conditions on the physicochemical properties of emulsions stabilized by soy protein isolate. Ultrason. Sonochem. 2018, 49, 283–293. [Google Scholar] [CrossRef]
- O’Sullivan, J.; Murray, B.; Flynn, C.; Norton, I. The effect of ultrasound treatment on the structural, physical and emulsifying properties of animal and vegetable proteins. Food Hydrocoll. 2016, 53, 141–154. [Google Scholar] [CrossRef] [Green Version]
- Chen, J.; Chen, W.T.; Duan, F.X.; Tang, Q.X.; Li, X.; Zeng, L.; Zhang, J.Q.; Xing, Z.H.; Dong, Y.; Jia, L.R.; et al. The synergistic gelation of okra polysaccharides with kappa-carrageenan and its influence on gel rheology, texture behaviour and microstructures. Food Hydrocoll. 2019, 87, 425–435. [Google Scholar] [CrossRef]
- Huang, M.G.; Liang, C.P.; Tan, C.; Huang, S.; Ying, R.F.; Wang, Y.S.; Wang, Z.J.; Zhang, Y.F. Liposome co-encapsulation as a strategy for the delivery of curcumin and resveratrol. Food Funct. 2019, 10, 6447–6458. [Google Scholar] [CrossRef]
- Farhat, I.A.; Orset, S.; Moreau, P.; Blanshard, J.M.V. FTIR Study of Hydration Phenomena in Protein-Sugar System. J. Colloid Interface Sci. 1998, 207, 200–208. [Google Scholar] [CrossRef] [PubMed]
- Xie, C.J.; Wang, Q.; Ying, R.F.; Wang, Y.S.; Wang, Z.J.; Huang, M.G. Binding a chondroitin sulfate-based nanocomplex with kappa-carrageenan to enhance the stability of anthocyanins. Food Hydrocoll. 2020, 100, 105448. [Google Scholar] [CrossRef]
- Yang, F.; Niu, Q.; Lan, Q.; Sun, D.J. Effect of dispersion pH on the formation and stability of Pickering emulsions stabilized by layered double hydroxides particles. J. Colloid Interface Sci. 2007, 306, 285–295. [Google Scholar] [CrossRef] [PubMed]



| Simulated Digestion | In Vitro Gastrointestinal Digestion | ||||
|---|---|---|---|---|---|
| Samples | Pure Olive Oil | HQpH3.0 | HQpH7.0 | HQpH7.5 | HQpH9.0 |
| Release rate (%) of free fatty acids | 29.40 | 16.35 | 15.40 | 13.50 | 11.75 |
| Before Digestion | After Digestion | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Samples | Pure Oil | HQpH3.0 | HQpH7.0 | HQpH7.5 | HQpH9.0 | Pure Oil | HQpH3.0 | HQpH7.0 | HQpH7.5 | HQpH9.0 |
| Retention rate of quercetin% | 100.00 | 77.39 | 74.01 | 72.50 | 69.10 | 98.25 | 69.59 | 58.52 | 66.80 | 66.33 |
| Bioavailability% | - | - | - | - | - | 1.75 | 7.80 | 15.49 | 5.70 | 2.77 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Q.; Wei, H.; Deng, C.; Xie, C.; Huang, M.; Zheng, F. Improving Stability and Accessibility of Quercetin in Olive Oil-in-Soy Protein Isolate/Pectin Stabilized O/W Emulsion. Foods 2020, 9, 123. https://doi.org/10.3390/foods9020123
Wang Q, Wei H, Deng C, Xie C, Huang M, Zheng F. Improving Stability and Accessibility of Quercetin in Olive Oil-in-Soy Protein Isolate/Pectin Stabilized O/W Emulsion. Foods. 2020; 9(2):123. https://doi.org/10.3390/foods9020123
Chicago/Turabian StyleWang, Qiang, Huaheng Wei, Chaofang Deng, Chenjing Xie, Meigui Huang, and Fuping Zheng. 2020. "Improving Stability and Accessibility of Quercetin in Olive Oil-in-Soy Protein Isolate/Pectin Stabilized O/W Emulsion" Foods 9, no. 2: 123. https://doi.org/10.3390/foods9020123





