Internal Doses of Glycidol in Children and Estimation of Associated Cancer Risk
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Study Population
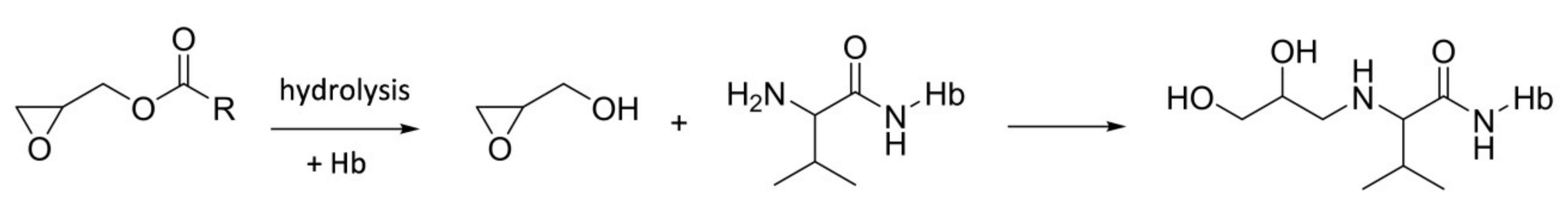
2.3. Synthesis of Internal Standard
2.4. Procedure for Hemoglobin Adduct Measurement
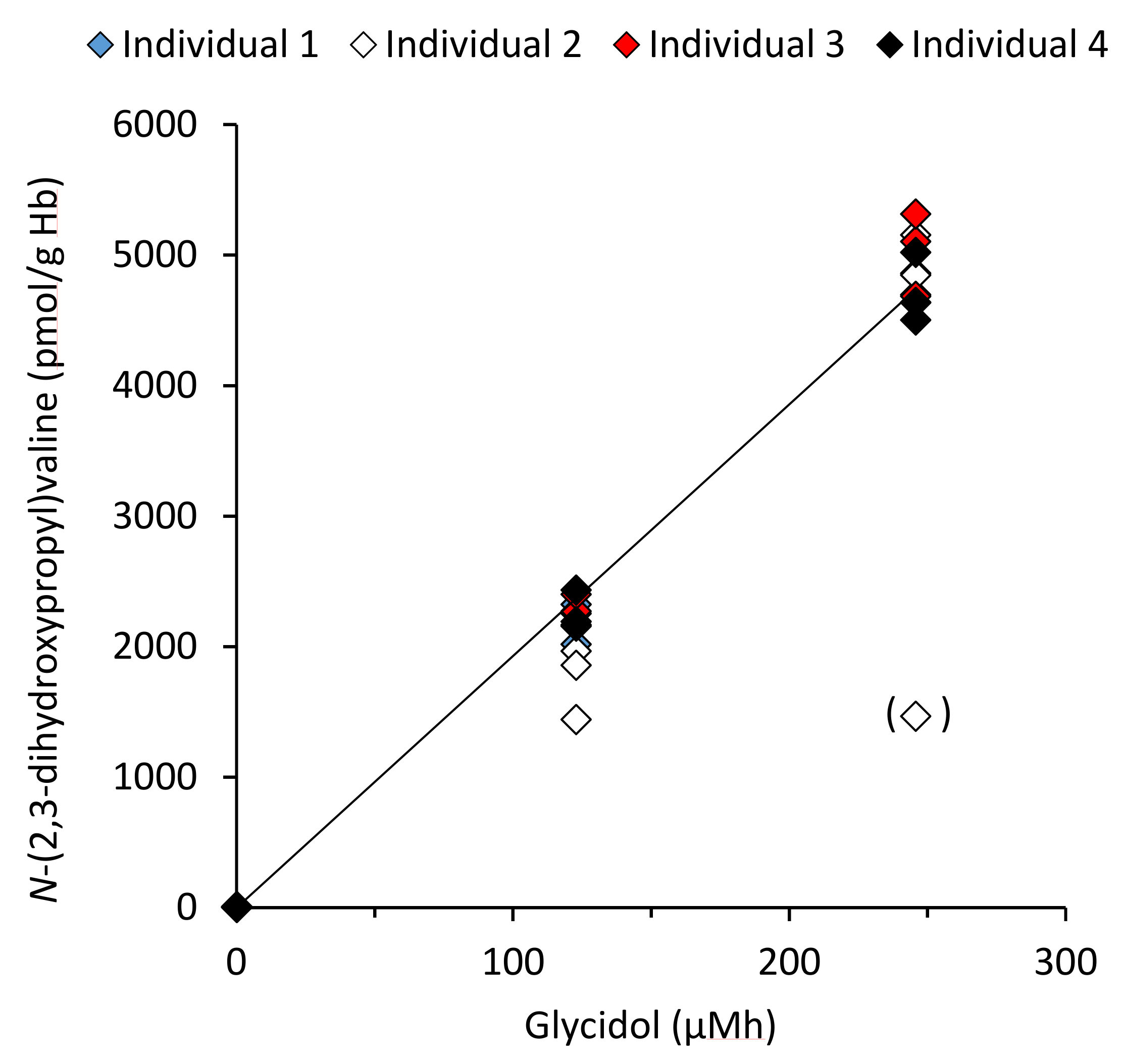
2.5. Measurement of Reaction Rate and Calculation of Internal Dose from Adduct Levels
2.6. LC/MS/MS System
2.7. Statistical Analysis
3. Results
4. Discussion
4.1. N-(2,3-dihydroxypropyl)valine Adduct Levels
4.2. In vivo dose and Intake of Glycidol
4.3. Human Cancer Risk
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Rappaport, S.M. Genetic Factors Are Not the Major Causes of Chronic Diseases. PLoS ONE 2016, 11, e0154387. [Google Scholar] [CrossRef] [PubMed]
- Nadler, D.L.; Zurbenko, I.G. Estimating Cancer Latency Times Using a Weibull Model. Adv. Epidemiol. 2014, 2014, 8. [Google Scholar] [CrossRef]
- EFSA (European Food Safety Authority). Risks for human health related to the presence of 3- and 2-monochloropropanediol (MCPD), and their fatty acid esters, and glycidyl fatty acid esters in food. EFSA J. 2016, 14, 159. [Google Scholar]
- BfR (Federal Institute for Risk Assessment). Initial Evaluation of the Assessment of Levels of Glycidol Fatty Acid Esters Detected in Refined Vegetable Fats. 2009. Available online: https://mobil.bfr.bund.de/cm/349/initial_evaluation_of_the_assessment_of_levels_of_glycidol_fatty_acid_esters.pdf (accessed on 30 March 2009).
- Cheng, W.; Liu, G.; Wang, L.; Liu, Z. Glycidyl Fatty Acid Esters in Refined Edible Oils: A Review on Formation, Occurrence, Analysis, and Elimination Methods. Compr. Rev. Food Sci. Food Saf. 2017, 16, 263–281. [Google Scholar] [CrossRef]
- MacMahon, S.; Begley, T.H.; Diachenko, G.W. Occurrence of 3-MCPD and glycidyl esters in edible oils in the United States. Food Addit Contam. Part A Chem. Anal. Control. Expo. Risk Assess. 2013, 30, 2081–2092. [Google Scholar] [CrossRef] [PubMed]
- Appel, K.E.; Abraham, K.; Berger-Preiss, E.; Hansen, T.; Apel, E.; Schuchardt, S.; Vogt, C.; Bakhiya, N.; Creutzenberg, O.; Lampen, A. Relative oral bioavailability of glycidol from glycidyl fatty acid esters in rats. Arch. Toxicol. 2013, 87, 1649–1659. [Google Scholar] [CrossRef] [PubMed]
- IARC (International Agency for Research on Cancer). Glycidol. Some industrial chemicals. In IARC Monographs on the Evaluation of Carcinogenic Risk of Chemicals to Humans; International Agency for Research on Cancer: Lyon, France, 2000; Volume 77, pp. 469–486. [Google Scholar]
- World Health Organization (WHO). Principles for Evaluating Health Risks in Children Associated with Exposure to Chemicals. In Environmental Health Criteria 23; World Health Organization: Geneva, Switzerland, 2006; p. 351. ISBN 978-92-4-157237-8. [Google Scholar]
- Törnqvist, M.; Mowrer, J.; Jensen, S.; Ehrenberg, L. Monitoring of environmental cancer initiators through hemoglobin adducts by a modified Edman degradation method. Anal. Biochem. 1986, 154, 255–266. [Google Scholar] [CrossRef]
- von Stedingk, H.; Rydberg, P.; Törnqvist, M. A new modified Edman procedure for analysis of N-terminal valine adducts in hemoglobin by LC-MS/MS. J. Chromatogr. B Anal. Technol. Biomed. Life. Sci. 2010, 878, 2483–2490. [Google Scholar] [CrossRef] [PubMed]
- Rydberg, P. Method for analyzing N-terminal protein adducts using isothiocyanate reagents. European Patent EP1738177 WO/2005/101020, 27 October 2005. [Google Scholar]
- Ehrenberg, L.; Moustacchi, E.; Osterman-Golkar, S. Dosimetry of genotoxic agents and dose-response relationships of their effects. Mutat. Res./Rev. Genetic Toxicol. 1983, 123, 121–182. [Google Scholar] [CrossRef]
- Vikström, A.C.; Abramsson-Zetterberg, L.; Naruszewicz, M.; Athanassiadis, I.; Granath, F.N.; Törnqvist, M.A. In vivo doses of acrylamide and glycidamide in humans after intake of acrylamide-rich food. Toxicol. Sci. 2011, 119, 41–49. [Google Scholar] [CrossRef] [PubMed]
- Aasa, J.; Abramsson-Zetterberg, L.; Carlsson, H.; Törnqvist, M. The genotoxic potency of glycidol established from micronucleus frequency and hemoglobin adduct levels in mice. Food Chem. Toxicol. 2016, 100, 168–174. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, M.; von Stedingk, H.; Botsivali, M.; Agramunt, S.; Alexander, J.; Brunborg, G.; Chatzi, L.; Fleming, S.; Fthenou, E.; Granum, B.; et al. Birth weight, head circumference, and prenatal exposure to acrylamide from maternal diet: The European prospective mother-child study (NewGeneris). Environ. Health Perspect. 2012, 120, 1739–1745. [Google Scholar] [CrossRef] [PubMed]
- Carlsson, H.; von Stedingk, H.; Nilsson, U.; Törnqvist, M. LC-MS/MS screening strategy for unknown adducts to N-terminal valine in hemoglobin applied to smokers and nonsmokers. Chem. Res. Toxicol. 2014, 27, 2062–2070. [Google Scholar] [CrossRef] [PubMed]
- Carlsson, H.; Aasa, J.; Kotova, N.; Vare, D.; Sousa, P.F.M.; Rydberg, P.; Abramsson-Zetterberg, L.; Törnqvist, M. Adductomic Screening of Hemoglobin Adducts and Monitoring of Micronuclei in School-Age Children. Chem. Res. Toxicol. 2017. [Google Scholar] [CrossRef] [PubMed]
- von Stedingk, H.; Vikström, A.C.; Rydberg, P.; Pedersen, M.; Nielsen, J.K.; Segerbäck, D.; Knudsen, L.E.; Törnqvist, M. Analysis of hemoglobin adducts from acrylamide, glycidamide, and ethylene oxide in paired mother/cord blood samples from Denmark. Chem. Res. Toxicol. 2011, 24, 1957–1965. [Google Scholar] [CrossRef] [PubMed]
- Törnqvist, M.; Fred, C.; Haglund, J.; Helleberg, H.; Paulsson, B.; Rydberg, P. Protein adducts: Quantitative and qualitative aspects of their formation, analysis and applications. J. Chromatogr. B Anal. Technol. Biomed. Life. Sci. 2002, 778, 279–308. [Google Scholar] [CrossRef]
- Shemin, D.; Rittenberg, D. The life span of the human red blood cell. J. Biol. Chem. 1946, 166, 627–636. [Google Scholar]
- Abraham, K.; Hielscher, J.; Kaufholz, T.; Mielke, H.; Lampen, A.; Monien, B. The hemoglobin adduct N-(2,3-dihydroxypropyl)-valine as biomarker of dietary exposure to glycidyl esters: A controlled exposure study in humans. Arch. Toxicol. 2018. [Google Scholar] [CrossRef]
- Rodgman, A.; Perfetti, T.A. The Chemical Components of Tobacco and Tobacco Smoke, 2nd ed.; CRC Press, Taylor & Francis Group: Boca Raton, FL, USA, 2013. [Google Scholar]
- Schumacher, J.N.; Green, C.R.; Best, F.W.; Newell, M.P. Smoke composition. An extensive investigation of the water-soluble portion of cigarette smoke. J. Agric. Food Chem. 1977, 25, 310–320. [Google Scholar] [CrossRef]
- Hielscher, J.; Monien, B.H.; Abraham, K.; Jessel, S.; Seidel, A.; Lampen, A. An isotope-dilution UPLC-MS/MS technique for the human biomonitoring of the internal exposure to glycidol via a valine adduct at the N-terminus of hemoglobin. J. Chromatogr. B Anal. Technol. Biomed. Life. Sci. 2017, 1059, 7–13. [Google Scholar] [CrossRef]
- Honda, H.; Onishi, M.; Fujii, K.; Ikeda, N.; Yamaguchi, T.; Fujimori, T.; Nishiyama, N.; Kasamatsu, T. Measurement of glycidol hemoglobin adducts in humans who ingest edible oil containing small amounts of glycidol fatty acid esters. Food Chem. Toxicol. 2011, 49, 2536–2540. [Google Scholar] [CrossRef] [PubMed]
- Hindsø Landin, H.; Grummt, T.; Laurent, C.; Tates, A. Monitoring of occupational exposure to epichlorohydrin by genetic effects and hemoglobin adducts. Mutat. Res./Fundam. Mol. Mech. Mutagenesis 1997, 381, 217–226. [Google Scholar] [CrossRef]
- Aasa, J.; Törnqvist, M.; Abramsson-Zetterberg, L. Measurement of micronuclei and internal dose in mice demonstrates that 3-monochloropropane-1,2-diol (3-MCPD) has no genotoxic potency in vivo. Food Chem. Toxicol. 2017, 109, 414–420. [Google Scholar] [CrossRef] [PubMed]
- Lemar, K.M.; Passa, O.; Aon, M.A.; Cortassa, S.; Muller, C.T.; Plummer, S.; O'Rourke, B.; Lloyd, D. Allyl alcohol and garlic (Allium sativum) extract produce oxidative stress in Candida albicans. Microbiology 2005, 151, 3257–3265. [Google Scholar] [CrossRef] [PubMed]
- Hindsø Landin, H.; Tareke, E.; Rydberg, P.; Olsson, U.; Törnqvist, M. Heating of food and haemoglobin adducts from carcinogens: Possible precursor role of glycidol. Food Chem. Toxicol. 2000, 38, 963–969. [Google Scholar] [CrossRef]
- Degner, A.; Carlsson, H.; Karlsson, I.; Eriksson, J.; Pujari, S.S.; Tretyakova, N.Y.; Törnqvist, M. Discovery of Novel N-(4-Hydroxybenzyl)valine Hemoglobin Adducts in Human Blood. Chem. Res. Toxicol. 2018. [Google Scholar] [CrossRef] [PubMed]
- NFA (National Food Agency). 2-MCPD, 3-MCPD och Glycidylfettsyraester i Livsmedel på den Svenska Marknaden. Riskhantering, Riskvärdering och Haltdata; Livsmedelsverkets Rapportserie Nr 35/2017; Livsmedelsverket: Uppsala, Sweden, 2017; p. 42. [Google Scholar]
- Mitlyng, B.L.; Singh, J.A.; Furne, J.K.; Ruddy, J.; Levitt, M.D. Use of breath carbon monoxide measurements to assess erythrocyte survival in subjects with chronic diseases. Am. J. Hematol. 2006, 81, 432–438. [Google Scholar] [CrossRef]
- Aasa, J.; Granath, F.; Törnqvist, M. Cancer risk estimation for glycidol based on rodent carcinogenicity studies and in vivo dosimetry. Food Chem. Toxicol. 2018. under review. [Google Scholar]
- Wakabayashi, K.; Kurata, Y.; Harada, T.; Tamaki, Y.; Nishiyama, N.; Kasamatsu, T. Species differences in toxicokinetic parameters of glycidol after a single dose of glycidol or glycidol linoleate in rats and monkeys. J. Toxicol. Sci. 2012, 37, 691–698. [Google Scholar] [CrossRef]
- National Toxicology Program. NTP Toxicology and Carcinogenesis Studies of Glycidol (CAS No. 556-52-5) In F344/N Rats and B6C3F1 Mice (Gavage Studies). Natl. Toxicol. Program. Tech. Rep. Ser. 1990, 374, 1–229. [Google Scholar]
- Irwin, R.D.; Eustis, S.L.; Stefanski, S.; Haseman, J.K. Carcinogenicity of glycidol in F344 rats and B6C3F1 mice. J. Appl. Toxicol. 1996, 16, 201–209. [Google Scholar] [CrossRef]
- CEPA (California Environmental Protection Agency), Office of Environmental Health Hazard Assessment (OEHHA). No Significant Risk Level (NSRL) for the Proposition 65 Carcinogen Glycidol; California Environmental Protection Agency: Sacramento, CA, USA, 2010; 16p. [Google Scholar]
- Granath, F.N.; Vaca, C.E.; Ehrenberg, L.G.; Törnqvist, M.A. Cancer risk estimation of genotoxic chemicals based on target dose and a multiplicative model. Risk Anal. 1999, 19, 309–320. [Google Scholar] [CrossRef] [PubMed]
- Fred, C.; Törnqvist, M.; Granath, F. Evaluation of cancer tests of 1,3-butadiene using internal dose, genotoxic potency, and a multiplicative risk model. Cancer Res. 2008, 68, 8014–8021. [Google Scholar] [CrossRef] [PubMed]
- Törnqvist, M.; Paulsson, B.; Vikström, A.C.; Granath, F. Approach for cancer risk estimation of acrylamide in food on the basis of animal cancer tests and in vivo dosimetry. J. Agric. Food Chem. 2008, 56, 6004–6012. [Google Scholar] [CrossRef] [PubMed]
- Cancerfonden. Cancerfondsrapporten 2017; Cancerfonden: Vindspelet Grafiska AB, Sweden, 2017. [Google Scholar]
- Kutanzi, K.R.; Lumen, A.; Koturbash, I.; Miousse, I.R. Pediatric Exposures to Ionizing Radiation: Carcinogenic Considerations. Int. J. Environ. Res. Public. Health. 2016, 13, 1057. [Google Scholar] [CrossRef] [PubMed]

| Group | A | B | |||
|---|---|---|---|---|---|
| Hb Adduct (pmol/g Hb) | Daily Hb Adduct Level Increment (pmol/g Hb) | Daily AUC b (nMh) | Daily Intake (µg/kg/day) | ||
| Subjects (n) | Mean a ± SD | Min–Max | Mean a ± SD | Mean ± SD | Mean ± SD |
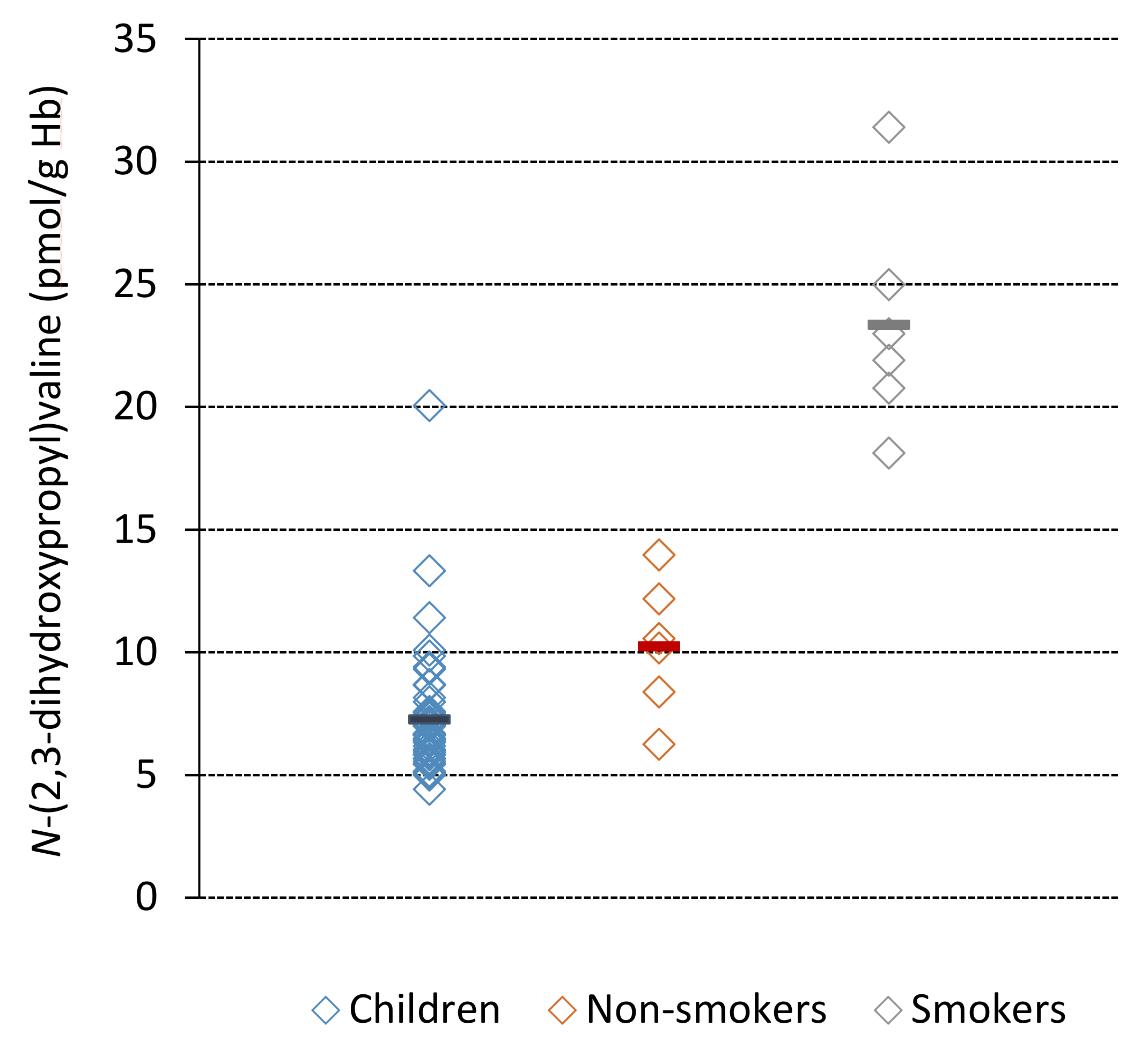
| All children (50) | 7.3 ± 2.5 | 4.4–20.1 | 0.11 ± 0.04 | 6.0 ± 2.1 | 1.4 ± 0.5 |
| Boys (35) c | 7.2 ± 1.8 | 4.4–13.3 | - | - | - |
| Girls (15) c | 7.4 ± 3.7 | 5.0–20.1 | - | - | - |
| Non-smokers (6) | 10.3 ± 2.7 | 6.3–14.0 | 0.16 ± 0.04 | 8.5 ± 2.3 | 2.0 ± 0.5 |
| Smokers (6) d | 23.4 ± 4.6 | 18.1–31.4 | 0.37 ± 0.07 | 19.3 ± 3.8 | 4.5 ± 0.9 |
| Studied Group | No. of Subjects | diHOPrVal (pmol/g globin) | Analytical Method | Reference |
|---|---|---|---|---|
| Non-smokers | 11 | 4.1 ± 0.8 a | LC/MS/MS | [22] |
| Non-smokers | 12 | 3.3 ± 0.8 a | LC/MS/MS | [25] |
| Non-smokers | 6 | 7.1 ± 3.1 b | GC/MS/MS | [26] |
| Non-smokers | 3 | 6.8 ± 3.2 b | GC/MS/MS | [27] |
| Non-smokers | 4 | 2.1 ± 1.1 b | GC/MS/MS | [27] |
| Non-smokers | 6 | 10.3 ± 2.7 a | LC/MS/MS | present study |
| Smokers | 6 | 13.1 ± 12.4 b | GC/MS/MS | [27] |
| Smokers | 6 | 9.5 ± 2.2 b | GC/MS/MS | [27] |
| Smokers | 6 | 23.4 ± 4.6 a | LC/MS/MS | present study |
| Studied Group | Number of Subjects | Daily Intake (μg/kg b.w./day) Mean [Min–Max] | Estimated Lifetime AUC (µMh) Mean (Approximately) |
|---|---|---|---|
| Present study | |||
| Children | 50 | 1.4 [0.9–3.9] | 150 |
| Adults (non-smokers) | 6 | 2.0 [1.2–2.7] | 230 |
| Intake estimated by authorities | |||
| Children (EFSA) | n.a. | 0.6 [0.4–0.9] | - |
| Adults (EFSA) | n.a. | 0.2 [0.2–0.3] | - |
| Adults (NFA) | n.a. | 0.1 | - |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aasa, J.; Vryonidis, E.; Abramsson-Zetterberg, L.; Törnqvist, M. Internal Doses of Glycidol in Children and Estimation of Associated Cancer Risk. Toxics 2019, 7, 7. https://doi.org/10.3390/toxics7010007
Aasa J, Vryonidis E, Abramsson-Zetterberg L, Törnqvist M. Internal Doses of Glycidol in Children and Estimation of Associated Cancer Risk. Toxics. 2019; 7(1):7. https://doi.org/10.3390/toxics7010007
Chicago/Turabian StyleAasa, Jenny, Efstathios Vryonidis, Lilianne Abramsson-Zetterberg, and Margareta Törnqvist. 2019. "Internal Doses of Glycidol in Children and Estimation of Associated Cancer Risk" Toxics 7, no. 1: 7. https://doi.org/10.3390/toxics7010007





