Polyphenolic-Protein-Polysaccharide Complexes from Hovenia dulcis: Insights into Extraction Methods on Their Physicochemical Properties and In Vitro Bioactivities
Abstract
:1. Introduction
2. Materials and Methods
2.1. Material and Chemicals
2.2. Extraction of Polyphenolic-Protein-Polysaccharide Complexes (PPPs) by Different Methods
2.2.1. Hot Water Extraction
2.2.2. Pressurized Water Extraction
2.2.3. Ultrasound-Assisted Extraction
2.2.4. Microwave-Assisted Extraction
2.2.5. Ultrasound-Assisted Enzymatic Extraction
2.2.6. Ultrasound-Microwave-Assisted Extraction
2.2.7. High-Speed Shearing Homogenization Extraction
2.3. Physicochemical Characterization of PPPs
2.3.1. Analysis of Chemical Compositions
2.3.2. Determination of Molecular Weights, Apparent Viscosities, Monosaccharide Compositions, and Amino Acid Compositions
2.3.3. Fourier Transform Infrared (FT-IR) Spectroscopy Analysis
2.3.4. Identification of Phenolic Compounds
2.4. Evaluation of In Vitro Bioactivities of PPPs
2.4.1. In Vitro Antioxidant Activities
2.4.2. In Vitro Antiglycation Activities
2.4.3. In Vitro α-Amylase and α-Glucosidase Inhibitory Activities
2.5. Statistical Analysis
3. Results and Discussions
3.1. Physicochemical Characteristics of PPPs
3.1.1. Chemical Compositions
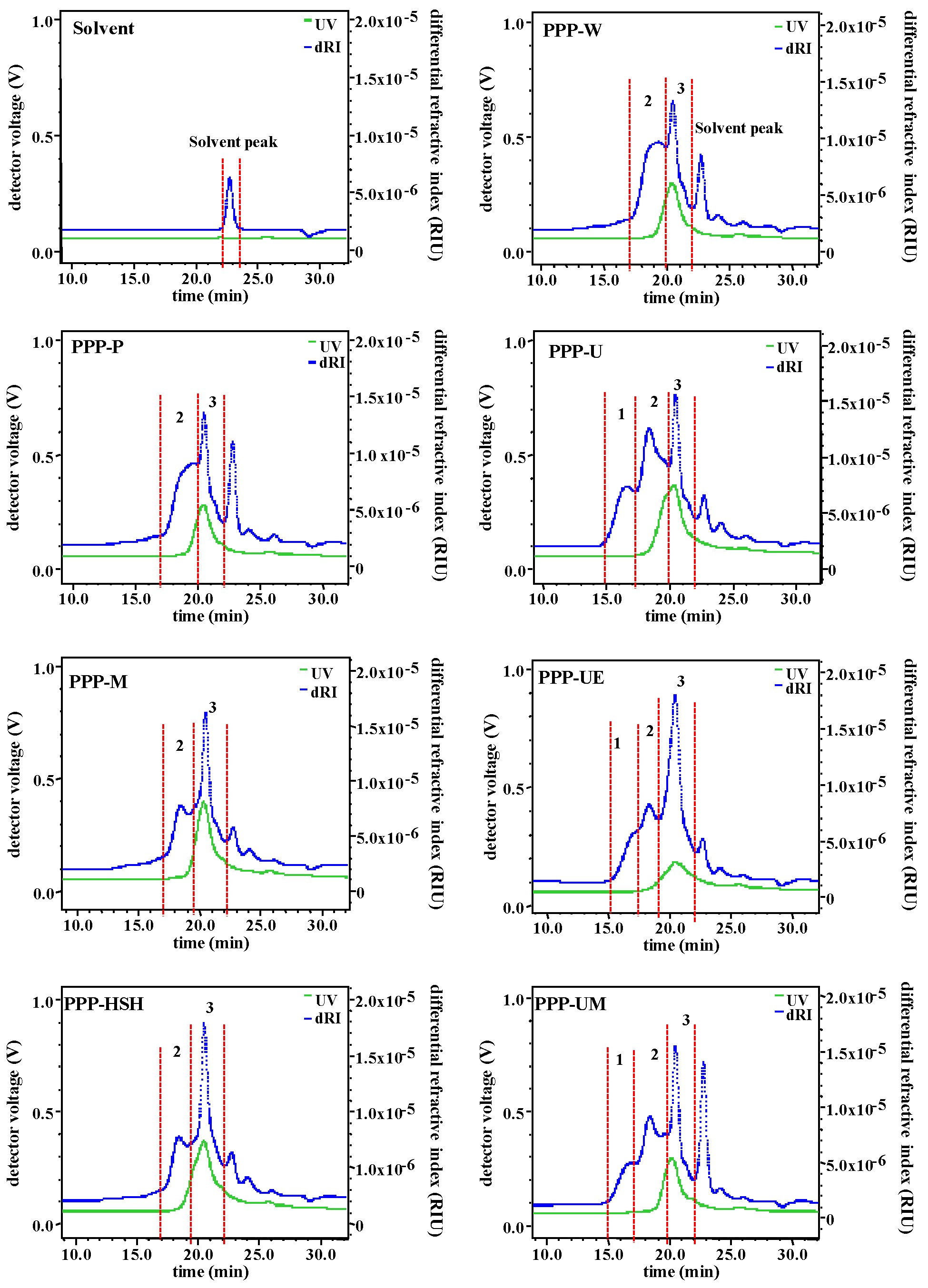
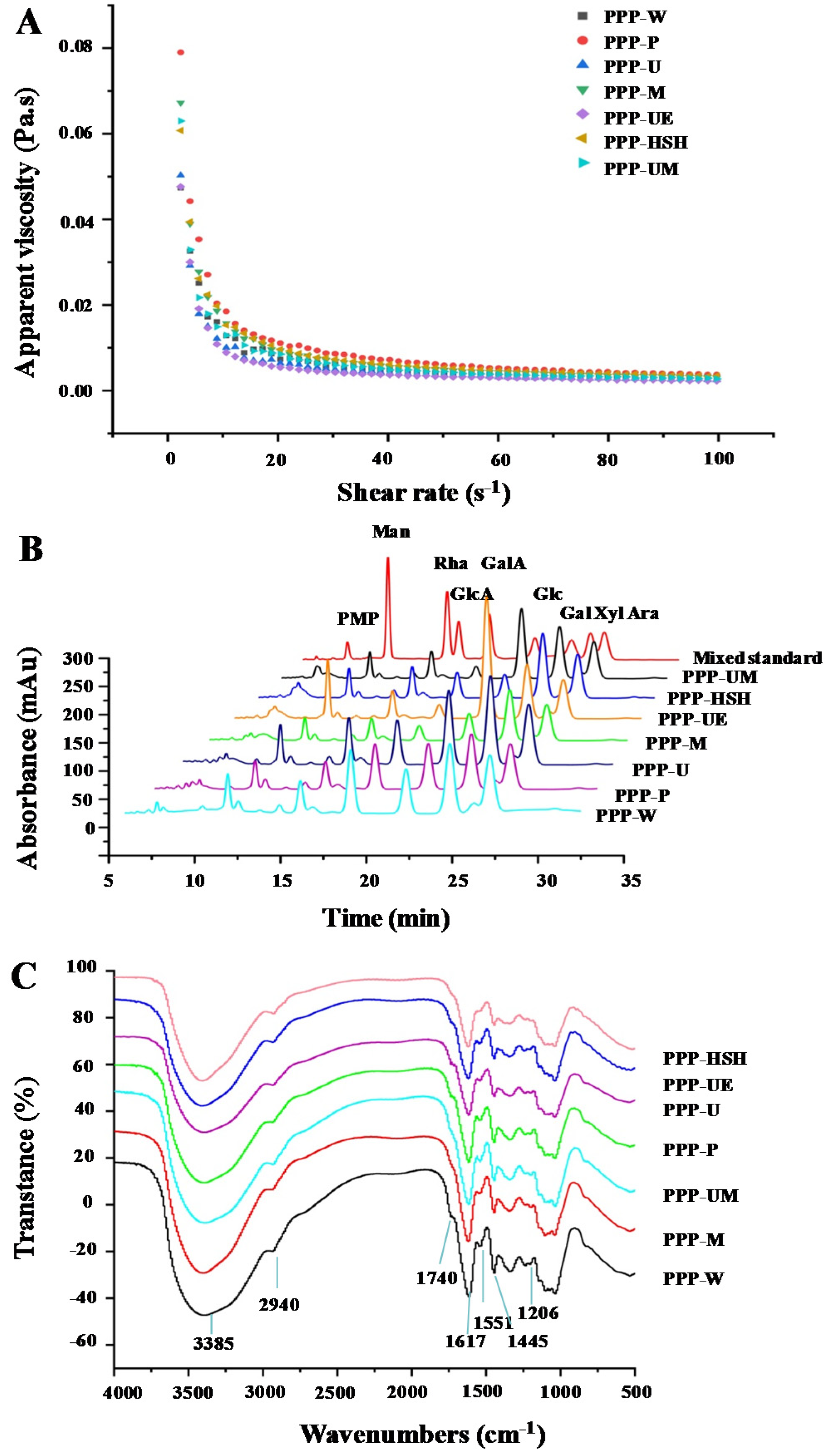
3.1.2. Molecular Weights, Apparent Viscosities, and Monosaccharide Compositions
3.1.3. Amino Acid Compositions and Phenolic Compositions
3.1.4. FT-IR Spectra
3.2. Impacts of Extraction Methods on the In Vitro Bioactivities of PPPs
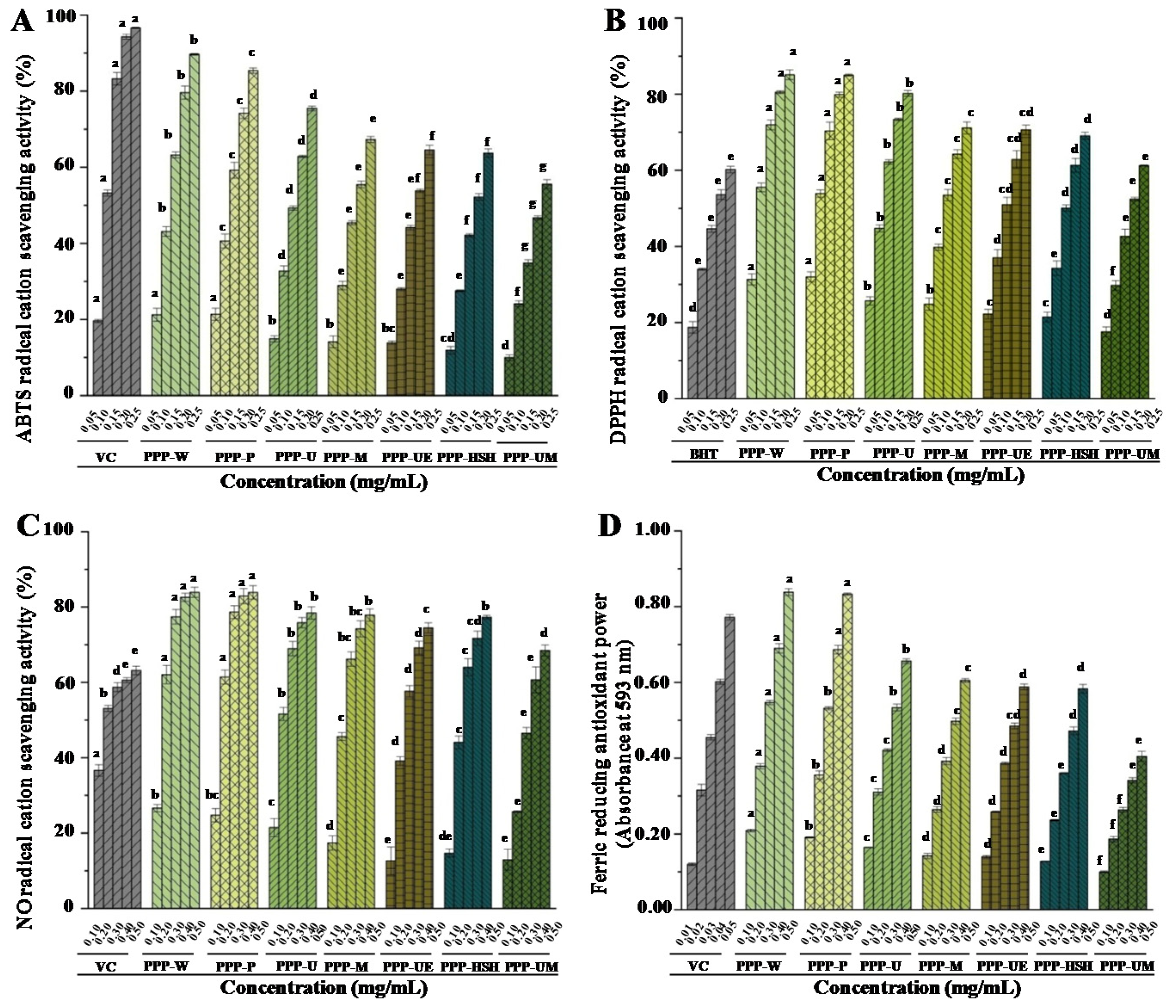
3.2.1. In Vitro Antioxidant Activities
3.2.2. In Vitro Antiglycation Activities
3.2.3. In Vitro Inhibitory Activities on α-Amylase and α-Glucosidase
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Sellimi, S.; Benslima, A.; Barragan-Montero, V.; Hajji, M.; Nasri, M. Polyphenolic-protein-polysaccharide ternary conjugates from Cystoseira barbata Tunisian seaweed as potential biopreservatives: Chemical, antioxidant and antimicrobial properties. Int. J. Biol. Macromol. 2017, 105, 1375–1383. [Google Scholar] [CrossRef] [PubMed]
- Šutovská, M.; Capek, P.; Kocmálová, M.; Fraňová, S.; Pawlaczyk, I.; Gancarz, R. Characterization and biological activity of Solidago canadensis complex. Int. J. Biol. Macromol. 2013, 52, 192–197. [Google Scholar] [CrossRef] [PubMed]
- Šutovská, M.; Capek, P.; Fraňová, S.; Pawlaczyk, I.; Gancarz, R. Antitussive and bronchodilatory effects of Lythrum salicaria polysaccharide-polyphenolic conjugate. Int. J. Biol. Macromol. 2012, 51, 794–799. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.C.; Zhu, P.L.; Jiang, C.X.; Ma, L.P.; Zhang, Z.J.; Zeng, X.X. Preliminary characterization, antioxidant activity in vitro and hepatoprotective effect on acute alcohol-induced liver injury in mice of polysaccharides from the peduncles of Hovenia dulcis. Food Chem. Toxicol. 2012, 50, 2964–2970. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Wu, Q.J.; Luo, Y.X.; Yang, Q.; Wei, X.Y.; Kan, J.Q. High-pressure ultrasonic-assisted extraction of polysaccharides from Hovenia dulcis: Extraction, structure, antioxidant activity and hypoglycemic. Int. J. Biol. Macromol. 2019, 137, 676–687. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Li, F.; Wang, P.; Liu, X.; He, J.J.; Xian, M.L.; Zhao, L.; Qin, W.; Gan, R.Y.; Wu, D.T. Effects of drying methods on the physicochemical characteristics and bioactivities of polyphenolic-protein-polysaccharide conjugates from Hovenia dulcis. Int. J. Biol. Macromol. 2020, 148, 1211–1221. [Google Scholar] [CrossRef]
- Wang, M.M.; Liu, Y.; Qiang, M.L.; Wang, J.H. Structural elucidation of a pectin-type polysaccharide from Hovenia dulcis peduncles and its proliferative activity on RAW264.7 cells. Int. J. Biol. Macromol. 2017, 104, 1246–1253. [Google Scholar] [CrossRef]
- Liu, Y.; Qiang, M.L.; Sun, Z.G.; Du, Y.Q. Optimization of ultrasonic extraction of polysaccharides from Hovenia dulcis peduncles and their antioxidant potential. Int. J. Biol. Macromol. 2015, 80, 350–357. [Google Scholar] [CrossRef]
- Liu, Y.; Cai, B.J.; Qiang, M.L. Characterization and bioactivities of polysaccharide from spent Hovenia dulcis peduncles by alkali pretreatment. Int. J. Food Prop. 2017, 20, S416–S429. [Google Scholar] [CrossRef] [Green Version]
- Yang, B.; Wu, Q.J.; Luo, Y.X.; Yang, Q.; Chen, G.J.; Wei, X.Y.; Kan, J.Q. Japanese grape (Hovenia dulcis) polysaccharides: New insight into extraction, characterization, rheological properties, and bioactivities. Int. J. Biol. Macromol. 2019, 134, 631–644. [Google Scholar] [CrossRef]
- Meng, Y.H.; Su, A.P.; Yuan, S.; Zhao, H.G.; Tan, S.Y.; Hu, C.Y.; Deng, H.; Guo, Y.R. Evaluation of total flavonoids, myricetin, and quercetin from Hovenia dulcis Thunb. as inhibitors of α-amylase andα-glucosidase. Plant Foods Hum. Nutr. 2016, 71, 444–449. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.C.; Jiang, C.X.; Ma, L.P.; Zhang, Z.J.; Cao, L.; Liu, J.; Zeng, X.X. Preparation, preliminary characterization and immunostimulatory activity of polysaccharide fractions from the peduncles of Hovenia dulcis. Food Chem. 2013, 138, 41–47. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Yan, X.T.; Liang, J.; Li, S.J.; He, H.R.; Xiong, Q.P.; Lai, X.P.; Hou, S.Z.; Huang, S. Comparison of different extraction methods for polysaccharides from Dendrobium officinale stem. Carbohydr. Polym. 2018, 198, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Bai, R.Y.; Liu, Y.P.; Zhang, X.; Kan, J.; Jin, C.H. Isolation, structural characterization and bioactivities of naturally occurring polysaccharide-polyphenolic conjugates from medicinal plants-A reivew. Int. J. Biol. Macromol. 2018, 107, 2242–2250. [Google Scholar] [CrossRef]
- Pawlaczyk-Graja, I.; Balicki, S.; Wilk, K.A. Effect of various extraction methods on the structure of polyphenolic-polysaccharide conjugates from Fragaria vesca L. leaf. Int. J. Biol. Macromol. 2019, 130, 664–674. [Google Scholar] [CrossRef]
- Wu, D.T.; Liu, W.; Han, Q.H.; Wang, P.; Xiang, X.R.; Ding, Y.; Zhao, L.; Zhang, Q.; Li, S.Q.; Wen, Q. Extraction optimization, structural characterization, and antioxidant activities of polysaccharides from cassia seed (Cassia obtusifolia). Molecules 2019, 24, 2817. [Google Scholar] [CrossRef] [Green Version]
- Yuan, Q.; Lin, S.; Fu, Y.; Nie, X.R.; Liu, W.; Su, Y.; Han, Q.H.; Zhao, L.; Zhang, Q.; Lin, D.R.; et al. Effects of extraction methods on the physicochemical characteristics and biological activities of polysaccharides from okra (Abelmoschus esculentus). Int. J. Biol. Macromol. 2019, 127, 178–186. [Google Scholar] [CrossRef]
- Xu, Y.Q.; Cai, F.; Yu, Z.Y.; Zhang, L.; Li, X.G.; Yang, Y.; Liu, G.J. Optimisation of pressurised water extraction of polysaccharides from blackcurrant and its antioxidant activity. Food Chem. 2016, 194, 650–658. [Google Scholar] [CrossRef]
- Han, Q.H.; Liu, W.; Li, H.Y.; He, J.L.; Guo, H.; Lin, S.; Zhao, L.; Chen, H.; Liu, Y.W.; Wu, D.T.; et al. Extraction optimization, physicochemical characteristics, and antioxidant activities of polysaccharides from kiwifruit (Actinidia chinensis Planch.). Molecules 2019, 24, 461. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.B.; Li, T.F.; Liu, F.C.; Liu, D.W.; Xu, Y.Q.; Yang, Y.; Zhao, Y.B.; Wei, H. Ultrasonic-assisted enzymatic extraction and characterization of polysaccharides from dandelion (Taraxacum officinale) leaves. Int. J. Biol. Macromol. 2019, 126, 846–856. [Google Scholar] [CrossRef]
- Xiao, Y.H.; Liu, S.C.; Shen, M.Y.; Jiang, L.; Ren, Y.M.; Luo, Y.; Wen, H.L.; Xie, J.H. Physicochemical, rheological and thermal properties of Mesona chinensis polysaccharides obtained by sodium carbonate assisted and cellulase assisted extraction. Int. J. Biol. Macromol. 2019, 126, 30–36. [Google Scholar] [CrossRef] [PubMed]
- Gharibzahedi, S.M.T.; Smith, B.; Guo, Y. Pectin extraction from common fig skin by different methods: The physicochemical, rheological, functional, and structural evaluations. Int. J. Biol. Macromol. 2019, 136, 275–283. [Google Scholar] [CrossRef] [PubMed]
- Gharibzahedi, S.M.T.; Smith, B.; Guo, Y. Ultrasound-microwave assisted extraction of pectin from fig (Ficus carica L.) skin: Optimization, characterization and bioactivity. Carbohydr. Polym. 2019, 222, 114992. [Google Scholar] [CrossRef]
- Guo, H.; Yuan, Q.; Fu, Y.; Liu, W.; Su, Y.H.; Liu, H.; Wu, C.Y.; Zhao, L.; Zhang, Q.; Lin, D.R.; et al. Extraction optimization and effects of extraction methods on the chemical structures and antioxidant activities of polysaccharides from snow chrysanthemum (Coreopsis tinctoria). Polymers 2019, 11, 215. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Su, D.L.; Li, P.J.; Quek, S.Y.; Huang, Z.Q.; Yuan, Y.J.; Li, G.Y.; Shan, Y. Efficient extraction and characterization of pectin from orange peel by a combined surfactant and microwave assisted process. Food Chem. 2019, 286, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Zhang, A.Q.; Deng, J.Y.; Liu, X.Q.; He, P.F.; He, L.; Zhang, F.M.; Linhardt, R.J.; Sun, P.L. Structure and conformation of alpha-glucan extracted from Agaricus blazei Murill by high-speed shearing homogenization. Int. J. Biol. Macromol. 2018, 113, 558–564. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.F.; Zhao, W.T.; Liao, X.J.; Hu, X.S.; Wu, J.H.; Wang, X. Extraction of pectin from the peels of pomelo by high-speed shearing homogenization and its characteristics. Lwt-Food Sci. Technol. 2017, 79, 640–646. [Google Scholar] [CrossRef]
- Wu, D.T.; Liu, W.; Han, Q.H.; Du, G.; Li, H.Y.; Yuan, Q.; Fu, Y.; Zhao, L.; Zhang, Q.; Li, S.Q.; et al. Physicochemical characteristics and antioxidant activities of non-starch polysaccharides from different kiwifruits. Int. J. Biol. Macromol. 2019, 136, 891–900. [Google Scholar] [CrossRef]
- Lin, S.; Guo, H.; Gong, J.D.B.; Lu, M.; Lu, M.Y.; Wang, L.; Zhang, Q.; Qin, W.; Wu, D.T. Phenolic profiles, β-glucan contents, and antioxidant capacities of colored Qingke (Tibetan hulless barley) cultivars. J. Cereal Sci. 2018, 81, 69–75. [Google Scholar] [CrossRef]
- Nie, X.R.; Li, H.Y.; Du, G.; Lin, S.; Hu, R.; Li, H.Y.; Zhao, L.; Zhang, Q.; Chen, H.; Wu, D.T.; et al. Structural characteristics, rheological properties, and biological activities of polysaccharides from different cultivars of okra (Abelmoschus esculentus) collected in China. Int. J. Biol. Macromol. 2019, 139, 459–467. [Google Scholar] [CrossRef]
- Chen, W.J.; Lv, R.L.; Muhammad, A.I.; Guo, M.M.; Ding, T.; Ye, X.Q.; Liu, D.H. Fabrication of (-)-epigallocatechin-3-gallate carrier based on glycosylated whey protein isolate obtained by ultrasound Maillard reaction. Ultrason. Sonochem. 2019, 58, 104678. [Google Scholar] [CrossRef] [PubMed]
- Renard, C.M.G.C.; Watrelot, A.A.; Le Bourvellec, C. Interactions between polyphenols and polysaccharides: Mechanisms and consequences in food processing and digestion. Trends Food Sci. Technol. 2017, 60, 43–51. [Google Scholar] [CrossRef]
- Wu, D.T.; Guo, H.; Lin, S.; Lam, S.C.; Zhao, L.; Lin, D.R.; Qin, W. Review of the structural characterization, quality evaluation, and industrial application of Lycium barbarum polysaccharides. Trends Food Sci. Technol. 2018, 79, 171–183. [Google Scholar] [CrossRef]
- Wang, W.J.; Ma, X.B.; Jiang, P.; Hu, L.; Zhi, Z.J.; Chen, J.L.; Ding, T.; Ye, X.Q.; Liu, D.H. Characterization of pectin from grapefruit peel: A comparison of ultrasound-assisted and conventional heating extractions. Food Hydrocoll. 2016, 61, 730–739. [Google Scholar] [CrossRef]
- Fu, Y.; Yuan, Q.; Lin, S.; Liu, W.; Du, G.; Zhao, L.; Zhang, Q.; Lin, D.R.; Liu, Y.T.; Qin, W.; et al. Physicochemical characteristics and biological activities of polysaccharides from the leaves of different loquat (Eriobotrya japonica) cultivars. Int. J. Biol. Macromol. 2019, 135, 274–281. [Google Scholar] [CrossRef]
- Bijak, M.; Saluk, J.; Tsirigotis-Maniecka, M.; Komorowska, H.; Wachowicz, B.; Zaczyńska, E.; Czarny, A.; Czechowski, F.; Nowak, P.; Pawlaczyk, I. The influence of conjugates isolated from Matricaria chamomilla L. on platelets activity and cytotoxicity. Int. J. Biol. Macromol. 2013, 61, 218–229. [Google Scholar] [CrossRef]
- Yan, J.K.; Wu, L.X.; Qiao, Z.R.; Cai, W.D.; Ma, H.L. Effect of different drying methods on the product quality and bioactive polysaccharides of bitter gourd (Momordica charantia L.) slices. Food Chem. 2019, 271, 588–596. [Google Scholar] [CrossRef]
- Yan, J.K.; Wang, W.Q.; Wu, J.Y. Recent advances in Cordyceps sinensis polysaccharides: Mycelial fermentation, isolation, structure, and bioactivities: A review. J. Funct. Foods 2014, 6, 33–47. [Google Scholar] [CrossRef]
- Wang, J.Q.; Hu, S.Z.; Nie, S.P.; Yu, Q.; Xie, M.Y. Reviews on mechanisms of in vitro antioxidant activity of polysaccharides. Oxidative Med. Cell. Longev. 2016, 2016, 5692852. [Google Scholar] [CrossRef] [Green Version]
- Li, X.M.; Li, X.L.; Zhou, A.G. Evaluation of antioxidant activity of the polysaccharides extracted from Lycium barbarum fruits in vitro. Eur. Polym. J. 2007, 43, 488–497. [Google Scholar] [CrossRef]
- Plumb, G.W.; de Pascual-Teresa, S.; Santos-Buelga, C.; Rivas-Gonzalo, J.C.; Williamson, G. Antioxidant properties of gallocatechin and prodelphinidins from pomegranate peel. Redox Rep. 2002, 7, 41–46. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rydlewski, A.A.; de Morais, D.R.; Rotta, E.M.; Claus, T.; Vagula, J.M.; da Silva, M.C.; Santos Junior, O.O.; Visentainer, J.V. Bioactive compounds, antioxidant capacity, and fatty acids in different parts of four unexplored fruits. J. Food Qual. 2017, 2017, 8401074. [Google Scholar] [CrossRef] [Green Version]
- Park, J.S.; Kim, I.S.; Shaheed Ur, R.; Na, C.S.; Yoo, H.H. HPLC determination of bioactive flavonoids in Hovenia dulcis fruit extracts. J. Chromatogr. Sci. 2016, 54, 130–135. [Google Scholar] [PubMed]
- Boots, A.W.; Haenen, G.R.M.M.; Bast, A. Health effects of quercetin: From antioxidant to nutraceutical. Eur. J. Pharmacol. 2008, 585, 325–337. [Google Scholar] [CrossRef] [PubMed]
- Leopoldini, M.; Russo, N.; Toscano, M. The molecular basis of working mechanism of natural polyphenolic antioxidants. Food Chem. 2011, 125, 288–306. [Google Scholar] [CrossRef]
- Lee, A.L.; Yu, Y.P.; Hsieh, J.F.; Kuo, M.I.; Ma, Y.S.; Lu, C.P. Effect of germination on composition profiling and antioxidant activity of the polysaccharide-protein conjugate in black soybean [Glycine max (L.) Merr.]. Int. J. Biol. Macromol. 2018, 113, 601–606. [Google Scholar] [CrossRef]
- Chen, Y.; Xie, M.Y.; Nie, S.P.; Li, C.; Wang, Y.X. Purification, composition analysis and antioxidant activity of a polysaccharide from the fruiting bodies of Ganoderma atrum. Food Chem. 2008, 107, 231–241. [Google Scholar] [CrossRef]
- Zhang, L.S.; Wang, X.; Dong, L.L. Antioxidation and antiglycation of polysaccharides from Misgurnus anguillicaudatus. Food Chem. 2011, 124, 183–187. [Google Scholar] [CrossRef]
- Zhu, R.G.; Zhang, X.Y.; Wang, Y.; Zhang, L.J.; Zhao, J.; Chen, G.; Fan, J.G.; Jia, Y.F.; Yan, F.W.; Ning, C. Characterization of polysaccharide fractions from fruit of Actinidia arguta and assessment of their antioxidant and antiglycated activities. Carbohydr. Polym. 2019, 210, 73–84. [Google Scholar] [CrossRef]
- Starowicz, M.; Zieliński, H. Inhibition of advanced glycation end-product formation by high antioxidant-leveled spices commonly used in european cuisine. Antioxidants 2019, 8, 100. [Google Scholar] [CrossRef] [Green Version]
- Yuan, Q.; He, Y.; Xiang, P.Y.; Huang, Y.J.; Cao, Z.W.; Shen, S.W.; Zhao, L.; Zhang, Q.; Qin, W.; Wu, D.T. Influences of different drying methods on the structural characteristics and multiple bioactivities of polysaccharides from okra (Abelmoschus esculentus). Int. J. Biol. Macromol. 2020, 147, 1053–1063. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.Y.; Ai, Z.Y.; Qu, F.F.; Chen, Y.Q.; Ni, D.J. Effect of steeping temperature on antioxidant and inhibitory activities of green tea extracts against α-amylase, α-glucosidase and intestinal glucose uptake. Food Chem. 2017, 234, 168–173. [Google Scholar] [CrossRef] [PubMed]
- Espinal-Ruiz, M.; Parada-Alfonso, F.; Restrepo-Sánchez, L.P.; Narváez-Cuenca, C.E. Inhibition of digestive enzyme activities by pectic polysaccharides in model solutions. Bioact. Carbohydr. Diet. Fibre 2014, 4, 27–38. [Google Scholar] [CrossRef]

| Chemical Compositions | PPPs Extracted from the Peduncles of H. dulcis | ||||||
|---|---|---|---|---|---|---|---|
| PPP-W | PPP-P | PPP-U | PPP-UE | PPP-UM | PPP-M | PPP-HSH | |
| Extraction yields (%) | 3.52 ± 0.28 a,b | 3.79 ± 0.22 a | 3.37 ± 0.15 b | 3.50 ± 0.30 a,b | 3.13 ± 0.18 b | 2.16 ± 0.20 c | 2.50 ± 0.16 c |
| Total polysaccharides (%) | 33.34 ± 0.53 b | 40.23 ± 1.09 a | 42.12 ± 1.56 a | 40.88 ± 1.01 a | 29.32 ± 1.08 c | 29.49 ± 1.76 c | 30.43 ± 1.32 c |
| Total uronic acids (%) | 4.95 ± 0.61 a | 4.02 ± 0.98 ab | 4.07 ± 0.69 a,b | 2.83 ± 0.69 bc | 2.91 ± 0.78 b,c | 2.60 ± 0.33 c | 3.08 ± 0.47 b,c |
| Total proteins (%) | 26.75 ± 0.56 a | 25.45 ± 0.94 b | 18.44 ± 0.23 f | 24.36 ± 0.36 c | 19.70 ± 0.49 e | 21.53 ± 0.64 d | 25.73 ± 0.82 a,b |
| Degrees of esterification (%) | 6.43 ± 0.18 b | 6.80 ± 0.21 a | 4.55 ± 0.15 c | 4.29 ± 0.20 c | 4.55 ± 0.22 c | 1.03 ± 0.05 e | 3.74 ± 0.10 d |
| TPC (mg GAE/g) | 277.56 ± 1.80 a (27.76%) | 273.52 ± 2.40 a (27.35%) | 226.92 ± 3.93 b (22.69%) | 189.73 ± 4.25 d (18.97%) | 156.59 ± 2.44 f (15.66%) | 199.03 ± 3.29 c (19.90%) | 179.71 ± 7.58 e (17.97%) |
| TFC (mg RE/g) | 141.10 ± 5.64 a (14.11%) | 139.57 ± 4.18 a (13.96%) | 112.69 ± 2.23 b (11.27%) | 96.71 ± 3.29 c (9.67%) | 80.09 ± 2.08 d (8.01%) | 97.30 ± 1.39 c (9.73%) | 85.63 ± 1.77 d (8.56%) |
| PPPs Extracted from the Peduncles of H. dulcis a | |||||||
|---|---|---|---|---|---|---|---|
| PPP-W | PPP-P | PPP-U | PPP-UE | PPP-UM | PPP-M | PPP-HSH | |
| Mw × 104 (Da, error) | |||||||
| Fraction 1 | - | - | 36.12 (±0.158%) | 27.68 (±0.147%) | 29.06 (±0.164%) | - | - |
| Fraction 2 | 5.077 (±0.303%) | 4.905 (±0.276%) | 7.753 (±0.167%) | 9.113 (±0.122%) | 7.563 (±0.165%) | 9.170 (±0.282%) | 8.474 (±0.610%) |
| Fraction 3 | 0.864 (±1.324%) | 0.794 (±0.990%) | 1.865 (±0.705%) | 1.351 (±0.789%) | 1.078 (±0.705%) | 1.763 (±0.721%) | 1.590 (±0.798%) |
| Mw/Mn | |||||||
| Fraction 1 | - | - | 1.155 (±0.218%) | 1.161 (±0.190%) | 1.156 (±0.217%) | - | - |
| Fraction 2 | 1.658 (±0.554%) | 1.702 (±0.489%) | 1.349 (±0.290%) | 1.076 (±0.177%) | 1.250 (±0.271%) | 1.083 (±0.396%) | 1.305 (±0.907%) |
| Fraction 3 | 1.074 (±1.761%) | 1.074 (±1.333%) | 1.104 (±0.984%) | 1.115 (±1.176%) | 1.154 (±0.986%) | 1.138 (±1.003%) | 1.105 (±1.115%) |
| Monosaccharide compositions (molar ratio) | |||||||
| Galacturonic acid | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| Galactose | 0.93 | 1.06 | 1.74 | 3.43 | 3.77 | 2.94 | 2.16 |
| Arabinose | 0.97 | 1.15 | 1.54 | 3.23 | 3.51 | 2.72 | 1.74 |
| Mannose | 0.43 | 0.29 | 0.42 | 1.95 | 1.07 | 0.69 | 0.55 |
| Rhamnose | 0.48 | 0.57 | 0.98 | 1.80 | 2.03 | 1.38 | 1.14 |
| Glucuronic acid | 0.05 | 0.06 | 0.09 | 0.14 | 0.17 | 0.16 | 0.12 |
| Glucose | 0.76 | 1.10 | 1.84 | 9.74 | 6.42 | 1.90 | 0.96 |
| Xylose | 0.06 | 0.08 | 0.07 | 0.21 | 0.21 | 0.14 | 0.24 |
| Amino Acids | PPPs Extracted from the Peduncles of H. dulcis a | ||||||
|---|---|---|---|---|---|---|---|
| PPP-W (%) | PPP-P (%) | PPP-U (%) | PPP-UE (%) | PPP-UM (%) | PPP-M (%) | PPP-HSH (%) | |
| Aspartic acid | 7.01 | 7.28 | 10.66 | 7.96 | 8.58 | 8.36 | 9.25 |
| Threonine | 4.83 | 4.53 | 7.41 | 5.61 | 6.36 | 6.10 | 9.26 |
| Serine | 6.80 | 7.23 | 8.91 | 7.35 | 8.14 | 8.41 | 8.13 |
| Glutamic acid | 13.39 | 12.98 | 13.82 | 14.81 | 13.45 | 15.77 | 9.19 |
| Proline | 2.54 | 3.10 | 5.45 | 2.99 | 3.50 | 3.06 | 5.04 |
| Glycine | 9.67 | 10.02 | 4.42 | 8.93 | 4.27 | 12.10 | 4.36 |
| Alanine | 9.60 | 9.84 | 9.47 | 9.13 | 9.04 | 11.20 | 8.62 |
| Cystine | 5.43 | 5.67 | 3.90 | 4.98 | 5.11 | 6.55 | 3.62 |
| Valine | 4.07 | 4.04 | 4.65 | 4.45 | 4.44 | 3.36 | 4.06 |
| Isoleucine | 7.16 | 6.57 | 3.07 | 7.05 | 6.69 | 3.40 | 6.41 |
| Leucine | 7.90 | 6.99 | 5.06 | 7.12 | 6.93 | 5.60 | 6.45 |
| Tyrosine | 6.78 | 7.12 | 2.40 | 5.74 | 5.51 | 3.38 | 4.74 |
| Phenylalanine | 4.96 | 4.63 | 3.23 | 4.50 | 4.68 | 3.92 | 3.86 |
| Lysine | 2.54 | 2.36 | 4.99 | 2.45 | 3.73 | 2.10 | 4.81 |
| Histidine | 1.10 | 0.95 | 0.78 | 0.59 | 0.66 | 0.79 | 0.65 |
| Arginine | 6.21 | 6.70 | 11.76 | 6.33 | 8.92 | 5.89 | 11.55 |
| Essential amino acids | 31.46 | 29.12 | 28.41 | 31.18 | 32.83 | 24.48 | 34.85 |
| Non-essential amino acids | 68.54 | 70.88 | 71.59 | 68.82 | 67.17 | 75.52 | 65.15 |
| No. | Retention Time (min) | Formula | Molecular Ion [M-H]− | Error (ppm) | Score (DB) | Score (MFG) | Identification |
|---|---|---|---|---|---|---|---|
| 1 | 1.173 | C7H6O4 | 153.0199 | 3.83 | 97.75 | 97.74 | Protocatechuic acid a,b |
| 2 | 1.688 | C15H14O7 | 305.0675 | 3.48 | 95.23 | 95.27 | Gallocatechin a,b,c |
| 3 | 2.188 | C7H6O3 | 137.0247 | 2.75 | 85.15 | 85.19 | p-Hydroxybenzoic acid a,b |
| 4 | 4.185 | C15H12O8 | 319.0467 | 2.51 | 97.32 | 97.27 | Ampelopsin a,b |
| 5 | 7.130 | C27H30O17 | 625.1430 | 3.00 | 94.51 | 94.49 | Quercetin-7,4′-diglucoside b |
| 6 | 7.729 | C15H12O7 | 303.0522 | 3.76 | 94.51 | 94.60 | Dihydroquercetin b |
| 7 | 8.345 | C27H30O16 | 609.1479 | 2.95 | 93.82 | 93.93 | Rutin a,b,c |
| 8 | 8.645 | C21H20O12 | 463.0894 | 2.46 | 97.09 | 97.06 | Myricitrin b |
| 9 | 10.575 | C15H10O8 | 317.0311 | 2.74 | 96.81 | 96.76 | Myricetin a,b,c |
| 10 | 12.239 | C15H10O7 | 301.0363 | 2.98 | 96.90 | 96.87 | Quercetin a,b,c |
| 11 | 12.256 | C15H10O6 | 285.0409 | 1.45 | 98.98 | 98.99 | Kaempferol a,b,c |
| 12 | 12.306 | C16H12O8 | 331.0466 | 1.99 | 97.94 | 97.90 | 5-Methylmyricetin b |
| 13 | 12.888 | C15H12O5 | 271.0618 | 3.16 | 91.03 | 91.03 | Naringenin a,b |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, D.-T.; Liu, W.; Xian, M.-L.; Du, G.; Liu, X.; He, J.-J.; Wang, P.; Qin, W.; Zhao, L. Polyphenolic-Protein-Polysaccharide Complexes from Hovenia dulcis: Insights into Extraction Methods on Their Physicochemical Properties and In Vitro Bioactivities. Foods 2020, 9, 456. https://doi.org/10.3390/foods9040456
Wu D-T, Liu W, Xian M-L, Du G, Liu X, He J-J, Wang P, Qin W, Zhao L. Polyphenolic-Protein-Polysaccharide Complexes from Hovenia dulcis: Insights into Extraction Methods on Their Physicochemical Properties and In Vitro Bioactivities. Foods. 2020; 9(4):456. https://doi.org/10.3390/foods9040456
Chicago/Turabian StyleWu, Ding-Tao, Wen Liu, Mei-Lin Xian, Gang Du, Xin Liu, Jing-Jing He, Ping Wang, Wen Qin, and Li Zhao. 2020. "Polyphenolic-Protein-Polysaccharide Complexes from Hovenia dulcis: Insights into Extraction Methods on Their Physicochemical Properties and In Vitro Bioactivities" Foods 9, no. 4: 456. https://doi.org/10.3390/foods9040456





