Removal of Nitrate from Drinking Water by Ion-Exchange Followed by nZVI-Based Reduction and Electrooxidation of the Ammonia Product to N2(g)
Abstract
:1. Introduction
Description of the Proposed Process
2. Materials and Methods
2.1. Materials
2.2. Ion-Exchange Experiments
2.3. Synthesis of Nano Zero Valent Iron (nZVI)
2.3.1. Synthesis of nZVI via Fe3+ Reduction
2.3.2. Synthesis of nZVI via Fe2+ Reduction
2.4. Nitrate Reduction by nZVI: Batch Experimental Procedure
2.5. Electrolysis Experiments
2.6. Analyses
2.7. Statistical Analysis
3. Results and Discussion
3.1. Nitrate Adsorption and Regeneration
3.2. nZVI-Induced Nitrate Reduction
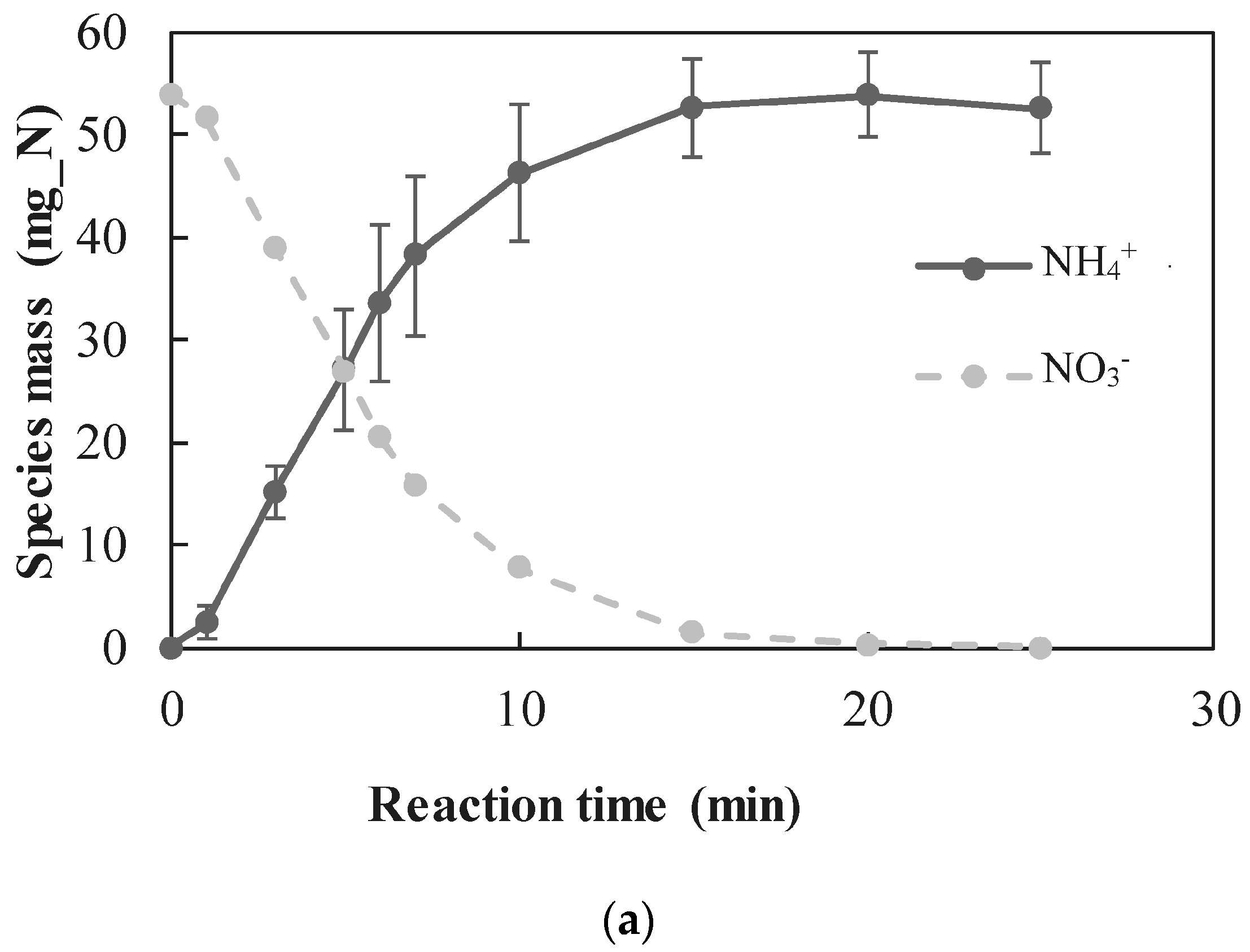
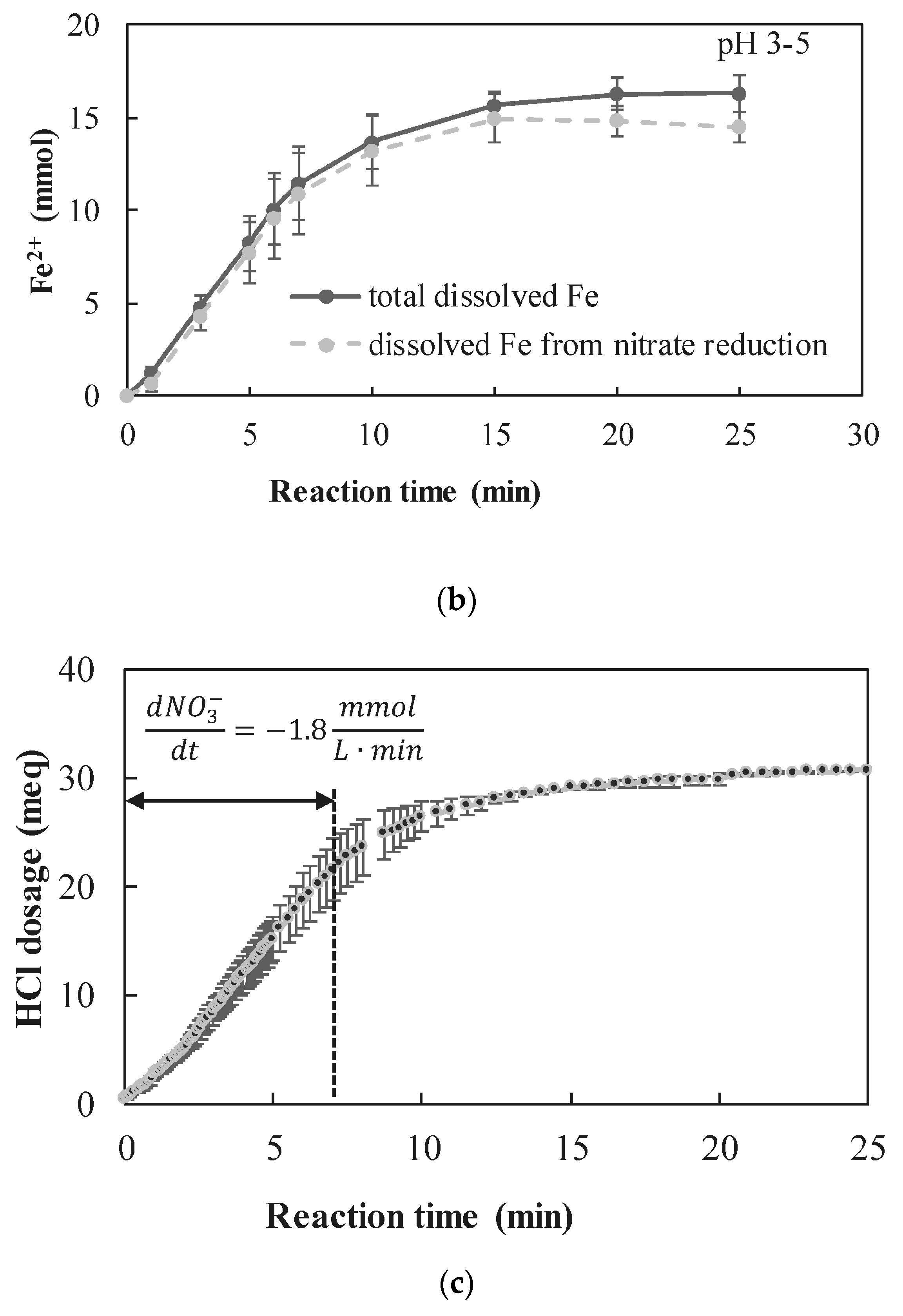
3.2.1. Quantitative Determination of the Dominant Reactions
3.2.2. Observed Reaction Rates
3.3. Effect of Cl− Ion Concentration on the Rate of Nitrate Reduction by nZVI
3.4. Effect of nZVI Excess over the Stoichiometric Ratio
3.5. Synthesizing nZVI from the Solution Resulting from the Nitrate Reduction Step
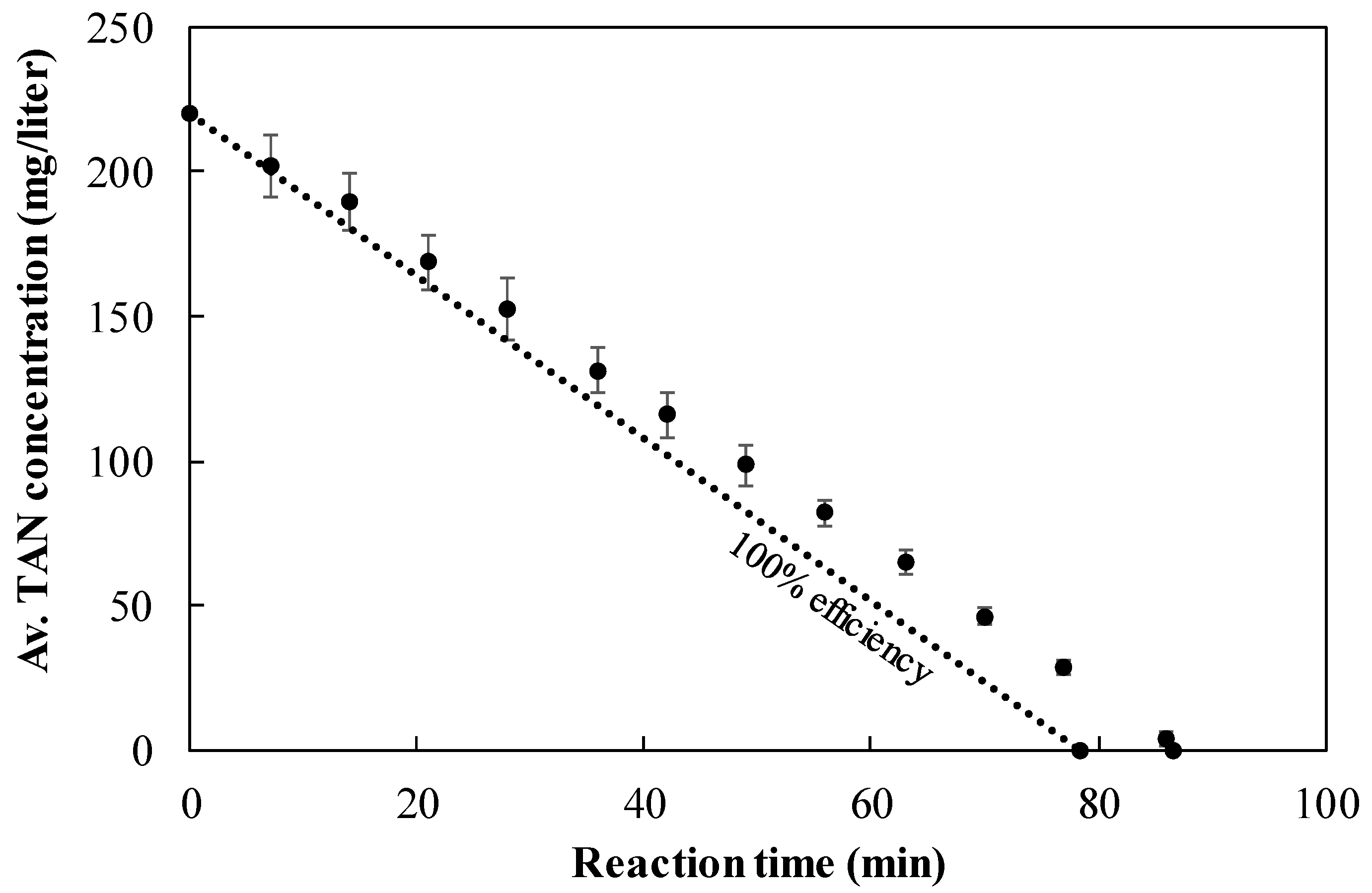
3.6. TAN Electrooxidation Step
4. Conclusions
- A new process for treating nitrate-polluted drinking water, consisting of anionic exchange followed by nano Fe-induced NO3− reduction and TAN electrooxidation, was described. The process consists of five steps and applies three different technologies: ion-exchange, chemical reduction by nZVI and electro-oxidation. In these steps, nitrate is separated from the polluted water, reduced to TAN at low pH, and, finally, TAN is oxidized into nitrogen gas. This sequence, plus a step in which new n-ZVI is generated from the effluent solution of the nitrate reduction step, allows reusing the regeneration solution for many cycles. A significant advantage of the presented process is that the chemical treatment is not carried out directly on the drinking water, making post treatment redundant. The process is sustainable in the sense that the end product is benign N2(g), the chemicals used (NaCl and nZVI) and also water are mostly (~80%, ~100% and ~80%, respectively) recovered and brine disposal is minimized by a factor of 5.
- ~80% of the main process commodities are recycled, resulting in a significant improvement over conventional IX processes, which neither recover brine nor reduce the disposed volume. Similarly, conventional nitrate reduction using nZVI is not accompanied by any Fe(II) recovery and TAN removal, as applied in the current process.
- A new approach for quantitative determination of the dominant reactions in nitrate reduction by nZVI was applied. The method is based on the measuring nitrogen species, dissolved Fe(II) concentrations, and acid consumption for maintaining constant pH. The data was analyzed using mass balance calculations and tested statistically to test its compatibility with previously published reactions. The Wilcoxon test was used to determine that only two reactions were dominant at acidic pH range, i.e., and Thus, the notion appearing in a few works, that NO3− is partly reduced by nZVI to N2(g), was disproved.
- Distinct operational conditions (corresponding to the expected characterization of ion-exchange regenerant solution) were tested for conducting the chemical reduction of nitrate by nZVI: high Cl− concentrations of 45 g Cl−/L along with high nitrate concentrations of 250 mg N/L. The effect of the presence of high Cl− concentration was found not to inhibit the nitrate reduction rate, implying that the use of nZVI for nitrate reduction within concentrated brines is feasible. Large excess of nZVI was found to increase the nitrate reduction rate compared to operation at close to stoichiometric ratio. In the proposed process, applying excess nZVI does not affect the economic feasibility, since the nZVI can be collected and reused.
- A drawback of the proposed process that should be addressed in future work relates to the removal of by-products from the nZVI synthesis procedure (e.g., B(OH)3), which may accumulate in the regenerant solution if not separated at predetermined intervals.
- The nZVI enhanced nitrate reduction was found to follow a zero order rate, with a rate coefficient of ~5 mmol NO3− L−1 min−1 when nZVI was in high excess and ~1.8 mmol NO3− L−1 min−1 when nZVI was only slightly above the stoichiometric dose.
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Pérez-martín, M.A.; Estrela, T. Science of the total environment measures required to reach the nitrate objectives in groundwater based on a long-term nitrate model for large river basins (Júcar, Spain). Sci. Total Environ. 2016, 566–567, 122–133. [Google Scholar] [CrossRef] [PubMed]
- Choe, J.K.; Mehnert, M.H.; Guest, J.S.; Strathmann, T.J.; Werth, C.J. Comparative assessment of the environmental sustainability of existing and emerging perchlorate treatment technologies for drinking water. Environ. Sci. Technol. 2013, 47, 4644–4652. [Google Scholar] [CrossRef] [PubMed]
- Pintar, A.; Batista, J.; Levec, J. Integrated ion exchange/catalytic process for efficient removal of nitrates from drinking water. Chem. Eng. Sci. 2001, 56, 1551–1559. [Google Scholar] [CrossRef]
- Kim, Y.; Kim, M.Y.; Choi, M. Synergistic integration of catalysis and ion-exchange for highly selective reduction of nitrate into N2. Chem. Eng. J. 2016, 289, 423–432. [Google Scholar] [CrossRef]
- Shi, J.; Long, C.; Li, A. Selective reduction of nitrate into nitrogen using Fe-Pd bimetallic nanoparticle supported on chelating resin at near-neutral pH. Chem. Eng. J. 2016, 286, 408–415. [Google Scholar] [CrossRef]
- Meng, X.; Vaccari, D.A.; Zhang, J.; Fiume, A. Bioregeneration of spent anion exchange resin for treatment of nitrate in water. Environ. Sci. Technol. 2014, 48, 1541–1548. [Google Scholar] [CrossRef] [PubMed]
- Dortsiou, M.; Katsounaros, I.; Polatides, C.; Kyriacou, G. Electrochemical removal of nitrate from the spent regenerant solution of the ion exchange. Desalination 2009, 248, 923–930. [Google Scholar] [CrossRef]
- Kabay, N.; Yüksel, M.; Samatya, S.; Arar, Ö.; Yüksel, Ü. Removal of Nitrate from Ground Water by a Hybrid Process Combining Electrodialysis and Ion Exchange Processes. Sep. Sci. Technol. 2007, 42, 2615–2627. [Google Scholar] [CrossRef]
- Bergquist, A.M.; Kwon, J.; Strathmann, T.J.; Werth, C.J. Evaluation of a hybrid ion exchange-catalyst treatment technology for nitrate removal from drinking water. Water Res. 2016, 96, 177–187. [Google Scholar] [CrossRef] [PubMed]
- Archna; Sharma, S.K.; Sobti, R.C. Nitrate removal from ground water. EJ. Chem. 2012, 9, 1667–1675. [Google Scholar]
- Ileri, B.; Ayyildiz, O.; Apaydin, O. Ultrasound-assisted activation of zero-valent magnesium for nitrate denitrification: Identification of reaction by-products and pathways. J. Hazard. Mater. 2015, 292, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.; Chakraborty, S. Chemical denitrification of water by zero-valent magnesium powder. J. Hazard. Mater. 2006, 135, 112–121. [Google Scholar] [CrossRef] [PubMed]
- Hwang, Y.-H.; Kim, D.-G.; Shin, H.-S. Mechanism study of nitrate reduction by nano zero valent iron. J. Hazard. Mater. 2011, 185, 1513–1521. [Google Scholar] [CrossRef] [PubMed]
- Ryu, A.; Jeong, S.; Jang, A.; Choi, H. Applied Catalysis B: Environmental Reduction of highly concentrated nitrate using nanoscale zero-valent iron: Effects of aggregation and catalyst on reactivity. Appl. Catal. B Environ. 2011, 105, 128–135. [Google Scholar] [CrossRef]
- Song, Y.; Song, S. Preparation, characterization, and kinetics of nanoscale iron in nitrate nitrogen removal from polluted water. Toxicol. Environ. Chem. 2016, 97, 379–387. [Google Scholar] [CrossRef]
- Choe, S.; Chang, Y.Y.; Hwang, K.Y.; Khim, J. Kinetics of reductive denitrification by nanoscale zero-valent iron. Chemosphere 2000, 41, 1307–1311. [Google Scholar] [CrossRef]
- Yang, G.C.C.; Lee, H.L. Chemical reduction of nitrate by nanosized iron: Kinetics and pathways. Water Res. 2005, 39, 884–894. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Z.; Zhao, D.; Pan, G. Rapid and controlled transformation of nitrate in water and brine by stabilized iron nanoparticles. J. Nanopart. Res. 2009, 11, 807–819. [Google Scholar] [CrossRef]
- Zhang, J.; Hao, Z.; Zhang, Z.; Yang, Y.; Xu, X. Kinetics of nitrate reductive denitrification by nanoscale zero-valent iron. Process. Saf. Environ. Prot. 2010, 88, 439–445. [Google Scholar] [CrossRef]
- Hwang, Y.H.; Kim, D.G.; Ahn, Y.T.; Moon, C.M.; Shin, H.S. Fate of nitrogen species in nitrate reduction by nanoscale zero valent iron and characterization of the reaction kinetics. Water Sci. Technol. 2010, 61, 705–712. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Jin, Z.; Li, T.; Zhang, H.; Gao, S. Preparation of spherical iron nanoclusters in ethanol–water solution for nitrate removal. Chemosphere 2006, 65, 1396–1404. [Google Scholar] [CrossRef] [PubMed]
- Noubactep, C. Comments on “Mechanism study of nitrate reduction by nano zero valent iron” by Hwang et al. J. Hazard. Mater. 2011, 186, 946–947. [Google Scholar] [CrossRef] [PubMed]
- Hwang, Y.; Kim, D.; Shin, H. Inhibition of nitrate reduction by NaCl adsorption on a nano-zero-valent iron surface during a concentrate treatment for water reuse. Environ. Technol. 2014, 3330, 1–31. [Google Scholar] [CrossRef] [PubMed]
- Siciliano, A. Use of nanoscale zero-valent iron (nZVI) particles for chemical denitrification under different operating conditions. Metals 2015, 5, 1507–1519. [Google Scholar] [CrossRef]
- Huang, C.-P.; Wang, H.-W.; Chiu, P.-C. Nitrate reduction by metallic iron. Water Res. 1998, 32, 2257–2264. [Google Scholar] [CrossRef]
- Choe, S.; Liljestrand, H.M.; Khim, J. Nitrate reduction by zero-valent iron under different pH regimes. Appl. Geochem. 2004, 19, 335–342. [Google Scholar] [CrossRef]
- Gendel, Y.; Lahav, O. Revealing the mechanism of indirect ammonia electrooxidation. Electrochim. Acta 2012, 63, 209–219. [Google Scholar] [CrossRef]
- Gendel, Y.; Lahav, O. A novel approach for ammonia removal from fresh-water recirculated aquaculture systems, comprising ion exchange and electrochemical regeneration. Aquac. Eng. 2013, 52, 27–38. [Google Scholar] [CrossRef]
- Wang, C.; Zhang, W. Synthesizing nanoscale iron particles for rapid and complete dechlorination of TCE and PCBs. Environ. Sci. Technol. 1997, 31, 2154–2156. [Google Scholar] [CrossRef]
- APHA. Standard Methods for the Examination of Water and Wastewater, 20th ed.; American Public Health Association, American Water Works Association and Water Environmental Federation: Washington, DC, USA, 1998. [Google Scholar]
- Willis, R.B.; Montgomery, M.E.; Allen, P.R. Improved method for manual, colorimetric determination of total kjeldahl nitrogen using salicylate. J. Agric. Food Chem. 1996, 44, 1804–1807. [Google Scholar] [CrossRef]
- Herrera, L.; Ruiz, P.; Aguillon, J.C.; Fehrmann, A. A new spectrophotometric method for the determination of ferrous iron in the presence of ferric iron. Chem. Technol. Biotechnol. 1989, 44, 171–181. [Google Scholar] [CrossRef]
- Primo, O.; Rivero, M.J.; Urtiaga, A.M.; Ortiz, I. Nitrate removal from electro-oxidized landfill leachate by ion exchange. J. Hazard. Mater. 2009, 164, 389–393. [Google Scholar] [CrossRef] [PubMed]
- Shams, S. Assessing Innovative Technologies for Nitrate Removal from Drinking Water. Master’s Thesis, University of Waterloo, Waterloo, ON, Canada, 2010. [Google Scholar]
- Morgan, B.; Lahav, O. The effect of pH on the kinetics of spontaneous Fe(II) oxidation by O2 in aqueous solution—Basic principles and a simple heuristic description. Chemosphere 2007, 68, 2080–2084. [Google Scholar] [CrossRef] [PubMed]
- Lahav, O.; Asher, R.B.; Gendel, Y. Potential applications of indirect electrochemical ammonia oxidation within the operation of freshwater and saline-water recirculating aquaculture systems. Aquac. Eng. 2015, 65, 55–64. [Google Scholar] [CrossRef]

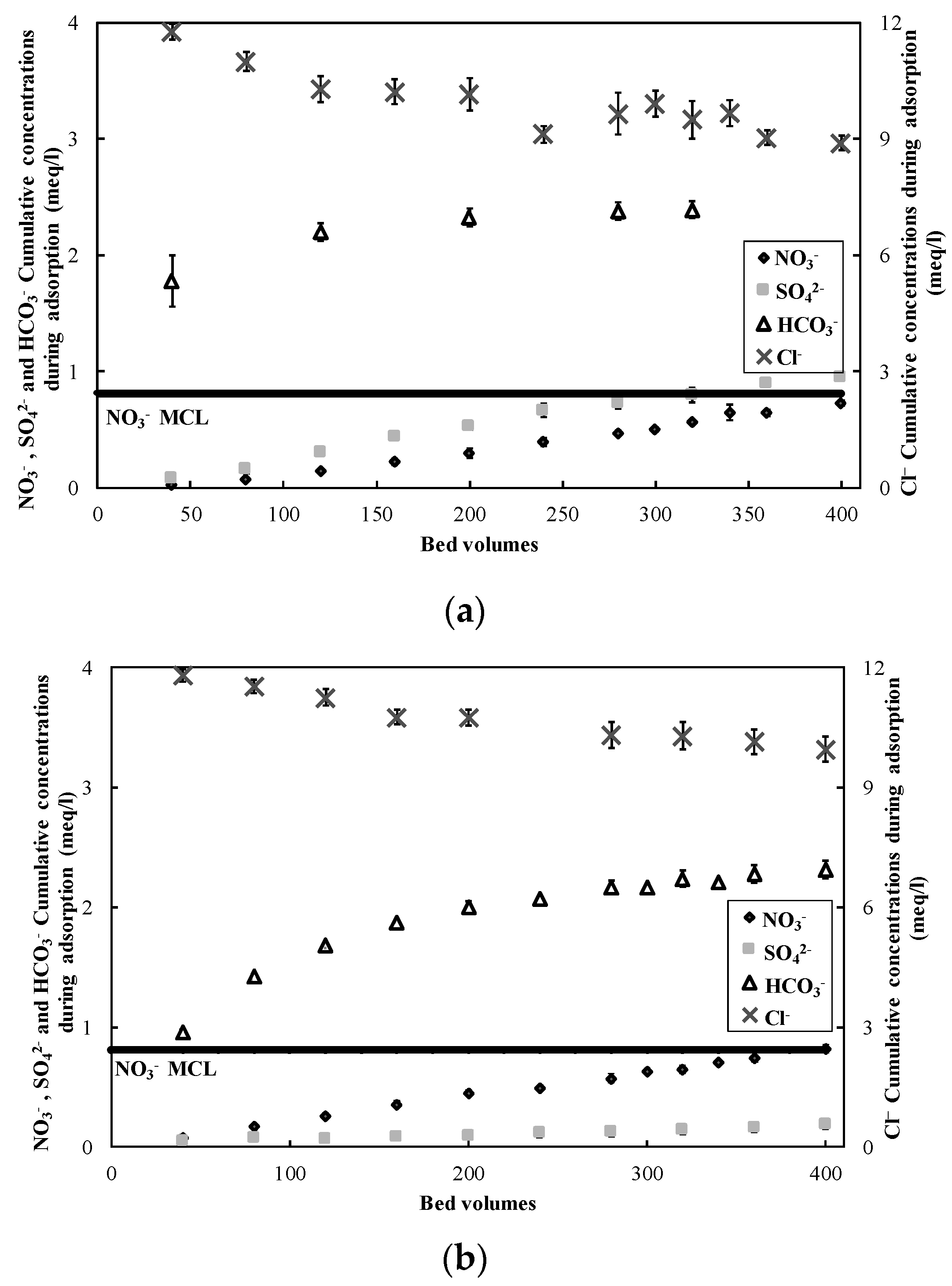
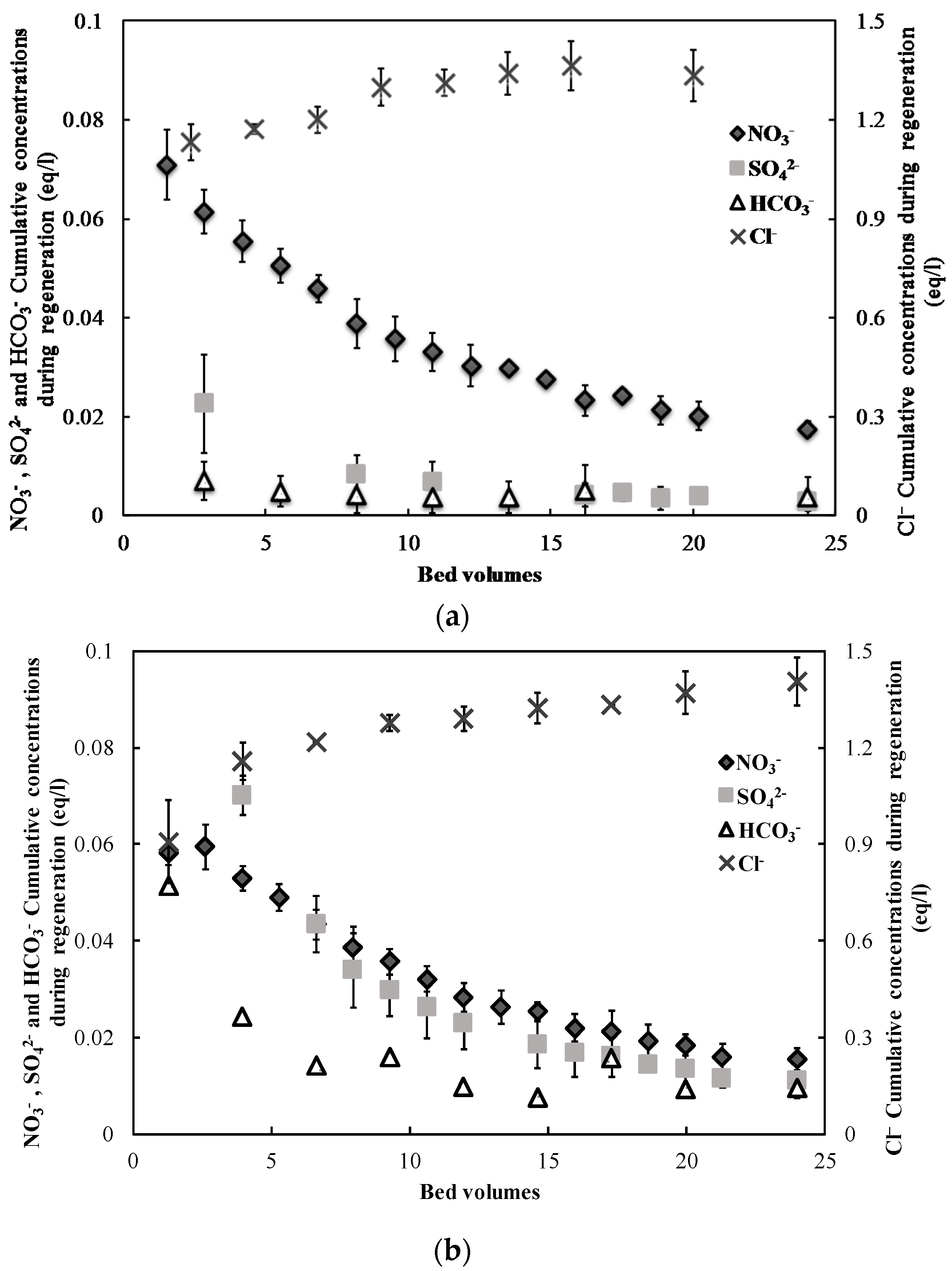
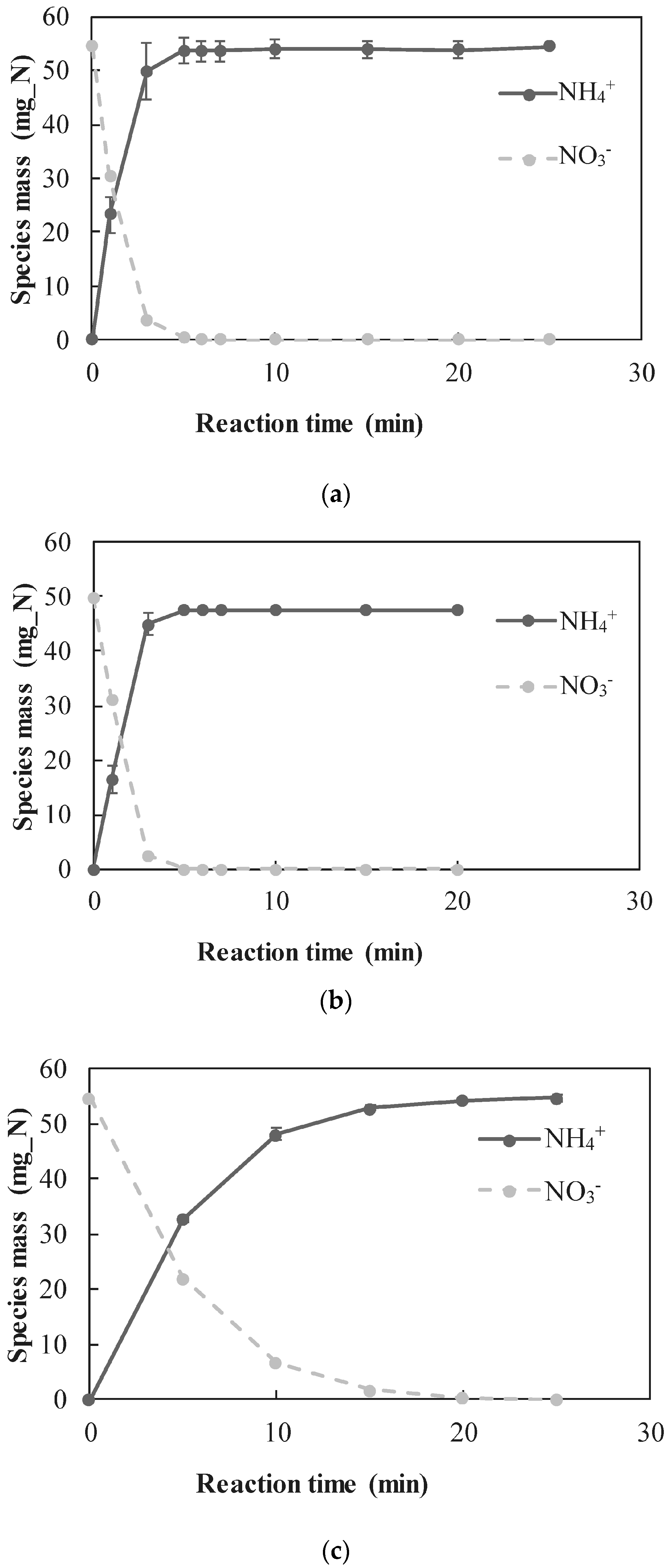
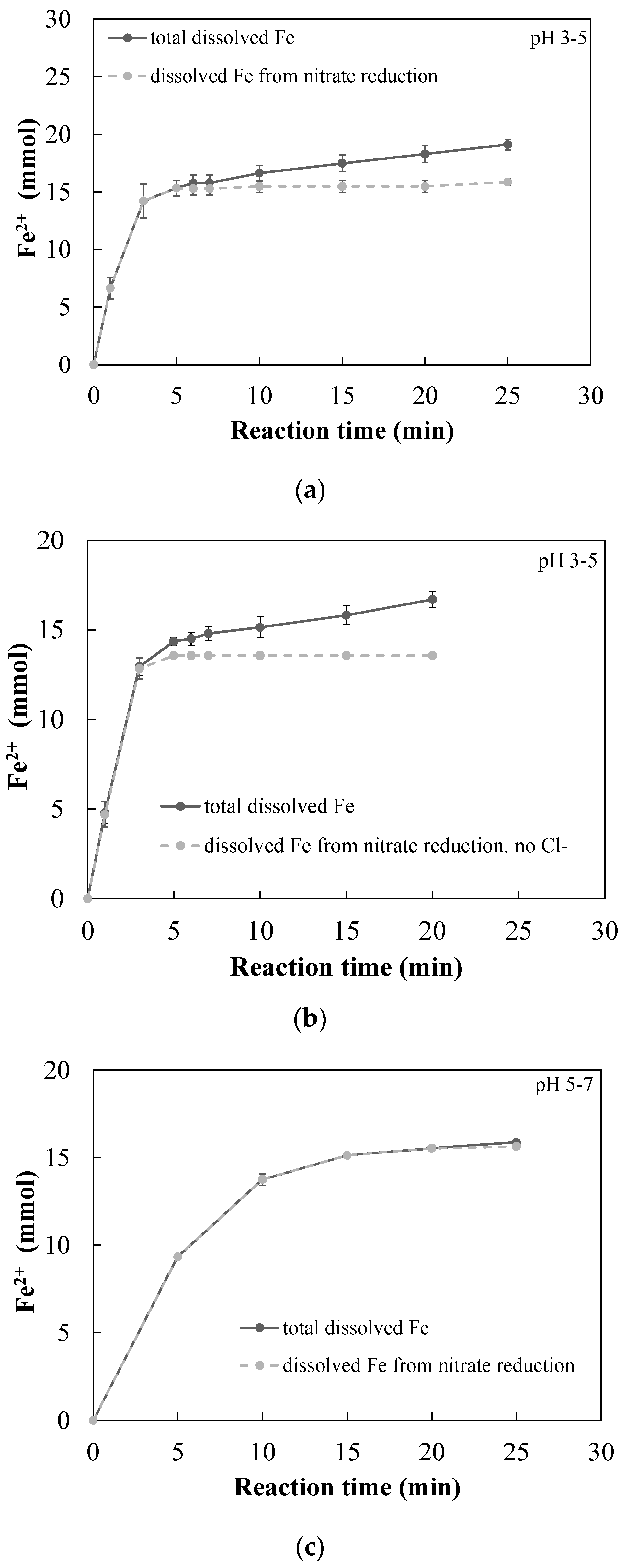

| Solution | SO42− (mg S/L) | NO3− (mg N/L) | Cl− (mg/L) | Alkalinity (mg CaCO3/L) |
|---|---|---|---|---|
| Regeneration solution | 44 | 253 | 45,246 | 162 |
| Synthetic solution | 64 | 256 | 0 | 281 |
| Exp. No. | pH Range | Duration | End Product Concentration Relative to [NO3−]initial | Ratio between Acid Dosage and Nitrate Reduced | Mass Balance Error (Actual vs. Anticipated Acid Consumption) | |
|---|---|---|---|---|---|---|
| min | TAN (%) | Fe2+ (mol/mol) | meq/mg NO3− as N | % | ||
| #1 | 2–4 | 41 | 90.13 | 8.074 ± 0.715 | 1.28 ± 0.09 | 0.054 |
| #2 | 3–5 | 52 | 91.76 | 7.277 ± 0.169 | 1.17 ± 0.02 | –253 |
| #3 | 4–6 | 38 | 87.89 | 6.968 ± 1.007 | 1.13 ± 0.11 | 0.939 |
| #4 | 5–7 | 30 | 98.70 | 4.300 ± 0.291 | 0.68 ± 0.12 | –12.153 |
| #5 | 3–5 | 25 | 100.0 | 4.673 ± 0.114 | 0.82 ± 0.01 | 0.676 |
| #6 * | 3–5 | 28 | 100.0 | 4.264 ± 0.158 | 0.73 ± 0.01 | –4.172 |
| #7 ** | 3–5 | 28 | 100.0 | 5.540 ± 0.403 | 0.89 ± 0.06 | –4.361 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fux, I.; Birnhack, L.; Tang, S.C.N.; Lahav, O. Removal of Nitrate from Drinking Water by Ion-Exchange Followed by nZVI-Based Reduction and Electrooxidation of the Ammonia Product to N2(g). ChemEngineering 2017, 1, 2. https://doi.org/10.3390/chemengineering1010002
Fux I, Birnhack L, Tang SCN, Lahav O. Removal of Nitrate from Drinking Water by Ion-Exchange Followed by nZVI-Based Reduction and Electrooxidation of the Ammonia Product to N2(g). ChemEngineering. 2017; 1(1):2. https://doi.org/10.3390/chemengineering1010002
Chicago/Turabian StyleFux, Inbal, Liat Birnhack, Samuel C.N. Tang, and Ori Lahav. 2017. "Removal of Nitrate from Drinking Water by Ion-Exchange Followed by nZVI-Based Reduction and Electrooxidation of the Ammonia Product to N2(g)" ChemEngineering 1, no. 1: 2. https://doi.org/10.3390/chemengineering1010002





