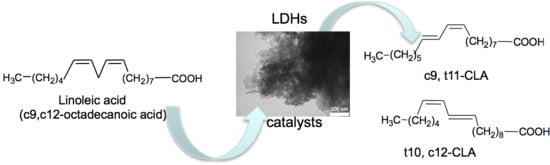
Layered Double Hydroxides for the Catalytic Isomerization of Linoleic Acid to Conjugated Linoleic Acids (CLAs)
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. Characterization of the Catalysts
3.2. Catalytic Activity
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- O’Quinn, P.R.; Nelssen, J.L.; Goodband, R.D.; Tokach, M.D. Conjugated linoleic acid. Anim. Health Res. Rev. 2000, 1, 35–46. [Google Scholar] [CrossRef] [PubMed]
- Whigham, L.D.; Cook, M.E.; Atkinson, R.L. Conjugated linoleic acid: Implications for human health. Pharmacol. Res. 2000, 42, 503–510. [Google Scholar] [CrossRef] [PubMed]
- Mougios, V.; Matsakas, A.; Petridou, A.; Ring, S.; Sagredos, A.; Melissopoulou, A.; Tsigilis, N.; Nikolaidis, M.J. Effect of supplementation with conjugated linoleic acid on human serum lipids and body fat. Nutr. Biochem. 2001, 12, 585–594. [Google Scholar] [CrossRef]
- Palombo, J.D.; Ganguly, A.; Bistrian, B.R.; Menard, M.P. The antiproliferative effects of biologically active isomers of conjugated linoleic acid on human colorectal and prostatic cancer cells. Cancer Lett. 2002, 177, 163–172. [Google Scholar] [CrossRef]
- Busch, S.; Zander, L.; Albiez, W.; Horlacher, P.; Westfechtel, A. Method for the production of conjugated linoleic acids. EP1527153A1, 4 May 2005. [Google Scholar]
- Bernas, A.; Kumar, N.; Mäki-Arvela, P.; Laine, E.; Holmbom, B.; Salmi, T.; Murzin, D. Conjugation of linoleic acid over a hydrogen pre-activated heterogeneous catalyst. Chem. Commun. 2002, 10, 1142–1143. [Google Scholar] [CrossRef]
- Bernas, A.; Laukkanen, P.; Kumar, N.; Mäki-Arvela, P.; Väyrynen, J.; Laine, E.; Holmbom, B.; Salmi, T.; Murzin, D. A new heterogeneously catalytic pathway for isomerization of linoleic acid over Ru/C and Ni/H–MCM-41 catalysts. J. Catal. 2002, 210, 354–366. [Google Scholar] [CrossRef]
- Bernas, A.; Kumar, N.; Mäki-Arvela, P.; Kul’kova, N.V.; Holmbom, B.; Salmi, T.; Murzin, D. Isomerization of linoleic acid over supported metal catalysts. Appl. Catal. A 2003, 245, 257–275. [Google Scholar] [CrossRef]
- Bernas, A.; Mäki-Arvela, P.; Kumar, N.; Holmbom, B.; Salmi, T.; Murzin, D. Heterogeneously catalytic isomerization of linoleic acid over supported ruthenium catalysts for production of anticarcinogenic food constituents. Ind. Eng. Chem. Res. 2003, 42, 718–727. [Google Scholar] [CrossRef]
- Bernas, A.; Murzin, D. Influence of hydrogen preactivation on the linoleic acid isomerization properties of supported ruthenium catalyst. React. Kinet. Catal. Lett. 2003, 78, 3–10. [Google Scholar] [CrossRef]
- Bernas, A.; Kumar, N.; Laukkanen, P.; Väyrynen, J.; Salmi, T.; Murzin, D. Influence of ruthenium precursor on catalytic activity of Ru/Al2O3 catalyst in selective isomerization of linoleic acid to cis-9,trans-11- andtrans-10,cis-12-conjugated linoleic acid. Appl. Catal. A 2004, 267, 121–133. [Google Scholar] [CrossRef]
- Bernas, A.; Kumar, N.; Mäki-Arvela, P.; Holmbom, B.; Salmi, T.; Murzin, D. Heterogeneous catalytic production of conjugated linoleic acid. Org. Process. Res. Dev. 2004, 8, 341–352. [Google Scholar] [CrossRef]
- Sim, K.S.; Hilaire, L.; Le Normand, F.; Touroude, R.; Paul-Boncour, V.; Percheron-Guegan, A. Catalysis by palladium–rare-earth-metal (REPd3) intermetallic compounds: hydrogenation of but-1-ene, buta-1,3-diene and but-1-yne. J. Chem. Soc. Faraday Trans. 1991, 87, 1453–1460. [Google Scholar] [CrossRef]
- Kreich, M.; Claus, P. Direct Conversion of Linoleic Acid over Silver Catalysts in the Presence of H2: An Unusual Way towards Conjugated Linoleic Acids. Angew. Chem. Int. Ed. 2005, 44, 7800–7804. [Google Scholar] [CrossRef] [PubMed]
- Bauer, P.; Horlacher, P.; Claus, P. Direct isomerization of linoleic acid to conjugated linoleic acids (CLA) using gold catalysts. Chem. Eng. Technol. 2009, 32, 2005–2010. [Google Scholar] [CrossRef]
- Simakova, O.; Lleino, A.; Campo, B.; Maki-Arvela, P.; Kordas, K.; Mikkola, J.; Murzin, D. Linoleic acid isomerization over mesoporous carbon supported gold catalysts. Catal. Today 2010, 150, 32–36. [Google Scholar] [CrossRef]
- Tanabe, K.; Hölderich, W. Industrial application of solid acid–base catalysts. Appl. Catal. A Gen. 1999, 181, 399–434. [Google Scholar] [CrossRef]
- Cardó, X.; Bergadà, O.; Cesteros, Y.; Salagre, P. Effect of catalyst acidity and porosity on the catalytic isomerization of linoleic acid to obtain conjugated linoleic acids (CLAs). Chem. Eng. J. 2012, 183, 459–465. [Google Scholar] [CrossRef]
- Oukaci, R.; Wu, J.C.S. Secondary reactions during CO hydrogenation on zeolite-supported metal catalysts: Influence of alkali cations. J. Catal. 1987, 107, 471–481. [Google Scholar] [CrossRef]
- Chen, J.; Chen, X.; Zheng, Y.; Li, Q. Synergetic effect of ruthenium and basicity sites in the Ru–MgAl catalyst for hydrogen-free production of conjugated linoleic acids. RSC Adv. 2015, 5, 20248–20255. [Google Scholar] [CrossRef]
- Vaccari, A. Clays and catalysis: A promising future. Appl. Clay Sci. 1999, 14, 161–198. [Google Scholar] [CrossRef]
- Rives, V.; Ulibarri, M.A. Layered double hydroxides (LDH) intercalated with metal coordination compounds and oxometalates. Coord. Chem. Rev. 1999, 181, 61–120. [Google Scholar] [CrossRef]
- Cavani, F.; Trifiro, F.; Vaccari, A. Hydrotalcite-type anionic clays: Preparation, properties and applications. Catal. Today 1991, 11, 173–302. [Google Scholar] [CrossRef]
- Cesteros, Y.; Salagre, P.; Medina, F.; Sueiras, J.E.; Tichit, D.; Coq, B. Hydrodechlorination of 1,2,4-trichlorobenzene on nickel-based catalysts prepared from several Ni/Mg/Al hydrotalcite-like precursors. Appl. Catal. B Environ. 2001, 32, 25–35. [Google Scholar] [CrossRef]
- Basile, F.; Fornasari, G.; Rosetti, V.; Trifirò, F.; Vaccari, A. Effect of the Mg/Al ratio of the hydrotalcite-type precursor on the dispersion and activity of Rh and Ru catalysts for the partial oxidation of methane. Catal. Today 2004, 91–92, 293–297. [Google Scholar] [CrossRef]
- Abelló, S.; Medina, F.; Tichit, D.; Pérez-Ramírez, J.; Groen, J.C.; Sueiras, J.E.; Salagre, P.; Cesteros, Y. Aldol Condensations Over Reconstructed Mg–Al Hydrotalcites: Structure–Activity Relationships Related to the Rehydration Method. Chem. Eur. J. 2005, 11, 728–739. [Google Scholar] [CrossRef] [PubMed]
- Debecker, D.P.; Gaigneaux, E.M.; Busca, G. Exploring, Tuning, and Exploiting the Basicity of Hydrotalcites for Applications in Heterogeneous Catalysis. Chemistry 2009, 15, 3920–3935. [Google Scholar] [CrossRef] [PubMed]
- Climent, M.J.; Corma, A.; Iborra, S.; Epping, K.; Velty, A. Increasing the basicity and catalytic activity of hydrotalcites by different synthesis procedures. J. Catal. 2004, 225, 316–326. [Google Scholar] [CrossRef]
- Di Cosimo, J.I.; Apesteguia, C.R.; Ginés, M.J.L.; Iglesia, E. Structural Requirements and Reaction Pathways in Condensation Reactions of Alcohols on MgyAlOx Catalysts. J. Catal. 2000, 190, 261–275. [Google Scholar] [CrossRef]
- Corma, A. Inorganic Solid Acids and Their Use in Acid-Catalyzed Hydrocarbon Reactions. Chem. Rev. 1995, 95, 559–614. [Google Scholar] [CrossRef]
- Bruce, S.M.; Zong, Z.; Chatzidimitriou, A.; Avci, L.E.; Bond, J.Q.; Carreon, M.A.; Wettstein, S.G. Small pore zeolite catalysts for furfural synthesis from xylose and switchgrass in a γ-valerolactone/water solvent. J. Mol. Catal. A Chem. 2016, 422, 18–22. [Google Scholar] [CrossRef]
- Jones, A.J.; Iglesia, E. The strength of Brønsted acid sites in microporous aluminosilicates. ACS Catal. 2015, 5, 5741–5755. [Google Scholar] [CrossRef]
- Hattori, H. Heterogeneous basic catalysis. Chem. Rev. 1995, 95, 537–558. [Google Scholar] [CrossRef]







| Catalysts | Crystalline Phases (XRD) | a (nm) | c (nm) | FWHM (003) (°) | Crystallite Size (nm) (XRD) a | B.E.T. Surface Area (m2/g) |
|---|---|---|---|---|---|---|
| HTMgAl1 | Hydrotalcite | 0.306 | 2.340 | 0.562 | 39.9 | 35 |
| HTMgAl2 | Hydrotalcite | 0.309 | 2.405 | 0.766 | 35.0 | 57 |
| HTMgAl3 | Hydrotalcite | 0.305 | 2.425 | 0.685 | 32.8 | 48 |
| HTNiMgAl1 | Hydrotalcite | 0.305 | 2.321 | 0.805 | 25.8 | 37 |
| HTNiMgAl2 | Hydrotalcite | 0.304 | 2.375 | 2.051 | 5.5 | 58 |
| HTCuZnCr | Hydrotalcite | 0.308 | 2.341 | 2.618 | - | 151 |
| HTCuCr | Hydrotalcite | 0.309 | 2.309 | 1.412 | - | 88 |
| cHTMgAl1 | MgO | - | - | - | 3.1 | 220 |
| cHTNiMgAl1 | MgO | - | - | - | 5.4 | 201 |
| MgO | MgO | - | - | - | 16.9 | 104 |
| B | Mg(OH)2 | - | - | - | 14.6 | 42 |
| Catalysts | Mg/Al | Ni/Al | Ni/Mg | Cu/Cr | Cu/Zn | Zn/Cr |
|---|---|---|---|---|---|---|
| HTMgAl1 | 7.30 (9) | - | - | - | - | - |
| HTMgAl2 | 8.82 (9) | - | - | - | - | - |
| HTMgAl3 | 2.91 (3) | - | - | - | - | - |
| HTNiMgAl1 | 0.19 (0.24) | 2.34 (2.4) | 9.58 (10) | - | - | - |
| HTNiMgAl2 | 2.31 (2.4) | 0.59 (0.6) | 0.26 (0.25) | - | - | - |
| HTCuZnCr | - | - | - | 0.87 (1) | 0.99 (1) | 0.97 (1) |
| HTCuCr | - | - | - | 3.94 (4) | - | - |
| Catalysts | Conversion (%) | Selectivity to c9, t11-CLA (%) | Selectivity to t10, c12-CLA (%) | Selectivity to t9, t11-CLA (%) | Other Products (%) |
|---|---|---|---|---|---|
| HTMgAl1 | 92 | 0.1 | 0.3 | 3.5 | 96.1 |
| HTMgAl2 | 87 | 2.4 | 2.5 | 8.7 | 86.4 |
| HTMgAl3 | 97 | 5.5 | 5.2 | 27.7 | 61.6 |
| HTNiMgAl1 | 73 | 0.3 | 0.3 | 2.9 | 96.5 |
| HTNiMgAl2 | 81 | 8.1 | 7.5 | 13.2 | 71.2 |
| HTCuZnCr | 84 | 0.4 | 0.7 | 1.2 | 97.7 |
| HTCuCr | 97 | - | - | - | 100 |
| cHTMgAl1 | 94 | 0.5 | 0.5 | 1.4 | 97.6 |
| cHTNiMgAl1 | 78 | 0.2 | 0.1 | 1.3 | 98.4 |
| MgO | 98 | 0 | 0 | 3.2 | 96.8 |
| B | 100 | 0 | 0 | 0 | 100 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cardó, X.; Salagre, P.; Cesteros, Y. Layered Double Hydroxides for the Catalytic Isomerization of Linoleic Acid to Conjugated Linoleic Acids (CLAs). ChemEngineering 2019, 3, 30. https://doi.org/10.3390/chemengineering3010030
Cardó X, Salagre P, Cesteros Y. Layered Double Hydroxides for the Catalytic Isomerization of Linoleic Acid to Conjugated Linoleic Acids (CLAs). ChemEngineering. 2019; 3(1):30. https://doi.org/10.3390/chemengineering3010030
Chicago/Turabian StyleCardó, Xavier, Pilar Salagre, and Yolanda Cesteros. 2019. "Layered Double Hydroxides for the Catalytic Isomerization of Linoleic Acid to Conjugated Linoleic Acids (CLAs)" ChemEngineering 3, no. 1: 30. https://doi.org/10.3390/chemengineering3010030