The Effect of Off-Spec Canola Biodiesel Blending on Fuel Properties for Cold Weather Applications
Abstract
1. Introduction
2. Experimental Details
3. Results and Discussion
3.1. Density
3.2. Viscosity
3.3. Flash Point
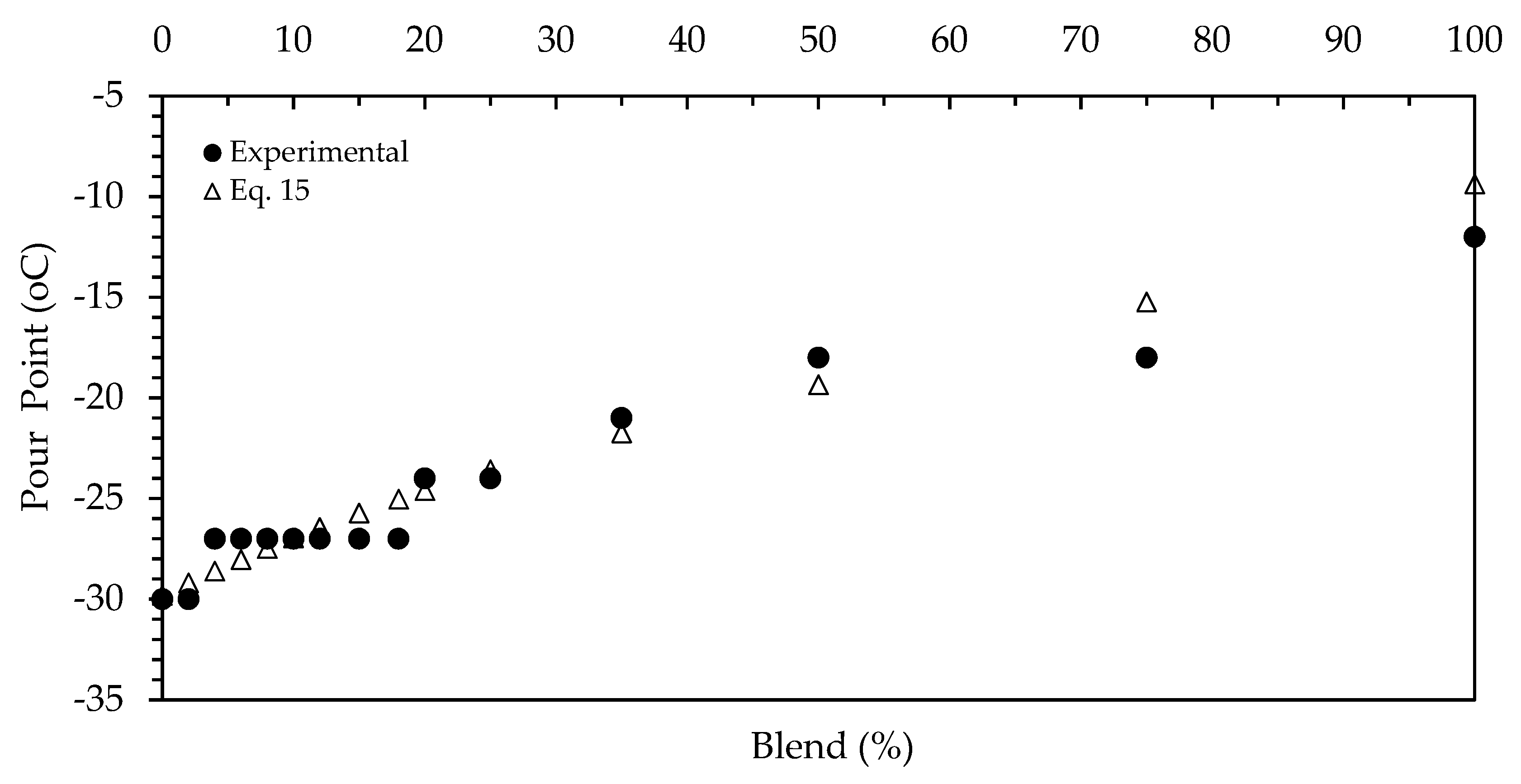
3.4. Cloud Point and Pour Point
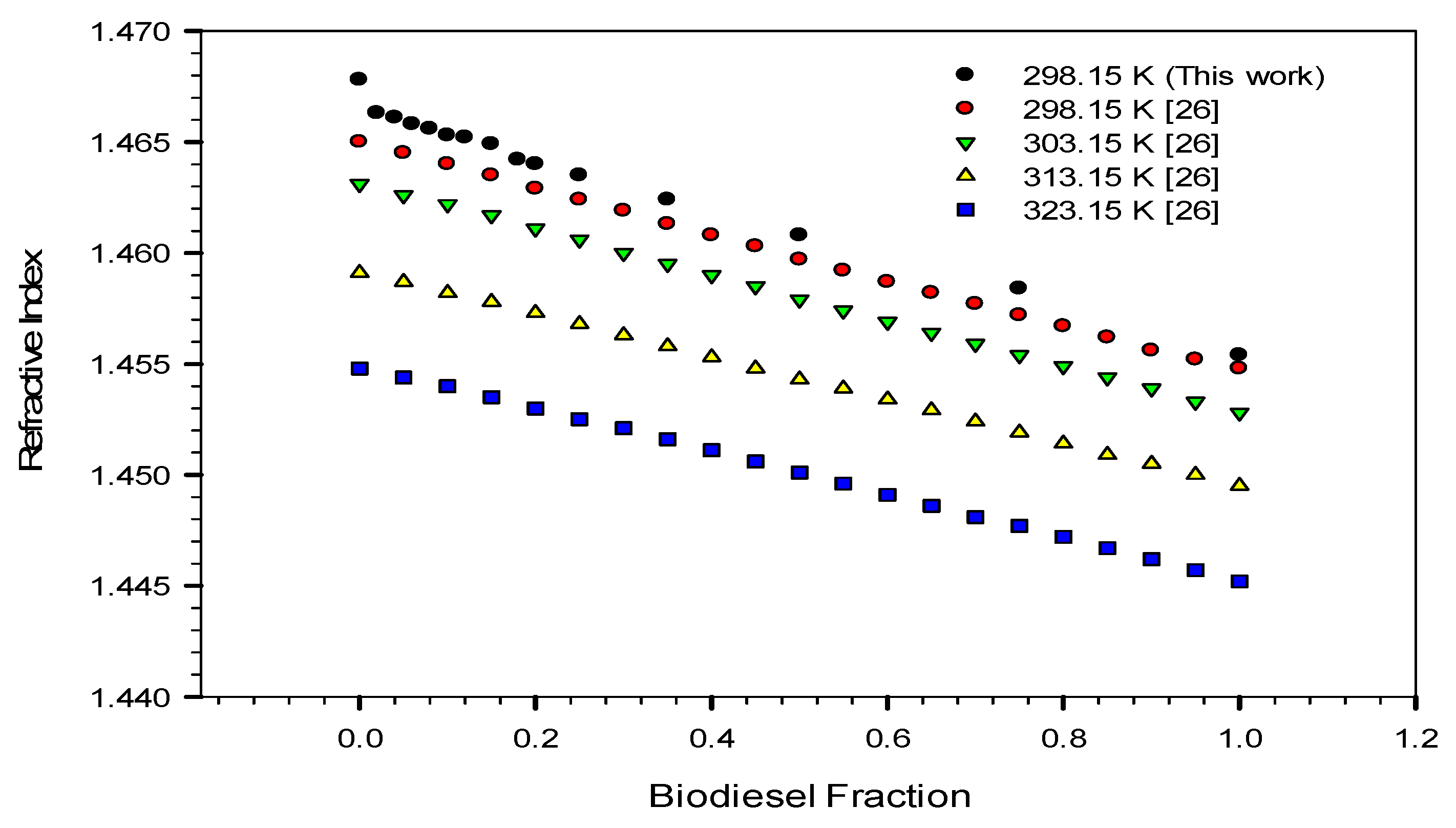
3.5. Refractive Index
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gerpen, J.V. Biodiesel processing and production. Fuel Process. Technol. 2005, 86, 1097–1107. [Google Scholar] [CrossRef]
- Armas, O.; Martínez, S.M.; Mata, M.; Pacheco, C. Alternative method for bulk modulus estimation of diesel fuels. Fuel 2016, 167, 199–207. [Google Scholar] [CrossRef]
- Mosesane, M.J.; Mbaya, R.K.; Tshabalala, L.C.; Kalombo, L. Characterization of fuel properties for the biodiesel-petro-diesel blends dosed with the FPC. Glob. J. Res. Eng. J. Gen. Eng. 2015, 15, 1–7. [Google Scholar]
- Mustafa, E.T.; Van Gerpen, J.H. The kinematic viscosity of biodiesel and its blends with diesel fuel. J. Am. Oil Chem. Soc. 1999, 76, 1511–1513. [Google Scholar]
- Kerschbaum, S.; Rinke, G. Measurement of the temperature dependent viscosity of biodiesel fuels. Fuel 2004, 83, 287–291. [Google Scholar] [CrossRef]
- Benjumea, P.; Agudelo, J.; Agudelo, A. Basic properties of palm oil biodiesel–diesel blends. Fuel 2008, 87, 2069–2075. [Google Scholar] [CrossRef]
- Alptekin, E.; Canakci, M. Characterization of the key fuel properties of methyl ester–diesel fuel blends. Fuel 2009, 88, 75–80. [Google Scholar] [CrossRef]
- Knothe, G.; Matheaus, A.C.; Ryan, T.W. Cetane numbers of branched and straight chain fatty esters determined in an ignition quality tester. Fuel 2003, 82, 971–975. [Google Scholar] [CrossRef]
- Knothe, G. Dependence of biodiesel fuel properties on the structure of fatty acid alkyl esters. Fuel Process. Technol. 2005, 86, 1059–1070. [Google Scholar] [CrossRef]
- Chevron Product Company. Diesel Fuels Technical Review (FTR-2); Chevron Product Company: San Ramon, CA, USA, 1998. [Google Scholar]
- Sivaramakrishnan, K. Determination of higher heating values of biodiesels. Int. J. Eng. Sci. Technol. 2011, 3, 7981–7987. [Google Scholar]
- Yoon, S.K.; Kim, M.S.; Kim, H.J.; Choi, N.J. Effects of canola oil biodiesel fuel blends on combustion, performance, and emissions reduction in a common rail diesel engine. Energies 2014, 7, 8132–8149. [Google Scholar] [CrossRef]
- National Biodiesel Board, OEM Warranty Statements and Use of Biodiesel Blends over 5% (B5). Available online: http://biodiesel.org/docs/default-source/ffs-engine_manufacturers/oem-warranty-statement-and-use-of-biodiesel-blends-over-5-(b5).pdf?sfvrsn=6 (accessed on 12 November 2017).
- Specifications for Biodiesel, National Biodiesel Board, December 2001. Available online: http://biodiesel.org/docs/default-source/ffs-production/fuel-quality-policy-update.pdf?sfvrsn=4 (accessed on 10 January 2018).
- Alptekin, E.; Canakci, M. Determination of the density and the viscosities of biodiesel–diesel fuel blends. Renew. Energy 2008, 33, 2623–2630. [Google Scholar] [CrossRef]
- Geacai, S.; Iulian, O.; Nita, I. Measurement, correlation and prediction of biodiesel blends viscosity. Fuel 2015, 143, 268–274. [Google Scholar] [CrossRef]
- Wakil, M.A.; Kalam, M.A.; Masjuki, H.H.; Atabani, A.E.; Fattah, I.M.R. Influence of biodiesel blending on physicochemical properties and importance of mathematical model for predicting the properties of biodiesel blend. Energy Convers. Manag. 2015, 94, 51–67. [Google Scholar] [CrossRef]
- Chang, A.; Pashikanti, K.; Liu, Y. Refinery Engineering; Wiley-VCH: Weinheim, Germany, 2012. [Google Scholar]
- Saxena, P.; Jawale, S.; Joshipura, M.H. A review on prediction of properties of biodiesel and blends of biodiesel. Procedia Eng. 2013, 51, 395–402. [Google Scholar] [CrossRef]
- Grunberg, L.; Nissan, A. Mixture Law for viscosity. Nature 1949, 164, 799–800. [Google Scholar] [CrossRef] [PubMed]
- Hasan, M.M.; Rahman, M.M. Performance and emission characteristics of biodiesel–diesel blend and environmental and economic impacts of biodiesel production: A review. Renew. Sustain. Energy Rev. 2017, 74, 938–948. [Google Scholar] [CrossRef]
- Muñoz, M.; Moreno, F.; Monné, C.; Morea, J.; Terradillos, J. Biodiesel improves lubricity of new low sulphur diesel fuels. Renew. Energy 2011, 36, 2918–2924. [Google Scholar] [CrossRef]
- Nita, I.; Geacai, S.; Neagu, A.; Geacai, E. Estimation of the refractive index of diesel fuel+biodiesel blends. Ovidius Univ. Ann. Chem. 2013, 24, 24–26. [Google Scholar] [CrossRef]







| Property | Canola Biodiesel | Diesel Fuel |
|---|---|---|
| Density at 15 °C g/cm3 | 0.8828 | 0.8424 |
| Viscosity at 40 °C, cSt | 4.3401 | 2.7109 |
| Flash point °C | 107 | 58 |
| Calculated Cetane number | 61.5 | 57.8 |
| Pour point, °C | −8 | −6 |
| Acid number, mg KOH/g | 0.16 | - |
| Free glycerin % mass | 0.010 | - |
| Total glycerin % mass | 0.12 | - |
| Ester content % mass | 99.2 | - |
| Distillation, °C | ||
| 10% | 350 | 228 |
| 50% | 352 | 283 |
| 90% | 359 | 350 |
| End point | 382 | 372 |
| Blend (%) | Density (kg/L) | Kin. Viscosity (cSt) | Flash Point (°C) | Pour Point (°C) | Cloud Point (°C) |
|---|---|---|---|---|---|
| 0 | 0.84224 | 3.012 | 71 | −30 | −12 |
| 2 | 0.84254 | 3.053 | 74.44 | −30 | −12.3 |
| 4 | 0.843 | 3.0647 | 72.22 | −27 | −12 |
| 6 | 0.84362 | 3.1109 | 73.33 | −27 | −12 |
| 8 | 0.8446 | 3.1735 | 73.33 | −27 | −11.5 |
| 10 | 0.8455 | 3.2382 | 74.44 | −27 | −11.8 |
| 12 | 0.8465 | 3.2912 | 74.44 | −27 | −11.7 |
| 15 | 0.8471 | 3.31627 | 75.55 | −27 | −10.7 |
| 18 | 0.8478 | 3.3546 | 74.44 | −27 | −10.3 |
| 20 | 0.848 | 3.39 | 75.55 | −24 | −10.4 |
| 25 | 0.84991 | 3.45 | 77.77 | −24 | −9.9 |
| 35 | 0.8535 | 3.6556 | 78.88 | −21 | −9.2 |
| 50 | 0.8585 | 3.9865 | 84.44 | −18 | −7.8 |
| 75 | 0.86792 | 4.4849 | 98.88 | −18 | −5.5 |
| 100 | 0.87654 | 5.2443 | 170 | −12 | −2.4 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hassan, U.; Al-Zubaidi, I.; Ibrahim, H. The Effect of Off-Spec Canola Biodiesel Blending on Fuel Properties for Cold Weather Applications. ChemEngineering 2018, 2, 30. https://doi.org/10.3390/chemengineering2030030
Hassan U, Al-Zubaidi I, Ibrahim H. The Effect of Off-Spec Canola Biodiesel Blending on Fuel Properties for Cold Weather Applications. ChemEngineering. 2018; 2(3):30. https://doi.org/10.3390/chemengineering2030030
Chicago/Turabian StyleHassan, Ubaid, Isam Al-Zubaidi, and Hussameldin Ibrahim. 2018. "The Effect of Off-Spec Canola Biodiesel Blending on Fuel Properties for Cold Weather Applications" ChemEngineering 2, no. 3: 30. https://doi.org/10.3390/chemengineering2030030
APA StyleHassan, U., Al-Zubaidi, I., & Ibrahim, H. (2018). The Effect of Off-Spec Canola Biodiesel Blending on Fuel Properties for Cold Weather Applications. ChemEngineering, 2(3), 30. https://doi.org/10.3390/chemengineering2030030






