Prenatal Exposure to Ambient PM2.5 and Early Childhood Growth Impairment Risk in East Africa
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area
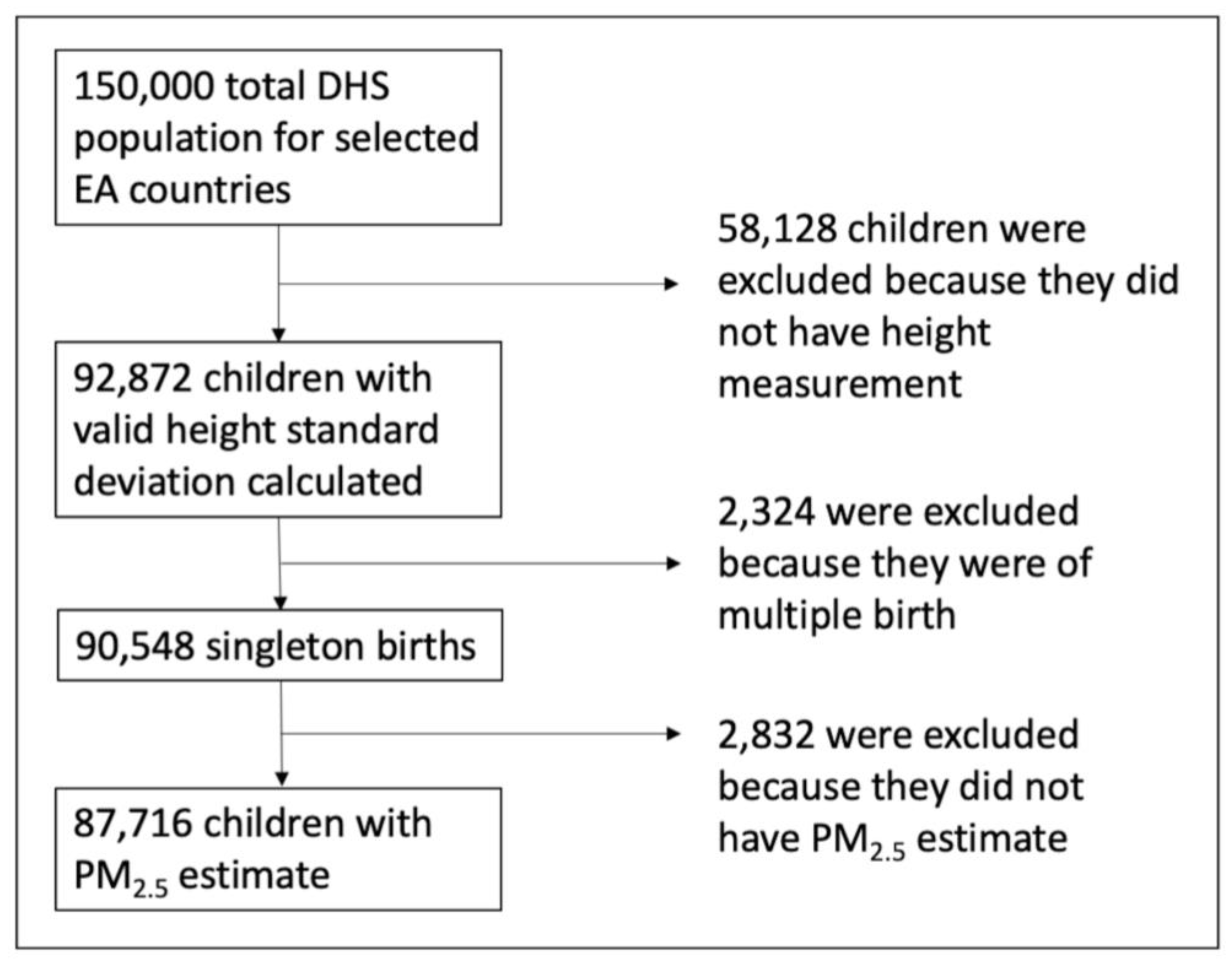
2.2. DHS Survey Data
2.3. Pre- and Postnatal Particulate Matter PM2.5 Exposure and Crop Estimation
2.3.1. Predicted PM2.5 Surfaces
2.3.2. Linkage of PM2.5 Data with DHS Data
2.3.3. Percent Crop Estimates
2.3.4. Prenatal and Postnatal Exposure Estimates
2.4. Statistical Analyses
Variables for Covariate Adjustment
3. Results
3.1. Summary and Descriptive Statistics
3.2. Bivariate Analyses
3.3. Association between Pprenatal PM2.5 Exposure and Height forAge
3.4. Association between Prenatal and Postnatal PM2.5 Exposure and Stunting
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Air Pollution and Child Health: Prescribing Clean Air; Summary; (WHO/CED/PHE/18.01); Licence: CC BY-NC-SA 3.0IGO; World Health Organization: Geneva, Switzerland, 2018. [Google Scholar]
- Monga, C.; Ogunleye, E.K.; Ezanin Koffi, A.M.; Baidoo, T.; Ngong, V. African Development Bank Group East Africa Economic Outlook: 2019; Avenue Joseph Anoma: Abijan, Côte d’Ivoire, 2019. [Google Scholar]
- World Bank Middle Income Countries Overview: Development News, Research, Data. Available online: https://www.worldbank.org/en/country/mic/overview (accessed on 13 July 2022).
- Tesema, G.A.; Teshale, A.B.; Tessema, Z.T. Incidence and predictors of under-five mortality in East Africa using multilevel Weibull regression modeling. Arch. Public Health 2021, 79, 196. [Google Scholar] [CrossRef] [PubMed]
- Fisher, S.; Bellinger, D.C.; Cropper, M.L.; Kumar, P.; Binagwaho, A.; Koudenoukpo, J.B.; Park, Y.; Taghian, G.; Landrigan, P.J. Air pollution and development in Africa: Impacts on health, the economy, and human capital. Lancet Planet. Health 2021, 5, e681–e688. [Google Scholar] [CrossRef]
- Bauer, S.E.; Im, U.; Mezuman, K.; Gao, C.Y. Desert dust, industrialization, and agricultural fires: Health impacts of outdoor air pollution in africa. J. Geophys. Res. Atmos. 2019, 124, 4104–4120. [Google Scholar] [CrossRef]
- Han, L.; Zhou, W.; Li, W. Increasing impact of urban fine particles (PM2.5) on areas surrounding Chinese cities. Sci. Rep. 2015, 5, 12467. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, L.; Wilson, J.P.; MacDonald, B.; Zhang, W.; Yu, T. The changing PM2.5 dynamics of global megacities based on long-term remotely sensed observations. Environ. Int. 2020, 142, 105862. [Google Scholar] [CrossRef]
- US EPA. EPA Health and Environmental Effects of Particulate Matter (PM). Available online: https://www.epa.gov/pm-pollution/health-and-environmental-effects-particulate-matter-pm (accessed on 4 August 2021).
- Wensu, Z.; Wen, C.; Fenfen, Z.; Wenjuan, W.; Li, L. The Association Between Long-Term Exposure to Particulate Matter and Incidence of Hypertension Among Chinese Elderly: A Retrospective Cohort Study. Front. Cardiovasc. Med. 2021, 8, 784800. [Google Scholar] [CrossRef]
- Wang, Y.; Shi, L.; Lee, M.; Liu, P.; Di, Q.; Zanobetti, A.; Schwartz, J.D. Long-term Exposure to PM2.5 and Mortality Among Older Adults in the Southeastern US. Epidemiology 2017, 28, 207–214. [Google Scholar] [CrossRef]
- Xing, Y.-F.; Xu, Y.-H.; Shi, M.-H.; Lian, Y.-X. The impact of PM2.5 on the human respiratory system. J. Thorac. Dis. 2016, 8, E69–E74. [Google Scholar] [CrossRef]
- US EPA; Air Quality Planning Unit. New England How Does PM Affect Human Health? Available online: https://www3.epa.gov/region1/airquality/pm-human-health.html (accessed on 13 October 2022).
- D’Errico, J.N.; Stapleton, P.A. Developmental onset of cardiovascular disease—Could the proof be in the placenta? Microcirculation 2019, 26, e12526. [Google Scholar] [CrossRef] [Green Version]
- Du, Y.; Xu, X.; Chu, M.; Guo, Y.; Wang, J. Air particulate matter and cardiovascular disease: The epidemiological, biomedical and clinical evidence. J. Thorac. Dis. 2016, 8, E8–E19. [Google Scholar] [CrossRef]
- Goyal, N.; Canning, D. Exposure to ambient fine particulate air pollution in utero as a risk factor for child stunting in bangladesh. Int. J. Environ. Res. Public Health 2017, 15, 22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Srám, R.J.; Binková, B.; Dejmek, J.; Bobak, M. Ambient air pollution and pregnancy outcomes: A review of the literature. Environ. Health Perspect. 2005, 113, 375–382. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sinharoy, S.S.; Clasen, T.; Martorell, R. Air pollution and stunting: A missing link? Lancet Glob. Health 2020, 8, e472–e475. [Google Scholar] [CrossRef] [Green Version]
- Ghosh, R.; Causey, K.; Burkart, K.; Wozniak, S.; Cohen, A.; Brauer, M. Ambient and household PM2.5 pollution and adverse perinatal outcomes: A meta-regression and analysis of attributable global burden for 204 countries and territories. PLoS Med. 2021, 18, e1003718. [Google Scholar] [CrossRef]
- Lewit, E.M.; Kerrebrock, N. Population-based growth stunting. Future Child. 1997, 7, 149–156. [Google Scholar] [CrossRef] [Green Version]
- WHO. Malnutrition in Children. Available online: https://www.who.int/data/nutrition/nlis/info/malnutrition-in-children (accessed on 22 September 2022).
- Spears, D.; Dey, S.; Chowdhury, S.; Scovronick, N.; Vyas, S.; Apte, J. The association of early-life exposure to ambient PM2.5 and later-childhood height-for-age in India: An observational study. Environ. Health 2019, 18, 62. [Google Scholar] [CrossRef] [Green Version]
- Tesema, G.A.; Yeshaw, Y.; Worku, M.G.; Tessema, Z.T.; Teshale, A.B. Pooled prevalence and associated factors of chronic undernutrition among under-five children in East Africa: A multilevel analysis. PLoS ONE 2021, 16, e0248637. [Google Scholar] [CrossRef]
- Vilcins, D.; Sly, P.D.; Jagals, P. Environmental Risk Factors Associated with Child Stunting: A Systematic Review of the Literature. Ann. Glob. Health 2018, 84, 551. [Google Scholar] [CrossRef]
- Aghasili, O.U. Fuel Choice, Acute Respiratory Infection and Child Growth in Uganda. Master’s Thesis, Purdue University, West Lafayette, IN, USA, 2015. Volume 564. [Google Scholar]
- Gao, P. The exposome in the era of one health. Environ. Sci. Technol. 2021, 55, 2790–2799. [Google Scholar] [CrossRef]
- DeBord, D.G.; Carreón, T.; Lentz, T.J.; Middendorf, P.J.; Hoover, M.D.; Schulte, P.A. Use of the “Exposome” in the Practice of Epidemiology: A Primer on -Omic Technologies. Am. J. Epidemiol. 2016, 184, 302–314. [Google Scholar] [CrossRef]
- Alok, K. Squatting with Dignity: Lesson from India; SAGE Publications India Pvt Ltd.: New Delhi, India, 2010; ISBN 9788132103059. [Google Scholar]
- Hammer, J.; Spears, D. Village Sanitation and Children’s Human Capital: Evidence from a Randomized Experiment by the Maharashtra Government. In World Bank Policy Research Working Paper No. 6580; World Bank: Washington DC, USA, 2013. [Google Scholar]
- Spears, D.; Ghosh, A.; Cumming, O. Open defecation and childhood stunting in India: An ecological analysis of new data from 112 districts. PLoS ONE 2013, 8, e73784. [Google Scholar] [CrossRef]
- Kartini, A.; Subagio, H.W.; Hadisaputro, S.; Kartasurya, M.I.; Suhartono, S.; Budiyono, B. Pesticide Exposure and Stunting among Children in Agricultural Areas. Int. J. Occup. Environ. Med. 2019, 10, 17–29. [Google Scholar] [CrossRef] [PubMed]
- Coker, E.; Chevrier, J.; Rauch, S.; Bradman, A.; Obida, M.; Crause, M.; Bornman, R.; Eskenazi, B. Association between prenatal exposure to multiple insecticides and child body weight and body composition in the VHEMBE South African birth cohort. Environ. Int. 2018, 113, 122–132. [Google Scholar] [CrossRef] [PubMed]
- Kansiime, W.K.; Mugambe, R.K.; Atusingwize, E.; Wafula, S.T.; Nsereko, V.; Ssekamatte, T.; Nalugya, A.; Coker, E.S.; Ssempebwa, J.C.; Isunju, J.B. Use of biomass fuels predicts indoor particulate matter and carbon monoxide concentrations; evidence from an informal urban settlement in Fort Portal city, Uganda. BMC Public Health 2022, 22, 1723. [Google Scholar] [CrossRef] [PubMed]
- Amegah, A.K.; Quansah, R.; Jaakkola, J.J.K. Household air pollution from solid fuel use and risk of adverse pregnancy outcomes: A systematic review and meta-analysis of the empirical evidence. PLoS ONE 2014, 9, e113920. [Google Scholar] [CrossRef] [PubMed]
- Mishra, V.; Retherford, R.D. Does biofuel smoke contribute to anaemia and stunting in early childhood? Int. J. Epidemiol. 2007, 36, 117–129. [Google Scholar] [CrossRef] [Green Version]
- Tielsch, J.M.; Katz, J.; Thulasiraj, R.D.; Coles, C.L.; Sheeladevi, S.; Yanik, E.L.; Rahmathullah, L. Exposure to indoor biomass fuel and tobacco smoke and risk of adverse reproductive outcomes, mortality, respiratory morbidity and growth among newborn infants in south India. Int. J. Epidemiol. 2009, 38, 1351–1363. [Google Scholar] [CrossRef] [Green Version]
- Kyu, H.H.; Georgiades, K.; Boyle, M.H. Maternal smoking, biofuel smoke exposure and child height-for-age in seven developing countries. Int. J. Epidemiol. 2009, 38, 1342–1350. [Google Scholar] [CrossRef] [Green Version]
- Amadu, I.; Seidu, A.-A.; Duku, E.; Boadu Frimpong, J.; Hagan Jnr, J.E.; Aboagye, R.G.; Ampah, B.; Adu, C.; Ahinkorah, B.O. Risk factors associated with the coexistence of stunting, underweight, and wasting in children under 5 from 31 sub-Saharan African countries. BMJ Open 2021, 11, e052267. [Google Scholar] [CrossRef]
- Brar, S.; Akseer, N.; Sall, M.; Conway, K.; Diouf, I.; Everett, K.; Islam, M.; Sène, P.I.S.; Tasic, H.; Wigle, J.; et al. Drivers of stunting reduction in Senegal: A country case study. Am. J. Clin. Nutr. 2020, 112, 860S–874S. [Google Scholar] [CrossRef]
- Keino, S.; Plasqui, G.; Ettyang, G.; van den Borne, B. Determinants of stunting and overweight among young children and adolescents in sub-Saharan Africa. Food Nutr. Bull. 2014, 35, 167–178. [Google Scholar] [CrossRef] [PubMed]
- Momberg, D.J.; Ngandu, B.C.; Voth-Gaeddert, L.E.; Cardoso Ribeiro, K.; May, J.; Norris, S.A.; Said-Mohamed, R. Water, sanitation and hygiene (WASH) in sub-Saharan Africa and associations with undernutrition, and governance in children under five years of age: A systematic review. J. Dev. Orig. Health Dis. 2021, 12, 6–33. [Google Scholar] [CrossRef] [PubMed]
- Mbuya, M.N.; Chidem, M.; Chasekwa, B.; Mishra, V.K. Biological, social, and environmental determinants of low birth weight and stunting among infants and young children in Zimbabwe. In Zimbabwe Working Papers; DHS: Rockville, MD, USA, 2010. [Google Scholar]
- Rehfuess, E.; Mehta, S.; Prüss-Üstün, A. Assessing Household Solid Fuel Use: Multiple Implications for the Millennium Development Goals. Environ. Health Perspect. 2006, 114, 373–378. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tishkoff, S.A.; Reed, F.A.; Friedlaender, F.R.; Ehret, C.; Ranciaro, A.; Froment, A.; Hirbo, J.B.; Awomoyi, A.A.; Bodo, J.-M.; Doumbo, O.; et al. The genetic structure and history of Africans and African Americans. Science 2009, 324, 1035–1044. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, A.; Ng’ang’a, D.; Gatari, M.J.; Kidane, A.W.; Alemu, Z.A.; Derrick, N.; Webster, M.J.; Bartington, S.E.; Thomas, G.N.; Avis, W.; et al. Air quality assessment in three East African cities using calibrated low-cost sensors with a focus on road-based hotspots. Environ. Res. Commun. 2021, 3, 075007. [Google Scholar] [CrossRef]
- Linard, C.; Gilbert, M.; Snow, R.W.; Noor, A.M.; Tatem, A.J. Population distribution, settlement patterns and accessibility across Africa in 2010. PLoS ONE 2012, 7, e31743. [Google Scholar] [CrossRef] [Green Version]
- Singh, A.; Avis, W.R.; Pope, F.D. Visibility as a proxy for air quality in East Africa. Environ. Res. Lett. 2020, 15, 084002. [Google Scholar] [CrossRef]
- WHO. Regional Office for Africa Air Pollution. Available online: https://www.afro.who.int/health-topics/air-pollution (accessed on 22 July 2022).
- ICF Macro. Demographic and Health Survey Supervisor’s and Editor’s Manual. In MEASURE DHS Basic Documentation No. 4; ICF Macro: Calverton, MD, USA, 2011. [Google Scholar]
- Hammer, M.S.; van Donkelaar, A.; Li, C.; Lyapustin, A.; Sayer, A.M.; Hsu, N.C.; Levy, R.C.; Garay, M.J.; Kalashnikova, O.V.; Kahn, R.A.; et al. Global Estimates and Long-Term Trends of Fine Particulate Matter Concentrations (1998–2018). Environ. Sci. Technol. 2020, 54, 7879–7890. [Google Scholar] [CrossRef]
- van Donkelaar, A.; Hammer, M.S.; Bindle, L.; Brauer, M.; Brook, J.R.; Garay, M.J.; Hsu, N.C.; Kalashnikova, O.V.; Kahn, R.A.; Lee, C.; et al. Monthly global estimates of fine particulate matter and their uncertainty. Environ. Sci. Technol. 2021, 55, 15287–15300. [Google Scholar] [CrossRef]
- European Space Agency. ESA/CCI Viewer. Available online: http://maps.elie.ucl.ac.be/CCI/viewer/download.php (accessed on 22 July 2022).
- Arino, O.; Gross, D.; Ranera, F.; Leroy, M.; Bicheron, P.; Brockman, C.; Defourny, P.; Vancutsem, C.; Achard, F.; Durieux, L.; et al. GlobCover: ESA service for global land cover from MERIS. In Proceedings of the 2007 IEEE International Geoscience and Remote Sensing Symposium, Barcelona, Spain, 23–28 July 2007; pp. 2412–2415. [Google Scholar]
- Li, W.; MacBean, N.; Ciais, P.; Defourny, P.; Lamarche, C.; Bontemps, S.; Houghton, R.A.; Peng, S. Gross and net land cover changes in the main plant functional types derived from the annual ESA CCI land cover maps (1992–2015). Earth Syst. Sci. Data 2018, 10, 219–234. [Google Scholar] [CrossRef]
- Li, W.; Ciais, P.; MacBean, N.; Peng, S.; Defourny, P.; Bontemps, S. Major forest changes and land cover transitions based on plant functional types derived from the ESA CCI Land Cover product. Int. J. Appl. Earth Obs. Geoinf. 2016, 47, 30–39. [Google Scholar] [CrossRef]
- Grant, R.L. Converting an odds ratio to a range of plausible relative risks for better communication of research findings. BMJ 2014, 348, f7450. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Converting between Probabilities, Odds (Ratios), and Risk Ratios. Available online: https://cran.r-project.org/web/packages/effectsize/vignettes/convert_p_OR_RR.html (accessed on 4 November 2022).
- Flegal, K.M. Bias in calculation of attributable fractions using relative risks from nonsmokers only. Epidemiology 2014, 25, 913–916. [Google Scholar] [CrossRef] [Green Version]
- WHO. Ambient (Outdoor) Air Pollution. Available online: https://www.who.int/news-room/fact-sheets/detail/ambient-(outdoor)-air-quality-and-health (accessed on 13 July 2021).
- Chirande, L.; Charwe, D.; Mbwana, H.; Victor, R.; Kimboka, S.; Issaka, A.I.; Baines, S.K.; Dibley, M.J.; Agho, K.E. Determinants of stunting and severe stunting among under-fives in Tanzania: Evidence from the 2010 cross-sectional household survey. BMC Pediatr. 2015, 15, 165. [Google Scholar] [CrossRef] [Green Version]
- Yisak, H.; Gobena, T.; Mesfin, F. Prevalence and risk factors for under nutrition among children under five at Haramaya district, Eastern Ethiopia. BMC Pediatr. 2015, 15, 212. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Novignon, J.; Aboagye, E.; Agyemang, O.S.; Aryeetey, G. Socioeconomic-related inequalities in child malnutrition: Evidence from the Ghana multiple indicator cluster survey. Health Econ. Rev. 2015, 5, 34. [Google Scholar] [CrossRef] [Green Version]
- Leroy, J.L.; Habicht, J.-P.; de Cossío, T.G.; Ruel, M.T. Maternal education mitigates the negative effects of higher income on the double burden of child stunting and maternal overweight in rural Mexico. J. Nutr. 2014, 144, 765–770. [Google Scholar] [CrossRef] [Green Version]
- Krishna, A.; Mejía-Guevara, I.; McGovern, M.; Aguayo, V.M.; Subramanian, S.V. Trends in inequalities in child stunting in South Asia. Matern. Child Nutr. 2018, 14 (Suppl. S4), e12517. [Google Scholar] [CrossRef]
- Pillai, V.K.; Maleku, A. Women’s education and child stunting reduction in India. J. Soc. Soc. Welf. 2019, 46, 111–130. [Google Scholar]
- Coker, E.S.; Amegah, A.K.; Mwebaze, E.; Ssematimba, J.; Bainomugisha, E. A land use regression model using machine learning and locally developed low cost particulate matter sensors in Uganda. Environ. Res. 2021, 199, 111352. [Google Scholar] [CrossRef] [PubMed]
- World Bank. GDP (Current US$)–Sub-Saharan Africa|Data. Available online: https://data.worldbank.org/indicator/NY.GDP.MKTP.CD?locations=ZG (accessed on 15 October 2022).
- Jorquera, H.; Montoya, L.D.; Rojas, N.Y. Urban Air Pollution. In Urban Climates in Latin America; Henríquez, C., Romero, H., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 137–165. ISBN 978-3-319-97012-7. [Google Scholar]
- Mauderly, J.L.; Burnett, R.T.; Castillejos, M.; Ozkaynak, H.; Samet, J.M.; Stieb, D.M.; Vedal, S.; Wyzga, R.E. Is the air pollution health research community prepared to support a multipollutant air quality management framework? Inhal. Toxicol. 2010, 22 (Suppl. S1), 1–19. [Google Scholar] [CrossRef] [PubMed]
- Jedrychowski, W.; Maugeri, U.; Jedrychowska-Bianchi, I. Body growth rate in preadolescent children and outdoor air quality. Environ. Res. 2002, 90, 12–20. [Google Scholar] [CrossRef] [PubMed]


| Country | DHS Wave Analyzed | Sample Size |
|---|---|---|
| Burundi | 2010 | 3376 |
| 2016–2017 | 5876 | |
| Ethiopia | 2011 | 8627 |
| 2016 | 8319 | |
| 2019 | 4967 | |
| Kenya | 2008–2009 | 4742 |
| 2014 | 17,716 | |
| Rwanda | 2010 | 3952 |
| 2014–2015 | 3402 | |
| 2019 | 3708 | |
| Tanzania | 2010 | 6352 |
| 2015–2016 | 8722 | |
| Uganda | 2006 | 1859 |
| 2011 | 1883 | |
| 2016 | 4215 |
| All Countries | Burundi | Rwanda | Kenya | Tanzania | Ethiopia | Uganda | |
|---|---|---|---|---|---|---|---|
| Number of children <60 months | 87716 | 9252 | 11,062 | 22,458 | 15,074 | 21,913 | 7957 |
| Mean prenatal PM2.5 (μg/m3) | 25.98 | 37.03 | 37.02 | 22.09 | 23.22 | 19.69 | 31.27 |
| Mean height-for-age z-score | −1.51 | −2.17 | −1.59 | −1.19 | −1.53 | −1.54 | −1.30 |
| Percent Stunted | 37.16 | 54.25 | 37.80 | 27.95 | 35.72 | 38.58 | 31.05 |
| Mean age in months | 28.69 | 28.72 | 29.09 | 28.99 | 27.66 | 29.12 | 27.94 |
| Mean birth order | 3.62 | 3.76 | 3.20 | 3.25 | 3.64 | 3.95 | 4.05 |
| Percent Girls | 49.44 | 49.47 | 49.49 | 49.34 | 50.02 | 49.03 | 49.65 |
| Percent Rural | 80.80 | 91.29 | 85.14 | 69.59 | 76.91 | 86.06 | 83.72 |
| Percent used polluting fuel for cooking | 97.48 | 99.85 | 98.99 | 93.41 | 99.37 | 97.53 | 99.63 |
| Percent ever breastfed | 98.47 | 99.30 | 99.60 | 98.73 | 98.81 | 96.99 | 98.45 |
| Covariate | Height-for-Age Mean (SD) | p-Value |
|---|---|---|
| Urban/rural status | <0.001 | |
| Urban | −1.03 (1.46) | |
| Rural | −1.59 (1.51) | |
| Maternal education | <0.001 | |
| No education | −1.68 (1.63) | |
| Primary | −1.51 (1.43) | |
| Secondary | −1.03 (1.39) | |
| Higher | −0.52 (1.35) | |
| Wealth index | <0.001 | |
| 1st quintile (poorest) | −1.65 (1.60) | |
| 2nd quintile | −1.66 (1.47) | |
| 3rd quintile | −1.56 (1.45) | |
| 4th quintile | −1.40 (1.44) | |
| 5th quintile (richest) | −0.94 (1.44) | |
| Polluting fuel use | <0.001 | |
| Clean | −0.53 (1.36) | |
| Polluting | −1.49 (1.51) | |
| Breastfeeding status | 0.2 | |
| Never | −1.53 (1.62) | |
| Ever | −1.47 (1.52) |
| Model 0 | Model 1 a β (95% CI) | Model 2 b β (95% CI) | Model 3 c β (95% CI) | |
|---|---|---|---|---|
| Prenatal PM2.5 exposure | −0.143 *** (−0.154, −0.131) | −0.080 *** (−0.98, −0.062) | −0.095 *** (−0.114, −0.076) | −0.069 *** (−0.097, −0.041) |
| Type of residence | ||||
| Urban | reference | |||
| Rural | −0.824 *** (−1.125, −0.524) | −0.836 *** (−1.167, −0.505) | −0.955 *** (−1.287, −0.623) | |
| Maternal education | ||||
| 1st quartile | reference | |||
| 2nd quartile | 0.272 * (0.031, 0.514) | 0.472 *** (0.218, 0.726) | 0.466 *** (0.211, 0.721) | |
| 3rd quartile | 2.247 *** (1.886, 2.607) | 2.602 *** (2.214, 2.990) | 2.623 *** (2.232, 3.013) | |
| 4th quartile | 4.707 *** (4.085, 5.329) | 5.111 *** (4.420, 5.803) | 5.266 *** (4.571, 5.962) | |
| Wealth index | ||||
| 1st quintile | reference | |||
| 2nd quintile | 0.418 ** (0.135, 0.701) | 0.366 * (0.064, 0.668) | 0.466 ** (0.163, 0.770) | |
| 3rd quintile | 1.464 *** (1.168, 1.761) | 1.321 *** (1.013, 1.642) | 1.431 *** (1.116, 1.747) | |
| 4th quintile | 2.740 *** (2.430, 3.051) | 2.616 *** (2.289, 2.944) | 2.736 *** (2.408, 3.065) | |
| 5th quintile | 5.788 *** (5.398, 6.179) | 5.692 *** (5.278, 6.106) | 5.775 *** (5.360, 6.191) | |
| Use of polluting fuel | ||||
| No | reference | |||
| Yes | −0.763 * (−1.472, −0.053) | −0.374 (−1.165, 0.418) | −0.455 (−1.250, 0.340) | |
| Ever Breastfed | ||||
| No | reference | |||
| Yes | 0.812 * (0.002, 1.622) | 0.804 ** (−0.006, 1.613) | ||
| Postnatal PM2.5 exposure | −0.050 *** (−0.080, −0.020) | |||
| Observations | 87,716 | 87,716 | 78,329 | 75,949 |
| AIC | 321,509 | 312,053 | 279,426 | 269,255 |
| Adjusted RR (95% CI) | |
|---|---|
| Prenatal PM2.5 exposure quartiles | |
| 4.55–19.4 μg/m3 | reference |
| 20.4–24 μg/m3 | 1.00 (0.97, 1.03) |
| 25–32.7 μg/m3 | 1.02 (0.98, 1.06) |
| 32.7–52.4 μg/m3 | 1.12 (1.07, 1.17) |
| Postnatal PM2.5 exposure quartiles | |
| 2.62–19.8 μg/m3 | reference |
| 20.8–24.1 μg/m3 | 1.02 (0.99, 1.04) |
| 25.1–33.1 μg/m3 | 1.02 (0.98, 1.06) |
| 34.1–74.7 μg/m3 | 1.11 (1.06, 1.16) |
| Type of residence | |
| Urban | reference |
| Rural | 1.06 (1.03, 1.09) |
| Maternal education | |
| 1st quartile | reference |
| 2nd quartile | 0.96 (0.94, 0.99) |
| 3rd quartile | 0.78 (0.74, 0.81) |
| 4th quartile | 0.58 (0.52, 0.64) |
| Wealth index | |
| 1st quintile | reference |
| 2nd quintile | 0.95 (0.92, 0.97) |
| 3rd quintile | 0.88 (0.86, 0.91) |
| 4th quintile | 0.77 (0.75, 0.80) |
| 5th quintile | 0.57 (0.54, 0.60) |
| Use of polluting fuel | |
| No | reference |
| Yes | 1.08 (0.99, 1.17) |
| Ever Breastfed | |
| No | reference |
| Yes | 0.91 (0.84, 0.98) |
| Observations | 75,949 |
| AIC | 94,760 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Clarke, K.; Rivas, A.C.; Milletich, S.; Sabo-Attwood, T.; Coker, E.S. Prenatal Exposure to Ambient PM2.5 and Early Childhood Growth Impairment Risk in East Africa. Toxics 2022, 10, 705. https://doi.org/10.3390/toxics10110705
Clarke K, Rivas AC, Milletich S, Sabo-Attwood T, Coker ES. Prenatal Exposure to Ambient PM2.5 and Early Childhood Growth Impairment Risk in East Africa. Toxics. 2022; 10(11):705. https://doi.org/10.3390/toxics10110705
Chicago/Turabian StyleClarke, Kayan, Adriana C. Rivas, Salvatore Milletich, Tara Sabo-Attwood, and Eric S. Coker. 2022. "Prenatal Exposure to Ambient PM2.5 and Early Childhood Growth Impairment Risk in East Africa" Toxics 10, no. 11: 705. https://doi.org/10.3390/toxics10110705




