Metabolic Syndrome and Air Pollution: A Narrative Review of Their Cardiopulmonary Effects
Abstract
:1. Introduction
2. Review/Search Strategy
Inclusion/Exclusion Criteria and Limitations
3. Epidemiological Studies
3.1. Epidemiology of MetSyn and PM Exposure
3.2. Epidemiology of CVD and PM Exposure
3.3. Epidemiology of Chronic Obstructive Pulmonary Disease (COPD) and PM Exposure
4. Biological Mechanisms Underlying PM-Induced Metabolic and Cardiopulmonary Diseases
4.1. Mechanisms of PM Associated MetSyn
4.2. PM Exposure and COPD
4.3. PM Exposure and CVD
5. MetSyn as a Risk Factor for COPD and CVD
6. Cardiopulmonary Effects of WTC-PM-Exposure
7. Conclusions and Future Investigations
Author Contributions
Funding
Conflicts of Interest
References
- Brocato, J.; Sun, H.; Shamy, M.; Kluz, T.; Alghamdi, M.A.; Khoder, M.I.; Chen, L.C.; Costa, M. Particulate matter from saudi arabia induces genes involved in inflammation, metabolic syndrome and atherosclerosis. J. Toxicol. Environ. Health A 2014, 77, 751–766. [Google Scholar] [CrossRef]
- Chen, J.C.; Schwartz, J. Metabolic syndrome and inflammatory responses to long-term particulate air pollutants. Environ. Health Persp. 2008, 116, 612–617. [Google Scholar] [CrossRef]
- World Health Organization. 9 Out of 10 People Worldwide Breathe Polluted Air, But More Countries Are Taking Action. 2 May 2018. Available online: https://www.who.int/news-room/detail/02-05-2018-9-out-of-10-people-worldwide-breathe-polluted-air-but-more-countries-are-taking-action (accessed on 29 January 2019).
- Peters, A.; Dockery, D.W.; Muller, J.E.; Mittleman, M.A. Increased particulate air pollution and the triggering of myocardial infarction. Circulation 2001, 103, 2810–2815. [Google Scholar] [CrossRef]
- Peters, A.; von Klot, S.; Heier, M.; Trentinaglia, I.; Hormann, A.; Wichmann, H.E.; Lowel, H. Exposure to traffic and the onset of myocardial infarction. New Engl J. Med. 2004, 351, 1721–1730. [Google Scholar] [CrossRef]
- Wellenius, G.A.; Schwartz, J.; Mittleman, M.A. Air pollution and hospital admissions for ischemic and hemorrhagic stroke among medicare beneficiaries. Stroke 2005, 36, 2549–2553. [Google Scholar] [CrossRef]
- Wellenius, G.A.; Yeh, G.Y.; Coull, B.A.; Suh, H.H.; Phillips, R.S.; Mittleman, M.A. Effects of ambient air pollution on functional status in patients with chronic congestive heart failure: A repeated-measures study. Environ. Health 2007, 6, 26. [Google Scholar] [CrossRef]
- Wellenius, G.A.; Coull, B.A.; Batalha, J.R.; Diaz, E.A.; Lawrence, J.; Godleski, J.J. Effects of ambient particles and carbon monoxide on supraventricular arrhythmias in a rat model of myocardial infarction. Inhal. Toxicol. 2006, 18, 1077–1082. [Google Scholar] [CrossRef]
- Wellenius, G.A.; Schwartz, J.; Mittleman, M.A. Particulate air pollution and hospital admissions for congestive heart failure in seven united states cities. Am. J. Cardiol. 2006, 97, 404–408. [Google Scholar] [CrossRef]
- Dominici, F.; Peng, R.D.; Bell, M.L.; Pham, L.; McDermott, A.; Zeger, S.L.; Samet, J.M. Fine particulate air pollution and hospital admission for cardiovascular and respiratory diseases. JAMA 2006, 295, 1127–1134. [Google Scholar] [CrossRef]
- Simkhovich, B.Z.; Kleinman, M.T.; Kloner, R.A. Particulate air pollution and coronary heart disease. Curr. Opin. Cardiol. 2009, 24, 604–609. [Google Scholar] [CrossRef]
- Crowley, G.; Kwon, S.; Haider, S.H.; Caraher, E.J.; Lam, R.; St-Jules, D.E.; Liu, M.; Prezant, D.J.; Nolan, A. Metabolomics of world trade center-lung injury: A machine learning approach. BMJ Open Respir. Res. 2018, 5, e000274. [Google Scholar] [CrossRef]
- Naveed, B.; Weiden, M.D.; Kwon, S.; Gracely, E.J.; Comfort, A.L.; Ferrier, N.; Kasturiarachchi, K.J.; Cohen, H.W.; Aldrich, T.K.; Rom, W.N.; et al. Metabolic syndrome biomarkers predict lung function impairment: A nested case-control study. Am. J. Respir. Crit. Care Med. 2012, 185, 392–399. [Google Scholar] [CrossRef]
- Tsukiji, J.; Cho, S.J.; Echevarria, G.C.; Kwon, S.; Joseph, P.; Schenck, E.J.; Naveed, B.; Prezant, D.J.; Rom, W.N.; Schmidt, A.M.; et al. Lysophosphatidic acid and apolipoprotein a1 predict increased risk of developing world trade center-lung injury: A nested case-control study. Biomarkers 2014, 19, 159–165. [Google Scholar] [CrossRef]
- Weiden, M.D.; Kwon, S.; Caraher, E.; Berger, K.I.; Reibman, J.; Rom, W.N.; Prezant, D.J.; Nolan, A. Biomarkers of world trade center particulate matter exposure: Physiology of distal airway and blood biomarkers that predict FEV(1) decline. In Seminars in respiratory and critical care medicine; NIH Public Access: Bethesda, MD, USA, 2015; Volume 36, p. 323. [Google Scholar]
- Holguin, F. The metabolic syndrome as a risk factor for lung function decline. Am. J. Respir. Crit. Care Med. 2012, 185, 352–353. [Google Scholar] [CrossRef]
- Balmes, J.R. Can we predict who will develop chronic sequelae of acute inhalational injury? Chest 2012, 142, 278–279. [Google Scholar] [CrossRef]
- Antao, V.C. The world trade center disaster: A tragic source of medical advancement. Eur. Respir. J. 2013, 41, 999–1001. [Google Scholar] [CrossRef]
- Grundy, S.M.; Cleeman, J.I.; Daniels, S.R.; Donato, K.A.; Eckel, R.H.; Franklin, B.A.; Gordon, D.J.; Krauss, R.M.; Savage, P.J.; Smith, S.C., Jr.; et al. Diagnosis and management of the metabolic syndrome: An american heart association/national heart, lung, and blood institute scientific statement. Circulation 2005, 112, 2735–2752. [Google Scholar] [CrossRef]
- Huang, W.; Wang, L.; Li, J.; Liu, M.; Xu, H.; Liu, S.; Chen, J.; Zhang, Y.; Morishita, M.; Bard, R.L.; et al. Short-term blood pressure responses to ambient fine particulate matter exposures at the extremes of global air pollution concentrations. Am. J. Hypertens. 2018, 31, 590–599. [Google Scholar]
- Brook, R.D.; Rajagopalan, S. Particulate matter, air pollution, and blood pressure. J. Am. Soc. Hypertens. 2009, 3, 332–350. [Google Scholar] [CrossRef]
- Chobanian, A.V.; Bakris, G.L.; Black, H.R.; Cushman, W.C.; Green, L.A.; IzzoJr, J.L.; Jones, D.W.; Materson, B.J.; Oparil, S.; WrightJr, J.T.; et al. National Heart, Institute Blood, and Committee National High Blood Pressure Education Program Coordinating. Seventh report of the joint national committee on prevention, detection, evaluation, and treatment of high blood pressure. Hypertension 2003, 42, 1206–1252. [Google Scholar] [CrossRef]
- Tenenbaum, A.; Fisman, E.Z.; Motro, M. Metabolic syndrome and type 2 diabetes mellitus: Focus on peroxisome proliferator activated receptors (PPAR). Cardiovasc. Diabetol. 2003, 2, 4. [Google Scholar] [CrossRef]
- Bowe, B.; Xie, Y.; Li, T.; Yan, Y.; Xian, H.; Al-Aly, Z. The 2016 global and national burden of diabetes mellitus attributable to PM2.5 air pollution. Lancet Planet. Health 2018, 2, e301–e312. [Google Scholar] [CrossRef]
- Wei, Y.; Zhang, J.J.; Li, Z.; Gow, A.; Chung, K.F.; Hu, M.; Sun, Z.; Zeng, L.; Zhu, T.; Jia, G.; et al. Chronic exposure to air pollution particles increases the risk of obesity and metabolic syndrome: Findings from a natural experiment in beijing. FASEB J. 2016, 30, 2115–2122. [Google Scholar] [CrossRef]
- Sun, Q.; Yue, P.; Deiuliis, J.A.; Lumeng, C.N.; Kampfrath, T.; Mikolaj, M.B.; Cai, Y.; Ostrowski, M.C.; Lu, B.; Parthasarathy, S.; et al. Ambient air pollution exaggerates adipose inflammation and insulin resistance in a mouse model of diet-induced obesity. Circulation 2009, 119, 538–546. [Google Scholar] [CrossRef]
- Gan, W.Q.; FitzGerald, J.M.; Carlsten, C.; Sadatsafavi, M.; Brauer, M. Associations of ambient air pollution with chronic obstructive pulmonary disease hospitalization and mortality. Am. J. Respir. Crit. Care Med. 2013, 187, 721–727. [Google Scholar] [CrossRef]
- Vujic, T.; Nagorni, O.; Maric, G.; Popovic, L.; Jankovic, J. Metabolic syndrome in patients with chronic obstructive pulmonary disease: Frequency and relationship with systemic inflammation. Hippokratia 2016, 20, 110–114. [Google Scholar]
- Samoli, E.; Stafoggia, M.; Rodopoulou, S.; Ostro, B.; Alessandrini, E.; Basagana, X.; Diaz, J.; Faustini, A.; Gandini, M.; Karanasiou, A.; et al. Which specific causes of death are associated with short term exposure to fine and coarse particles in southern europe? Results from the med-particles project. Environ. Int. 2014, 67, 54–61. [Google Scholar] [CrossRef]
- Tankersley, C.G.; Champion, H.C.; Takimoto, E.; Gabrielson, K.; Bedja, D.; Misra, V.; El-Haddad, H.; Rabold, R.; Mitzner, W. Exposure to inhaled particulate matter impairs cardiac function in senescent mice. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2008, 295, R252–R263. [Google Scholar] [CrossRef] [Green Version]
- Sun, Q.; Wang, A.; Jin, X.; Natanzon, A.; Duquaine, D.; Brook, R.D.; Aguinaldo, J.G.; Fayad, Z.A.; Fuster, V.; Lippmann, M.; et al. Long-term air pollution exposure and acceleration of atherosclerosis and vascular inflammation in an animal model. JAMA 2005, 294, 3003–3010. [Google Scholar] [CrossRef]
- Devlin, R.B.; Smith, C.B.; Schmitt, M.T.; Rappold, A.G.; Hinderliter, A.; Graff, D.; Carraway, M.S. Controlled exposure of humans with metabolic syndrome to concentrated ultrafine ambient particulate matter causes cardiovascular effects. Toxicol. Sci. 2014, 140, 61–72. [Google Scholar] [CrossRef]
- Park, S.K.; Auchincloss, A.H.; O’Neill, M.S.; Prineas, R.; Correa, J.C.; Keeler, J.; Barr, R.G.; Kaufman, J.D.; Diez Roux, A.V. Particulate air pollution, metabolic syndrome, and heart rate variability: The multi-ethnic study of atherosclerosis (MESA). Environ. Health Perspect. 2010, 118, 1406–1411. [Google Scholar] [CrossRef]
- Chang, C.C.; Chen, P.S.; Yang, C.Y. Short-term effects of fine particulate air pollution on hospital admissions for cardiovascular diseases: A case-crossover study in a tropical city. J. Toxicol. Environ. Health Part A 2015, 78, 267–277. [Google Scholar] [CrossRef]
- Miller, K.A.; Siscovick, D.S.; Sheppard, L.; Shepherd, K.; Sullivan, J.H.; Anderson, G.L.; Kaufman, J.D. Long-term exposure to air pollution and incidence of cardiovascular events in women. New Eng. Med. 2007, 356, 447–458. [Google Scholar] [CrossRef]
- Monteiro, R.; Azevedo, I. Chronic inflammation in obesity and the metabolic syndrome. Mediat. Inflamm. 2010, 2010. [Google Scholar] [CrossRef]
- Brook, R.D.; Rajagopalan, S.; Pope, C.A., 3rd; Brook, J.R.; Bhatnagar, A.; Diez-Roux, A.V.; Holguin, F.; Hong, Y.; Luepker, R.V.; Mittleman, M.A.; et al. Kaufman, Epidemiology American Heart Association Council on, Council on the Kidney in Cardiovascular Disease Prevention, Physical Activity Council on Nutrition, and Metabolism. Particulate matter air pollution and cardiovascular disease: An update to the scientific statement from the american heart association. Circulation 2010, 121, 2331–2378. [Google Scholar]
- Hutcheson, R.; Rocic, P. The metabolic syndrome, oxidative stress, environment, and cardiovascular disease: The great exploration. Exp. diAbetes Res. 2012, 2012. [Google Scholar] [CrossRef]
- Webber, M.P.; Lee, R.; Soo, J.; Gustave, J.; Hall, C.B.; Kelly, K.; Prezant, D. Prevalence and incidence of high risk for obstructive sleep apnea in world trade center-exposed rescue/recovery workers. Sleep Breath. 2011, 15, 283–294. [Google Scholar] [CrossRef]
- Moore, J.X.; Chaudhary, N.; Akinyemiju, T. Metabolic syndrome prevalence by race/ethnicity and sex in the United States, national health and nutrition examination survey, 1988–2012. Prev. Chronic. Dis. 2017, 14, E24. [Google Scholar] [CrossRef]
- Martinelli, N.; Olivieri, O.; Girelli, D. Air particulate matter and cardiovascular disease: A narrative review. Eur. J. Intern. Med. 2013, 24, 295–302. [Google Scholar] [CrossRef]
- Sint, T.; Donohue, J.F.; Ghio, A.J. Ambient air pollution particles and the acute exacerbation of chronic obstructive pulmonary disease. Inhal. Toxicol. 2008, 20, 25–29. [Google Scholar] [CrossRef]
- Ling, S.H.; van Eeden, S.F. Particulate matter air pollution exposure: Role in the development and exacerbation of chronic obstructive pulmonary disease. Int. J. Chronic Obstr. Pulmon. Dis. 2009, 4, 233–243. [Google Scholar] [CrossRef]
- Hotamisligil, G.S. Inflammation and metabolic disorders. Nature 2006, 444, 860–867. [Google Scholar] [CrossRef]
- Tsai, D.H.; Amyai, N.; Marques-Vidal, P.; Wang, J.L.; Riediker, M.; Mooser, V.; Paccaud, F.; Waeber, G.; Vollenweider, P.; Bochud, M. Effects of particulate matter on inflammatory markers in the general adult population. Part. Fibre Toxicol. 2012, 9, 24. [Google Scholar] [CrossRef] [Green Version]
- Xu, X.; Yavar, Z.; Verdin, M.; Ying, Z.; Mihai, G.; Kampfrath, T.; Wang, A.; Zhong, M.; Lippmann, M.; Chen, L.C.; et al. Effect of early particulate air pollution exposure on obesity in mice: Role of p47phox. Arterioscler. Thromb. Vas. Biol. 2010, 30, 2518–2527. [Google Scholar] [CrossRef]
- Bellavia, A.; Urch, B.; Speck, M.; Brook, R.D.; Scott, J.A.; Albetti, B.; Behbod, B.; North, M.; Valeri, L.; Bertazzi, P.A.; et al. DNA hypomethylation, ambient particulate matter, and increased blood pressure: Findings from controlled human exposure experiments. J. Am. Heart Assoc. 2013, 2, e000212. [Google Scholar] [CrossRef]
- Urch, B.; Silverman, F.; Corey, P.; Brook, J.R.; Lukic, K.Z.; Rajagopalan, S.; Brook, R.D. Acute blood pressure responses in healthy adults during controlled air pollution exposures. Environ. Health Perspect. 2005, 113, 1052–1055. [Google Scholar] [CrossRef]
- Cosio, M.G.; Saetta, M.; Agusti, A. Immunologic aspects of chronic obstructive pulmonary disease. New Engl. J. Med. 2009, 360, 2445–2454. [Google Scholar] [CrossRef]
- Davies, D.E.; Wicks, J.; Powell, R.M.; Puddicombe, S.M.; Holgate, S.T. Airway remodeling in asthma: New insights. J. Allergy Clin. Immunol. 2003, 111, 215–225. [Google Scholar]
- Barnes, P.J. Chronic obstructive pulmonary disease: Effects beyond the lungs. PLoS Med. 2010, 7, e1000220. [Google Scholar] [CrossRef]
- Zureik, M.; Benetos, A.; Neukirch, C.; Courbon, D.; Bean, K.; Thomas, F.; Ducimetiere, P. Reduced pulmonary function is associated with central arterial stiffness in men. Am. J. Respir. Crit. Care Med. 2001, 164, 2181–2185. [Google Scholar] [CrossRef]
- Tockman, M.S.; Pearson, J.D.; Fleg, J.L.; Metter, E.J.; Kao, S.Y.; Rampal, K.G.; Cruise, L.J.; Fozard, J.L. Rapid decline in FEV1. A new risk factor for coronary heart disease mortality. Am. J. Respir. Crit. Care Med. 1995, 151, 390–398. [Google Scholar] [CrossRef]
- Mannino, D.M.; Thorn, D.; Swensen, A.; Holguin, F. Prevalence and outcomes of diabetes, hypertension and cardiovascular disease in chronic obstructive pulmonary disease. Eur. Respir. J. 2008, 32, 962–969. [Google Scholar] [CrossRef]
- Taraseviciene-Stewart, L.; Scerbavicius, R.; Choe, K.H.; Moore, M.; Sullivan, A.; Nicolls, M.R.; Fontenot, A.P.; Tuder, R.M.; Voelkel, N.F. An animal model of autoimmune emphysema. Am. J. Respir. Crit. Care Med. 2005, 171, 734–742. [Google Scholar]
- Voelkel, N.; Taraseviciene-Stewart, L. Emphysema: An autoimmune vascular disease? PATS 2005, 2, 23–25. [Google Scholar] [CrossRef]
- Mills, N.L.; Tornqvist, H.; Gonzalez, M.C.; Vink, E.; Robinson, S.D.; Soderberg, S.; Boon, N.A.; Donaldson, K.; Sandstrom, T.; Blomberg, A.; et al. Ischemic and thrombotic effects of dilute diesel-exhaust inhalation in men with coronary heart disease. New Engl. J. Med. 2007, 357, 1075–1082. [Google Scholar] [CrossRef]
- Tornqvist, H.; Mills, N.L.; Gonzalez, M.; Miller, M.R.; Robinson, S.D.; Megson, I.L.; Macnee, W.; Donaldson, K.; Soderberg, S.; Newby, D.E.; et al. Persistent endothelial dysfunction in humans after diesel exhaust inhalation. Am. J. Respir. Crit. Care Med. 2007, 176, 395–400. [Google Scholar] [CrossRef]
- Broekhuizen, R.; Wouters, E.F.; Creutzberg, E.C.; Schols, A.M. Raised CRP levels mark metabolic and functional impairment in advanced copd. Thorax 2006, 61, 17–22. [Google Scholar] [CrossRef]
- Gan, W.Q.; Man, S.F.; Senthilselvan, A.; Sin, D.D. Association between chronic obstructive pulmonary disease and systemic inflammation: A systematic review and a meta-analysis. Thorax 2004, 59, 574–580. [Google Scholar] [CrossRef]
- Koenig, W. Inflammation and coronary heart disease: An overview. Cardiol. Rev. 2001, 9, 31–35. [Google Scholar] [CrossRef]
- Hoffmeister, A.; Rothenbacher, D.; Bazner, U.; Frohlich, M.; Brenner, H.; Hombach, V.; Koenig, W. Role of novel markers of inflammation in patients with stable coronary heart disease. Am. J. Cardiol. 2001, 87, 262–266. [Google Scholar] [CrossRef]
- Sin, D.D.; Man, S.F. Why are patients with chronic obstructive pulmonary disease at increased risk of cardiovascular diseases? The potential role of systemic inflammation in chronic obstructive pulmonary disease. Circulation 2003, 107, 1514–1519. [Google Scholar] [CrossRef]
- Brook, R.D. Cardiovascular effects of air pollution. Clin. Sci. 2008, 115, 175–187. [Google Scholar] [CrossRef]
- Gurgueira, S.A.; Lawrence, J.; Coull, B.; Murthy, G.G.; Gonzalez-Flecha, B. Rapid increases in the steady-state concentration of reactive oxygen species in the lungs and heart after particulate air pollution inhalation. Environ. Health Perspect. 2002, 110, 749–755. [Google Scholar] [CrossRef]
- Du, Y.; Xu, X.; Chu, M.; Guo, Y.; Wang, J. Air particulate matter and cardiovascular disease: The epidemiological, biomedical and clinical evidence. J. Thorac. Dis. 2016, 8, E8–E19. [Google Scholar]
- Franchini, M.; Mannucci, P.M. Thrombogenicity and cardiovascular effects of ambient air pollution. Blood 2011, 118, 2405–2412. [Google Scholar] [CrossRef] [Green Version]
- Watz, H.; Waschki, B.; Kirsten, A.; Muller, K.C.; Kretschmar, G.; Meyer, T.; Holz, O.; Magnussen, H. The metabolic syndrome in patients with chronic bronchitis and copd: Frequency and associated consequences for systemic inflammation and physical inactivity. Chest 2009, 136, 1039–1046. [Google Scholar] [CrossRef]
- Zheng, Y.; Stein, R.; Kwan, T.; Yu, C.; Kwan, J.; Chen, S.L.; Hu, D. Evolving cardiovascular disease prevalence, mortality, risk factors, and the metabolic syndrome in china. Clin. Cardiol. 2009, 32, 491–497. [Google Scholar] [CrossRef]
- Liu, J.; Wang, H.; Zuo, Y.; Farmer, S.R. Functional interaction between peroxisome proliferator-activated receptor gamma and beta-catenin. Mol. Cell. Biol. 2006, 26, 5827–5837. [Google Scholar] [CrossRef]
- Ford, E.S.; Giles, W.H.; Dietz, W.H. Prevalence of the metabolic syndrome among us adults: Findings from the third national health and nutrition examination survey. JAMA 2002, 287, 356–359. [Google Scholar] [CrossRef]
- Hunninghake, D.B. Cardiovascular disease in chronic obstructive pulmonary disease. PATS 2005, 2, 44–49. [Google Scholar] [CrossRef]
- Pope, C.A., 3rd; Turner, M.C.; Burnett, R.T.; Jerrett, M.; Gapstur, S.M.; Diver, W.R.; Krewski, D.; Brook, R.D. Relationships between fine particulate air pollution, cardiometabolic disorders, and cardiovascular mortality. Circ. Res. 2015, 116, 108–115. [Google Scholar]
- Friedman, S.M.; Maslow, C.B.; Reibman, J.; Pillai, P.S.; Goldring, R.M.; Farfel, M.R.; Stellman, S.D.; Berger, K.I. Case-control study of lung function in world trade center health registry area residents and workers. Am. J. Respir. Crit. Care Med. 2011, 184, 582–589. [Google Scholar] [CrossRef]
- Banauch, G.I.; Hall, C.; Weiden, M.; Cohen, H.W.; Aldrich, T.K.; Christodoulou, V.; Arcentales, N.; Kelly, K.J.; Prezant, D.J. Pulmonary function after exposure to the world trade center collapse in the new york city fire department. Am. J. Respir. Crit. Care Med. 2006, 174, 312–319. [Google Scholar]
- Prezant, D.J.; Weiden, M.; Banauch, G.I.; McGuinness, G.; Rom, W.N.; Aldrich, T.K.; Kelly, K.J. Cough and bronchial responsiveness in firefighters at the world trade center site. New Engl. J. Med. 2002, 347, 806–815. [Google Scholar]
- Aldrich, T.K.; Gustave, J.; Hall, C.B.; Cohen, H.W.; Webber, M.P.; Zeig-Owens, R.; Cosenza, K.; Christodoulou, V.; Glass, L.; Al-Othman, F.; et al. Lung function in rescue workers at the world trade center after 7 years. New Engl. J. Med. 2010, 362, 1263–1272. [Google Scholar] [CrossRef]
- Zeig-Owens, R.; Webber, M.P.; Hall, C.B.; Schwartz, T.; Jaber, N.; Weakley, J.; Rohan, T.E.; Cohen, H.W.; Derman, O.; Aldrich, T.K.; et al. Early assessment of cancer outcomes in New York city firefighters after the 9/11 attacks: An observational cohort study. Lancet 2011, 378, 898–905. [Google Scholar] [CrossRef]
- Webber, M.P.; Gustave, J.; Lee, R.; Niles, J.K.; Kelly, K.; Cohen, H.W.; Prezant, D.J. Trends in respiratory symptoms of firefighters exposed to the world trade center disaster: 2001–2005. Environ. Health Perspect. 2009, 117, 975–980. [Google Scholar]
- Webber, M.P.; Glaser, M.S.; Weakley, J.; Soo, J.; Ye, F.; Zeig-Owens, R.; Weiden, M.D.; Nolan, A.; Aldrich, T.K.; Kelly, K.; et al. Physician-diagnosed respiratory conditions and mental health symptoms seven to nine years following the world trade center disaster. Am. J. Ind. Med. 2011, 54, 661–671. [Google Scholar] [CrossRef]
- Weakley, J.; Webber, M.P.; Gustave, J.; Kelly, K.; Cohen, H.W.; Hall, C.B.; Prezant, D.J. Trends in respiratory diagnoses and symptoms of firefighters exposed to the world trade center disaster: 2005–2010. Prev. Med. 2011, 53, 364–369. [Google Scholar]
- Weiden, M.D.; Naveed, B.; Kwon, S.; Jung Cho, S.; Comfort, A.L.; Prezant, D.J.; Rom, W.N.; Nolan, A. Cardiovascular disease biomarkers predict susceptibility or resistance to lung injury in world trade center dust exposed firefighters. Eur. Respir. J. 2012, 1023–1030. [Google Scholar]
- Soo, J.; Webber, M.P.; Gustave, J.; Lee, R.; Hall, C.B.; Cohen, H.W.; Kelly, K.J.; Prezant, D.J. Trends in probable PTSD in firefighters exposed to the world trade center disaster, 2001–2010. Disaster Med. Public 2011, 5 (Suppl. 2), S197–S203. [Google Scholar] [CrossRef]
- Rom, W.N.; Reibman, J.; Rogers, L.; Weiden, M.D.; Oppenheimer, B.; Berger, K.; Goldring, R.; Harrison, D.; Prezant, D. Emerging exposures and respiratory health: World trade center dust. PATS 2010, 7, 142–145. [Google Scholar] [CrossRef]
- Banauch, G.I.; Alleyne, D.; Sanchez, R.; Olender, K.; Cohen, H.W.; Weiden, M.; Kelly, K.J.; Prezant, D.J. Persistent hyperreactivity and reactive airway dysfunction in firefighters at the world trade center. Am. J. Respir. Crit. Care Med. 2003, 168, 54–62. [Google Scholar] [CrossRef]
- Fireman, E.M.; Lerman, Y.; Ganor, E.; Greif, J.; Fireman-Shoresh, S.; Lioy, P.J.; Banauch, G.I.; Weiden, M.; Kelly, K.J.; Prezant, D.J. Induced sputum assessment in new york city firefighters exposed to world trade center dust. Environ. Health Perspect. 2004, 112, 1564–1569. [Google Scholar] [CrossRef]
- Banauch, G.; Weiden, N.; Hall, C.; Cohen, H.W.; Aldrich, T.K.; Arcentales, N.; Kelly, K.J.; Prezant, D.J. Accelerated pulmonary function decline after world trade center particulate exposure in the new york city fire department workforce. Chest 2005, 128, 213S. [Google Scholar] [CrossRef]
- Jordan, H.T.; Stellman, S.D.; Morabia, A.; Miller-Archie, S.A.; Alper, H.; Laskaris, Z.; Brackbill, R.M.; Cone, J.E. Cardiovascular disease hospitalizations in relation to exposure to the september 11, 2001 world trade center disaster and posttraumatic stress disorder. J. Am. Heart Assoc. 2013, 2, e000431. [Google Scholar] [CrossRef]
- Lin, S.; Gomez, M.I.; Gensburg, L.; Liu, W.; Hwang, S.A. Respiratory and cardiovascular hospitalizations after the world trade center disaster. Arch. Environ. Occup. Health 2010, 65, 12–20. [Google Scholar] [CrossRef]
- Perritt, K.R.; Herbert, R.; Levin, S.M.; Moline, J. Work-related injuries and illnesses reported by world trade center response workers and volunteers. Prehosp. Disaster Med. 2011, 26, 401–407. [Google Scholar] [CrossRef]
- Caplan-Shaw, C.E.; Yee, H.; Rogers, L.; Abraham, J.L.; Parsia, S.S.; Naidich, D.P.; Borczuk, A.; Moreira, A.; Shiau, M.C.; Ko, J.P.; et al. Lung pathologic findings in a local residential and working community exposed to world trade center dust, gas, and fumes. J. Occup. Environ. Med. 2011, 53, 981–991. [Google Scholar] [CrossRef]
- Nolan, A.; Naveed, B.; Comfort, A.L.; Ferrier, N.; Hall, C.B.; Kwon, S.; Kasturiarachchi, K.J.; Cohen, H.W.; Zeig-Owens, R.; Glaser, M.S.; et al. Inflammatory biomarkers predict airflow obstruction after exposure to world trade center dust. Chest 2012, 142, 412–418. [Google Scholar] [CrossRef]
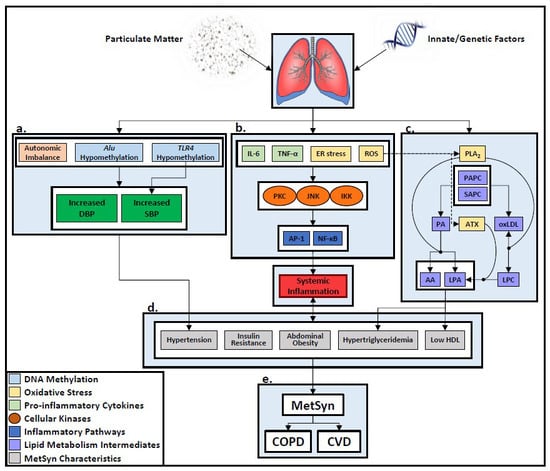
 ) indicate enzymatic contribution to downstream catabolic reactions.
) indicate enzymatic contribution to downstream catabolic reactions.
 ) indicate enzymatic contribution to downstream catabolic reactions.
) indicate enzymatic contribution to downstream catabolic reactions.
| Disease | Study | Country | Study Population | Significant Findings |
|---|---|---|---|---|
| METSYN | Animal Studies | |||
| Brocato [1] | USA | Murine model | PM exposure enhances the expression of genes located in pathways associated with MetSyn. | |
| Wei [25] | China | Murine model | Chronic exposure to PM increases the risk of MetSyn. | |
| Sun [26] | USA | Murine model | Long-term PM exposure exacerbates MetSyn. | |
| Human Studies | ||||
| Huang [20] | China, USA | Longitudinal cohort | High PM2.5 exposure promotes BP elevations in healthy and overweight individuals. | |
| Bowe [24] | USA | Longitudinal cohort | Inhalation of PM2.5 is significantly associated with increased risk for developing diabetes mellitus (HR, 1.15; 95% CI, 1.08–1.22). | |
| Naveed [13] | USA | Longitudinal cohort | MetSyn biomarkers—abnormal triglycerides and HDL (OR, 3.03; 95% CI, 1.39–6.16) and elevated heart rate (OR, 2.20; 95% CI, 1.14–4.24) and leptin (OR, 3.00; 95% CI, 1.35–6.66)—are risk factors of lung function impairment after WTC PM exposure. | |
| COPD | Human Studies | |||
| Gan [27] | Canada | Population-based cohort | Exposure to particulates in traffic-related air pollution was associated with a 6% increase in the risk of COPD hospitalization (95% CI, 2–10%). | |
| Dominici [10] | USA | Population-based cohort | Increased PM exposure doubled hospital admissions for COPD exacerbations. | |
| Vujic [28] | Serbia | Cross-sectional | Systemic inflammatory markers are higher in COPD patients with MetSyn than in those without MetSyn. Individuals with MetSyn have a higher leukocyte count (OR, 1.321; 95% CI, 1.007–1.628) and C-reactive protein level (OR, 1.184; 95% CI, 1.020–1.376) compared to those without MetSyn. | |
| Samoli [29] | Europe | Cross-sectional | PM2.5 is positively associated with mortality due to diabetes (1.23%; 95% CI, 1.63–4.17%), cardiac causes (1.33%; 95% CI, 0.27–2.40%), COPD (2.53%; 95% CI, 0.01–5.14%), and to a lesser degree to cerebrovascular causes (1.37%; 95% CI, 1.94–4.78%). | |
| CVD | Animal Studies | |||
| Tankersley [30] | USA | Murine model | Carbon black exposure led to impaired cardiac function in senescent mice | |
| Sun [31] | USA | Murine model | Long-term PM exposure altered vasomotor tone, induced vascular inflammation, and potentiated atherosclerosis. | |
| Human Studies | ||||
| Devlin [32] | USA | Case-crossover | MetSyn patients with no overt CVD experienced PM-induced cardiovascular changes. | |
| Park [33] | USA | Longitudinal cohort | As a result of PM exposure, individuals with MetSyn had significantly larger decreases in heart rate variability measures than those without MetSyn. Patients with MetSyn experienced a 2.1% decrease in the root mean square of successive differences (95% CI, −4.2–0.0) and a 1.8% decrease in the standard deviation of normal-to-normal intervals (95% CI, −3.7–0.1). | |
| Chang [34] | Taiwan | Case-crossover | Short-term PM exposure increases hospital admissions for CVD. On cool days, PM2.5 exposure was associated with a 47% (95% CI, 39–56%), 48% (95% CI, 40–56%), 47% (95% CI, 34–61%), and 51% (95% CI, 34–70%) increase in ischemic heart disease, stroke, congestive heart failure, and arrhythmias hospital admissions, respectively. | |
| Miller [35] | USA | Prospective cohort | Long-term PM exposure was related to cardiovascular disease and mortality. Each increase of 10 microgram per cubic meter of PM2.5 was associated with a 24% increase in the risk of cardiovascular event (HR, 1.24; 95% CI, 1.09–1.44) and a 76% increase in the risk of death from CVD (HR, 1.76; 95% CI, 1.25–2.47). | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Clementi, E.A.; Talusan, A.; Vaidyanathan, S.; Veerappan, A.; Mikhail, M.; Ostrofsky, D.; Crowley, G.; Kim, J.S.; Kwon, S.; Nolan, A. Metabolic Syndrome and Air Pollution: A Narrative Review of Their Cardiopulmonary Effects. Toxics 2019, 7, 6. https://doi.org/10.3390/toxics7010006
Clementi EA, Talusan A, Vaidyanathan S, Veerappan A, Mikhail M, Ostrofsky D, Crowley G, Kim JS, Kwon S, Nolan A. Metabolic Syndrome and Air Pollution: A Narrative Review of Their Cardiopulmonary Effects. Toxics. 2019; 7(1):6. https://doi.org/10.3390/toxics7010006
Chicago/Turabian StyleClementi, Emily A., Angela Talusan, Sandhya Vaidyanathan, Arul Veerappan, Mena Mikhail, Dean Ostrofsky, George Crowley, James S. Kim, Sophia Kwon, and Anna Nolan. 2019. "Metabolic Syndrome and Air Pollution: A Narrative Review of Their Cardiopulmonary Effects" Toxics 7, no. 1: 6. https://doi.org/10.3390/toxics7010006