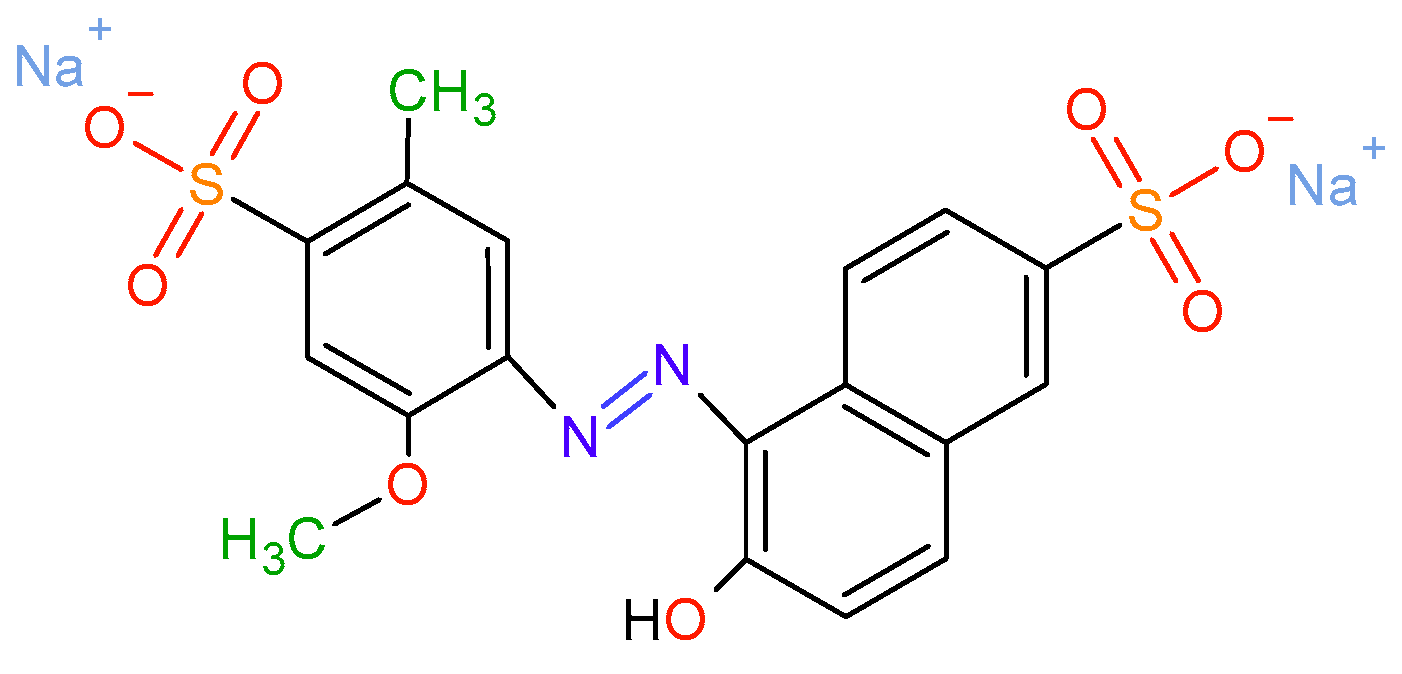
Oxidation of Allura Red AC Using the NaHCO3-activated H2O2 System Catalyzed with Cobalt Supported on Al-PILC
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Synthesis and Characterization
2.3. Catalytic Tests
2.4. Experimental Design—Statistical Analysis of the Models
2.5. Chromatographic Analysis of Reaction By-Products
3. Results and Discussion
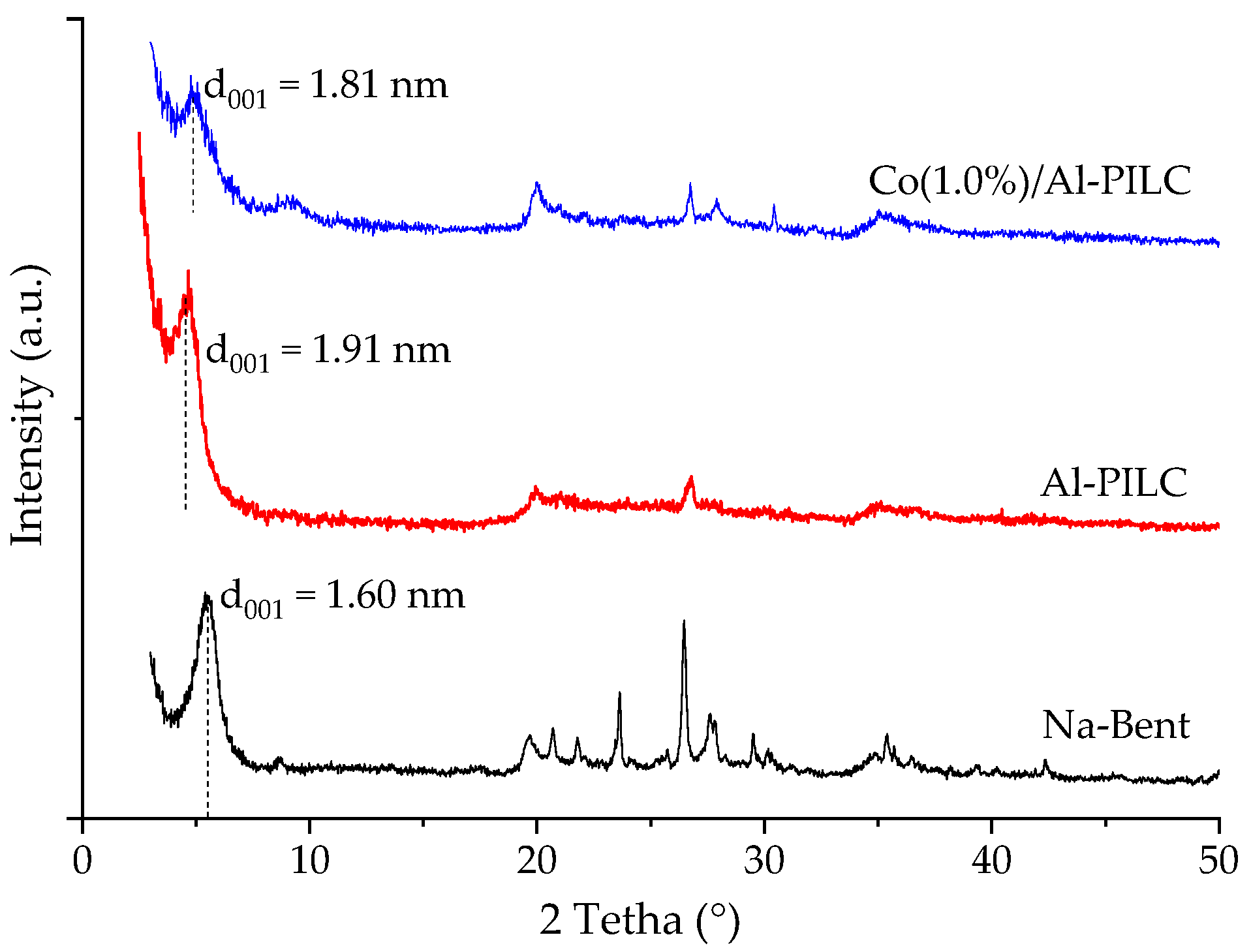
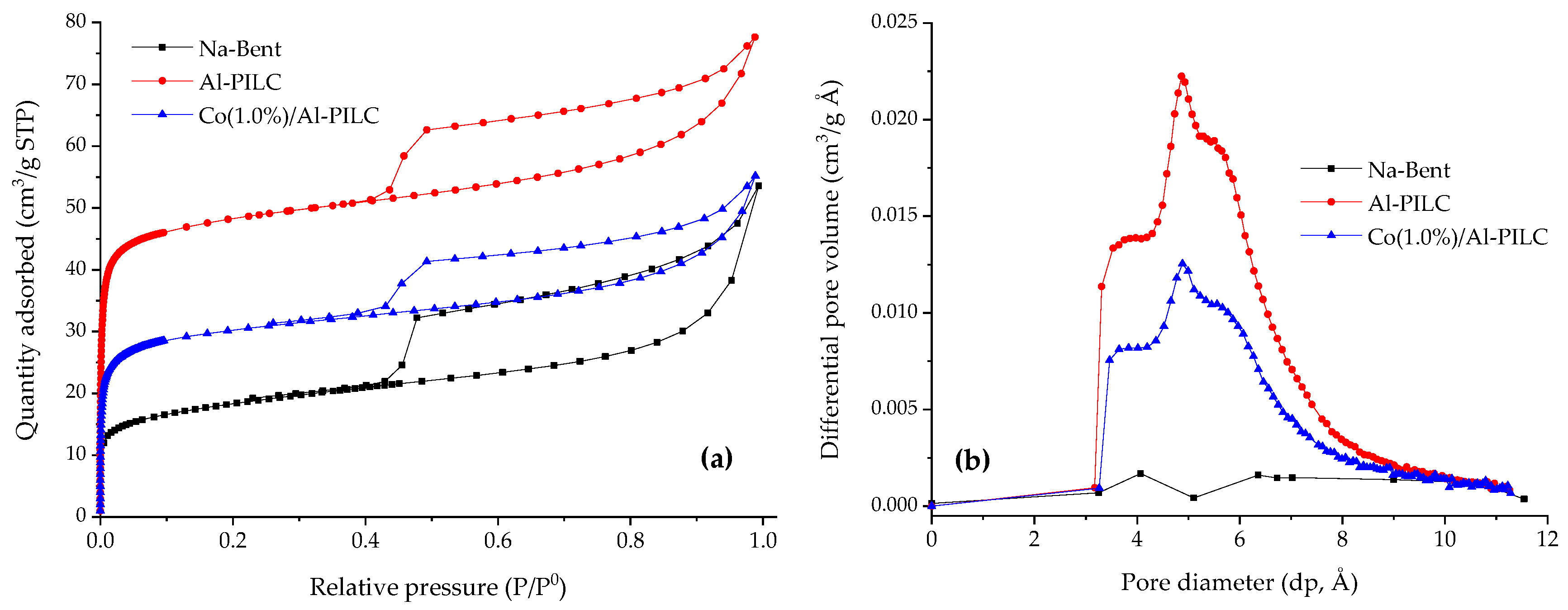
3.1. Sample Characterization
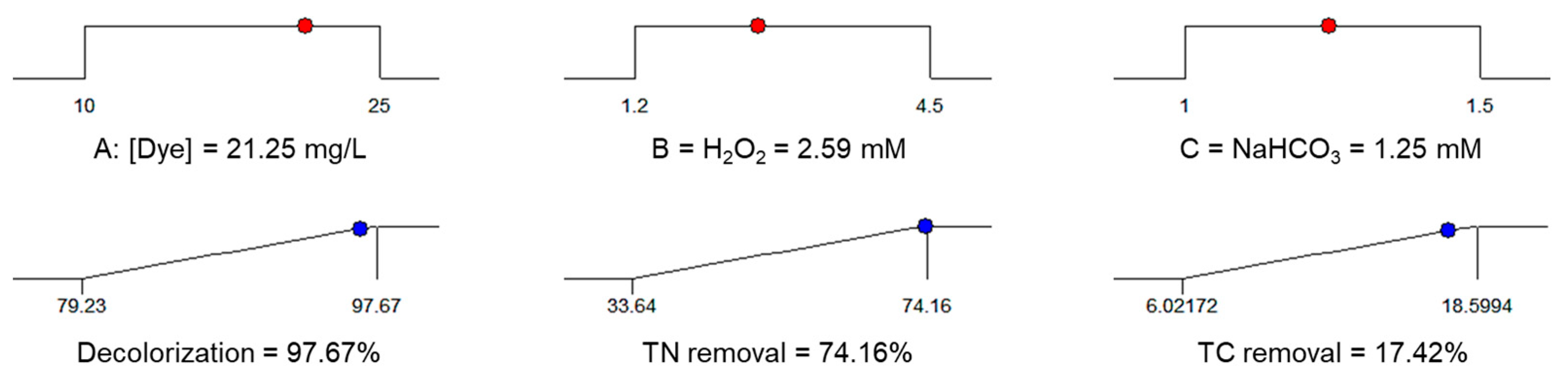
3.2. Catalytic Tests—Experimental Design
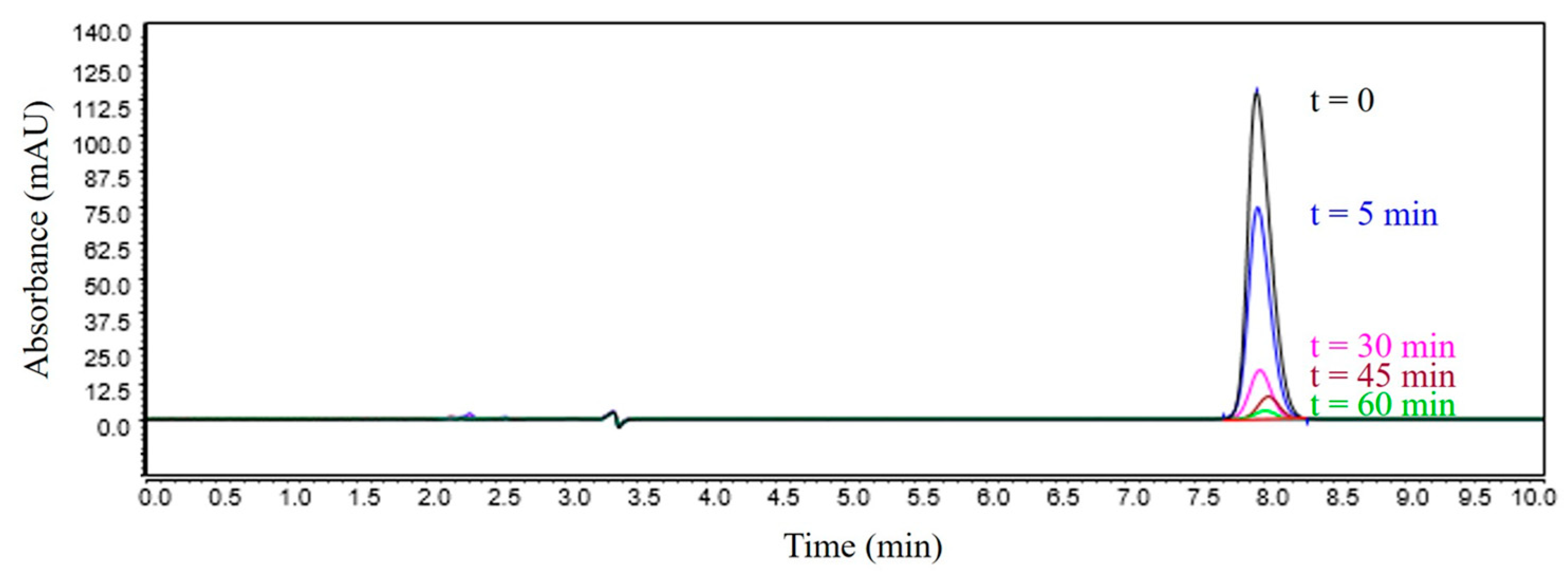
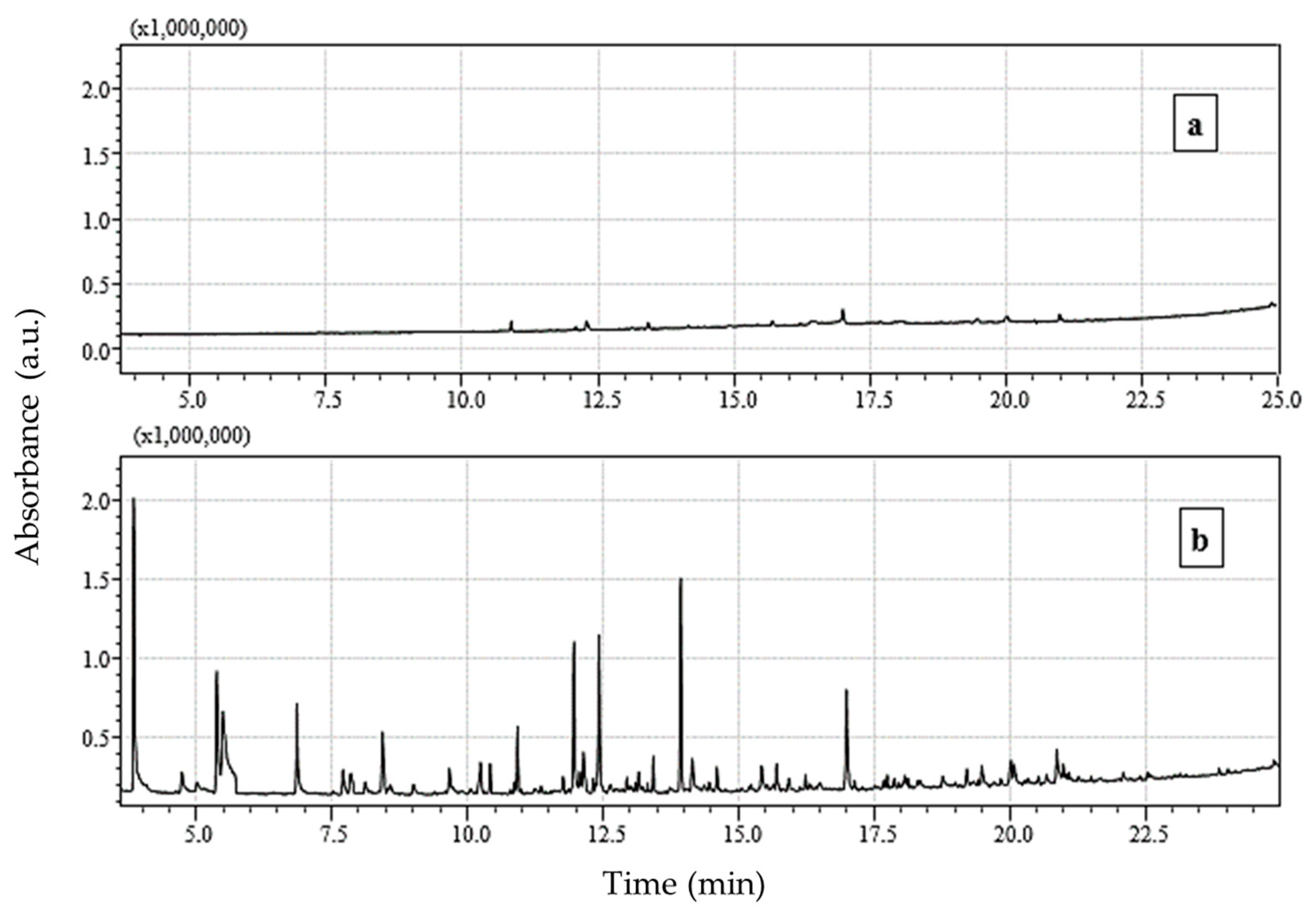
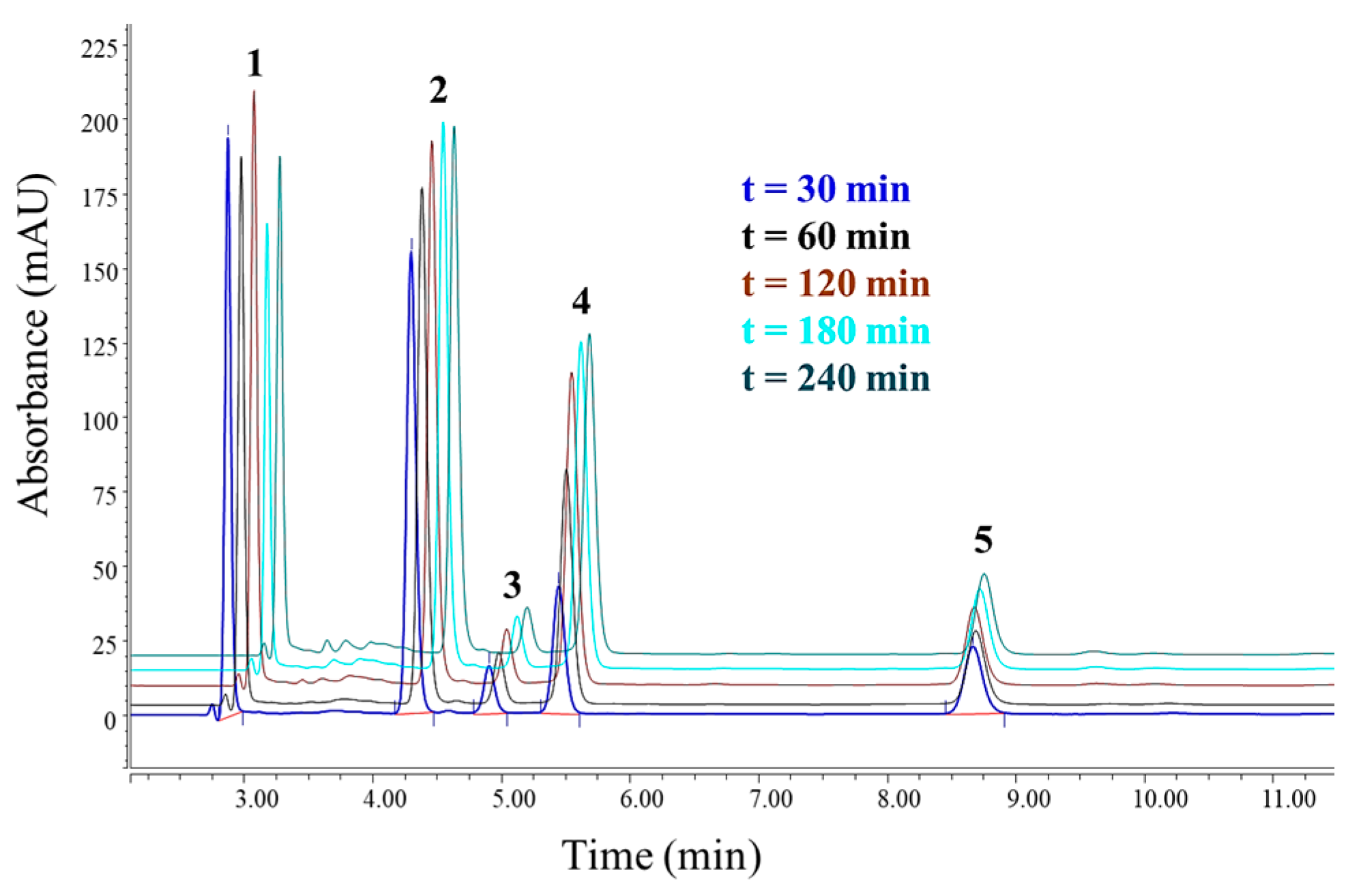
3.3. Chromatographic Analysis of Reaction By-Products
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Alalewi, A.; Jiang, C. Bacterial influence on textile wastewater decolorization. J. Environ. Prot. 2012, 3, 889–903. [Google Scholar] [CrossRef]
- Thiam, A.; Sirés, I.; Brillas, E. Treatment of a mixture of food color additives (E122, E124 and E129) in different water matrices by UVA and solar photoelectro-Fenton. Water Res. 2015, 81, 178–187. [Google Scholar] [CrossRef]
- Solís, M.; Solís, A.; Pérez, H.I.; Manjarrez, N.; Flores, M. Microbial decolouration of azo dyes: A review. Process Biochem. 2012, 47, 1723–1748. [Google Scholar] [CrossRef]
- Brillas, E.; Martínez-Huitle, C.A. Decontamination of wastewaters containing synthetic organic dyes by electrochemical methods. An updated review. Appl. Catal. B Envrion. 2015, 166–167, 603–643. [Google Scholar] [CrossRef]
- Paszczynski, A.; Pasti-Grigsby, M.B.; Goszczynski, S.; Crawford, R.L.; Crawford, D.L. Mineralization of sulfonated azo dyes and sulfanilic acid by Phanerochaete chrysosporium and Streptomyces chromofuscus. Appl. Environ. Microbiol. 1992, 58, 3598–3604. [Google Scholar] [CrossRef]
- Papić, S.; Koprivanac, N.; Bozić, A.L.; Vujević, D.; Dragicević, S.K.; Kusić, H.; Peternel, I. Advanced oxidation processes in azo dye wastewater treatment. Water Environ. Res. 2006, 78, 572–579. [Google Scholar] [CrossRef]
- Kusic, H.; Juretic, D.; Koprivanac, N.; Marin, V.; Božić, A.L. Photooxidation processes for an azo dye in aqueous media: Modeling of degradation kinetic and ecological parameters evaluation. J. Hazard. Mater. 2011, 185, 1558–1568. [Google Scholar] [CrossRef]
- Lellis, B.; Fávaro-Polonio, C.Z.; Pamphile, J.A.; Polonio, J.C. Effects of textile dyes on health and the environment and bioremediation potential of living organisms. Biotechnol. Res. Innov. 2019, 3, 275–290. [Google Scholar] [CrossRef]
- Saharan, V.K.; Pinjari, D.V.; Gogate, P.R.; Pandit, A.B. Chapter 3—Advanced Oxidation Technologies for Wastewater Treatment: An Overview. In Industrial Wastewater Treatment, Recycling and Reuse; Ranade, V.V., Bhandari, V.M., Eds.; Elsevier: Oxford, UK, 2014; pp. 141–191. [Google Scholar]
- Farooque Lanjwani, M.; Tuzen, M.; Yar Khuhawar, M.; Saleh, T.A. Trends in photocatalytic degradation of organic dye pollutants using nanoparticles: A Review. Inorg. Chem. Commun. 2024, 159, 111613. [Google Scholar] [CrossRef]
- Martínez-Huitle, C.A.; Brillas, E. Decontamination of wastewaters containing synthetic organic dyes by electrochemical methods: A general review. Appl. Catal. B Envrion. 2009, 87, 105–145. [Google Scholar] [CrossRef]
- Guo, H.; Pan, S.; Hu, Z.; Wang, Y.; Jiang, W.; Yang, Y.; Wang, Y.; Han, J.; Wu, Y.; Wang, T. Persulfate activated by non-thermal plasma for organic pollutants degradation: A review. Chem. Eng. J. 2023, 470, 144094. [Google Scholar] [CrossRef]
- Ramos, M.D.N.; Santana, C.S.; Velloso, C.C.V.; da Silva, A.H.M.; Magalhães, F.; Aguiar, A. A review on the treatment of textile industry effluents through Fenton processes. Process Saf. Environ. Prot. 2021, 155, 366–386. [Google Scholar] [CrossRef]
- Guo, X.; Li, H.; Zhao, S. Fast degradation of Acid Orange II by bicarbonate-activated hydrogen peroxide with a magnetic S-modified CoFe2O4 catalyst. J. Taiwan Inst. Chem. Eng. 2015, 55, 90–100. [Google Scholar] [CrossRef]
- Macías-Quiroga, I.F.; Pérez-Flórez, A.; Arcila, J.S.; Giraldo-Goméz, G.I.; Sanabria-Gonzalez, N.R. Synthesis and characterization of Co/Al-PILCs for the oxidation of an azo dye using the bicarbonate-activated hydrogen peroxide system. Catal. Lett. 2022, 152, 1905–1916. [Google Scholar] [CrossRef]
- Pan, H.; Gao, Y.; Li, N.; Zhou, Y.; Lin, Q.; Jiang, J. Recent advances in bicarbonate-activated hydrogen peroxide system for water treatment. Chem. Eng. J. 2021, 408, 127332. [Google Scholar] [CrossRef]
- Jawad, A.; Li, Y.; Lu, X.; Chen, Z.; Liu, W.; Yin, G. Controlled leaching with prolonged activity for Co–LDH supported catalyst during treatment of organic dyes using bicarbonate activation of hydrogen peroxide. J. Hazard. Mater. 2015, 289, 165–173. [Google Scholar] [CrossRef]
- Long, X.; Yang, Z.; Wang, H.; Chen, M.; Peng, K.; Zeng, Q.; Xu, A. Selective degradation of orange II with the cobalt(II)–bicarbonate–hydrogen peroxide system. Ind. Eng. Chem. Res. 2012, 51, 11998–12003. [Google Scholar] [CrossRef]
- Xu, A.; Li, X.; Ye, S.; Yin, G.; Zeng, Q. Catalyzed oxidative degradation of methylene blue by in situ generated cobalt (II)-bicarbonate complexes with hydrogen peroxide. Appl. Catal. B Envrion. 2011, 102, 37–43. [Google Scholar] [CrossRef]
- Li, Y.; Li, L.; Chen, Z.-X.; Zhang, J.; Gong, L.; Wang, Y.-X.; Zhao, H.-Q.; Mu, Y. Carbonate-activated hydrogen peroxide oxidation process for azo dye decolorization: Process, kinetics, and mechanisms. Chemosphere 2018, 192, 372–378. [Google Scholar] [CrossRef]
- Richardson, D.E.; Yao, H.; Frank, K.M.; Bennett, D.A. Equilibria, kinetics, and mechanism in the bicarbonate activation of hydrogen peroxide: MaciOxidation of sulfides by peroxymonocarbonate. J. Am. Chem. Soc. 2000, 122, 1729–1739. [Google Scholar] [CrossRef]
- Lin, J.-M.; Liu, M. Singlet oxygen generated from the decomposition of peroxymonocarbonate and its observation with chemiluminescence method. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2009, 72, 126–132. [Google Scholar] [CrossRef]
- Yang, Z.; Wang, H.; Chen, M.; Luo, M.; Xia, D.; Xu, A.; Zeng, Q. Fast degradation and biodegradability improvement of reactive brilliant red X-3B by the cobalt(II)/bicarbonate/hydrogen peroxide system. Ind. Eng. Chem. Res. 2012, 51, 11104–11111. [Google Scholar] [CrossRef]
- Zhou, L.; Song, W.; Chen, Z.; Yin, G. Degradation of organic pollutants in wastewater by bicarbonate-activated hydrogen peroxide with a supported cobalt catalyst. Environ. Sci. Technol. 2013, 47, 3833–3839. [Google Scholar] [CrossRef]
- Duan, L.; Chen, Y.; Zhang, K.; Luo, H.; Huang, J.; Xu, A. Catalytic degradation of acid orange 7 with hydrogen peroxide using CoxOy-N/GAC catalysts in a bicarbonate aqueous solution. RSC Adv. 2015, 5, 84303–84310. [Google Scholar] [CrossRef]
- Mora-Bonilla, K.Y.; Macías-Quiroga, I.F.; Sanabria-González, N.R.; Dávila-Arias, M.T. Bicarbonate-activated hydrogen peroxide for an azo dye degradation: Experimental design. ChemEngineering 2023, 7, 86. [Google Scholar] [CrossRef]
- Macías-Quiroga, I.F.; Giraldo-Gómez, G.I.; Sanabria-González, N.R. Characterization of Colombian clay and its potential use as adsorbent. Sci. World J. 2018, 2018, 5969178. [Google Scholar] [CrossRef]
- Carriazo, J.G.; Guelou, E.; Barrault, J.; Tatibouët, J.M.; Moreno, S. Catalytic wet peroxide oxidation of phenol over Al–Cu or Al–Fe modified clays. Appl. Clay Sci. 2003, 22, 303–308. [Google Scholar] [CrossRef]
- Cañizares, P.; Valverde, J.L.; Sun Kou, M.R.; Molina, C.B. Synthesis and characterization of PILCs with single and mixed oxide pillars prepared from two different bentonites. A comparative study. Microporous Mesoporous Mater. 1999, 29, 267–281. [Google Scholar] [CrossRef]
- Gregg, S.J.; Sing, K.S.W. Adsorption, Surface Area and Porosity, 2nd ed.; Academic Press: London, UK, 1982; pp. 41–61. [Google Scholar]
- Harkins, W.D.; Jura, G. Surfaces of solids. XIII. A vapor adsorption method for the determination of the area of a solid without the assumption of a molecular area, and the areas occupied by nitrogen and other molecules on the surface of a solid. J. Am. Chem. Soc. 1944, 66, 1366–1373. [Google Scholar] [CrossRef]
- Horvath, G.; Kawazoe, K. Method for the calculation of effective pore size distribution in molecular sieve carbon. J. Chem. Eng. Jpn. 1983, 16, 470–475. [Google Scholar] [CrossRef]
- Guechi, E.-K.; Hamdaoui, O. Biosorption of methylene blue from aqueous solution by potato (Solanum tuberosum) peel: Equilibrium modelling, kinetic, and thermodynamic studies. Desalin. Water Treat. 2016, 57, 10270–10285. [Google Scholar] [CrossRef]
- Leone, V.O.; Pereira, M.C.; Aquino, S.F.; Oliveira, L.C.A.; Correa, S.; Ramalho, T.C.; Gurgel, L.V.A.; Silva, A.C. Adsorption of diclofenac on a magnetic adsorbent based on maghemite: Experimental and theoretical studies. New J. Chem. 2018, 42, 437–449. [Google Scholar] [CrossRef]
- Gosetti, F.; Chiuminatto, U.; Mazzucco, E.; Calabrese, G.; Gennaro, M.C.; Marengo, E. Identification of photodegradation products of allura red AC (E129) in a beverage by ultra high performance liquid chromatography–quadrupole-time-of-flight mass spectrometry. Catal. Today 2012, 746, 84–89. [Google Scholar] [CrossRef]
- Sivrikaya, O.; Uzal, B.; Ozturk, Y.E. Practical charts to identify the predominant clay mineral based on oxide composition of clayey soils. Appl. Clay Sci. 2017, 135, 532–537. [Google Scholar] [CrossRef]
- Ramsey, M.H.; Potts, P.J.; Webb, P.C.; Watkins, P.; Watson, J.S.; Coles, B.J. An objective assessment of analytical method precision: Comparison of ICP-AES and XRF for the analysis of silicate rocks. Chem. Geol. 1995, 124, 1–19. [Google Scholar] [CrossRef]
- Occelli, M.L.; Bertrand, J.A.; Gould, S.A.C.; Dominguez, J.M. Physicochemical characterization of a Texas montmorillonite pillared with polyoxocations of aluminum: Part I: The microporous structure. Microporous Mesoporous Mater. 2000, 34, 195–206. [Google Scholar] [CrossRef]
- Marković, M.; Marinović, S.; Mudrinić, T.; Ajduković, M.; Jović-Jovičić, N.; Mojović, Z.; Orlić, J.; Milutinović-Nikolić, A.; Banković, P. Co(II) impregnated Al(III)-pillared montmorillonite—Synthesis, characterization and catalytic properties in Oxone® activation for dye degradation. Appl. Clay Sci. 2019, 182, 105276. [Google Scholar] [CrossRef]
- Milovanović, B.; Marinović, S.; Vuković, Z.; Milutinović-Nikolić, A.; Petrović, R.; Banković, P.; Mudrinić, T. The influence of cobalt loading on electrocatalytic performance toward glucose oxidation of pillared montmorillonite-supported cobalt. J. Electroanal. Chem. 2022, 915, 116332. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef]
- Vicente, M.A.; Belver, C.; Trujillano, R.; Rives, V.; Álvarez, A.C.; Lambert, J.F.; Korili, S.A.; Gandía, L.M.; Gil, A. Preparation and characterisation of Mn- and Co-supported catalysts derived from Al-pillared clays and Mn- and Co-complexes. Appl. Catal. A Gen. 2004, 267, 47–58. [Google Scholar] [CrossRef]
- Colín L, J.A.; de los Reyes, J.A.; Vázquez, A.; Montoya, A. Pillar effects in MoS2 catalysts supported on Al and Zr pillared clays in a hydrotreatment reaction: A preliminary study. Appl. Surf. Sci. 2005, 240, 48–62. [Google Scholar] [CrossRef]
- Vicente, M.A.; Lambert, J.-F. Synthesis of Pt pillared clay nanocomposite catalysts from [PtII(NH3)4]Cl2 precursor. Phys. Chem. Chem. Phys. 2001, 3, 4843–4852. [Google Scholar] [CrossRef]
- Carriazo, J.G.; Molina, R.; Moreno, S. Fractal dimension and energetic heterogeneity of gold-modified Al–Fe–Ce pilc’s. Appl. Surf. Sci. 2008, 255, 3354–3360. [Google Scholar] [CrossRef]
- Gil, A.; Montes, M. Analysis of the microporosity in pillared clays. Langmuir 1994, 10, 291–297. [Google Scholar] [CrossRef]
- Aguiar, J.E.; Bezerra, B.T.C.; Siqueira, A.C.A.; Barrera, D.; Sapag, K.; Azevedo, D.C.S.; Lucena, S.M.P.; Silva, I.J. Improvement in the adsorption of anionic and cationic dyes from aqueous solutions: A comparative study using aluminium pillared clays and activated carbon. Sep. Sci. Technol. 2014, 49, 741–751. [Google Scholar] [CrossRef]
- Ramírez, M.J.; Giraldo, G.I.; Orrego, C.E. Modeling and stability of polyphenol in spray-dried and freeze-dried fruit encapsulates. Powder Technol. 2015, 277, 89–96. [Google Scholar] [CrossRef]
- Kanafin, Y.N.; Abdirova, P.; Kanafina, D.; Arkhangelsky, E.; Kyzas, G.Z.; Poulopoulos, S.G. UV and zero-valent iron (ZVI) activated continuous flow persulfate oxidation of municipal wastewater. Catalysts 2023, 13, 25. [Google Scholar] [CrossRef]
- Samarghandi, M.R.; Dargahi, A.; Shabanloo, A.; Nasab, H.Z.; Vaziri, Y.; Ansari, A. Electrochemical degradation of methylene blue dye using a graphite doped PbO2 anode: Optimization of operational parameters, degradation pathway and improving the biodegradability of textile wastewater. Arab. J. Chem. 2020, 13, 6847–6864. [Google Scholar] [CrossRef]
- Ravikumar, K.; Deebika, B.; Balu, K. Decolourization of aqueous dye solutions by a novel adsorbent: Application of statistical designs and surface plots for the optimization and regression analysis. J. Hazard. Mater. 2005, 122, 75–83. [Google Scholar] [CrossRef]
- Roudi, A.M.; Salem, S.; Abedini, M.; Maslahati, A.; Imran, M. Response surface methodology (RSM)-based prediction and optimization of the Fenton process in landfill leachate decolorization. Processes 2021, 9, 2284. [Google Scholar] [CrossRef]
- Thiam, A.; Sirés, I.; Centellas, F.; Cabot, P.L.; Brillas, E. Decolorization and mineralization of Allura Red AC azo dye by solar photoelectro-Fenton: Identification of intermediates. Chemosphere 2015, 136, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Ruiz, E.J.; Hernández-Ramírez, A.; Peralta-Hernández, J.M.; Arias, C.; Brillas, E. Application of solar photoelectro-Fenton technology to azo dyes mineralization: Effect of current density, Fe2+ and dye concentrations. Chem. Eng. J. 2011, 171, 385–392. [Google Scholar] [CrossRef]
- Thiam, A.; Sirés, I.; Garrido, J.A.; Rodríguez, R.M.; Brillas, E. Decolorization and mineralization of Allura Red AC aqueous solutions by electrochemical advanced oxidation processes. J. Hazard. Mater. 2015, 290, 34–42. [Google Scholar] [CrossRef] [PubMed]
- Thiam, A.; Brillas, E.; Garrido, J.A.; Rodríguez, R.M.; Sirés, I. Routes for the electrochemical degradation of the artificial food azo-colour Ponceau 4R by advanced oxidation processes. Appl. Catal. B Envrion. 2016, 180, 227–236. [Google Scholar] [CrossRef]
- Thiam, A.; Sirés, I.; Garrido, J.A.; Rodríguez, R.M.; Brillas, E. Effect of anions on electrochemical degradation of azo dye carmoisine (acid red 14) using a BDD anode and air-diffusion cathode. Sep. Purif. Technol. 2015, 140, 43–52. [Google Scholar] [CrossRef]
- Zhou, Z.; Liang, Z.; Liu, Y.; Ma, Y.; Zhu, X.; Zhang, X.; Li, Q.; Ren, Z. Intensification of degradation of sunset yellow using packed bed in a pulsed high-voltage hybrid gas-liquid discharge system: Optimization of operating parameters, degradation mechanism and pathways. Chem. Eng. Process Process Intensif. 2017, 115, 23–33. [Google Scholar] [CrossRef]

| Independent Variable | Range and Level | ||||
|---|---|---|---|---|---|
| –1.414 | –1 | 0 | +1 | +1.414 | |
| A: Dye, mg/L | 6.89 | 10.0 | 17.50 | 25.00 | 28.11 |
| B: H2O2, mM | 0.52 | 1.20 | 2.85 | 4.50 | 5.18 |
| C: NaHCO3, mM | 0.90 | 1.00 | 1.25 | 1.50 | 1.60 |
| Sample | Concentration (wt.%) | SiO2/Al2O3 | ||||||
|---|---|---|---|---|---|---|---|---|
| SiO2 | Al2O3 | Fe2O3 | CaO | MgO | Na2O | Co | ||
| Na–Bent | 61.53 | 17.23 | 7.93 | 2.80 | 2.44 | 1.35 | ND | 3.58 |
| Al–PILC | 59.09 | 24.61 | 6.71 | 0.93 | 2.30 | 0.23 | ND | 2.40 |
| Co/Al–PILC | 57.61 | 23.93 | 6.41 | 0.96 | 2.23 | 0.21 | 1.05 | 2.41 |
| Sample | SSA (m2/g) | Smicro (m2/g) | Sext (m2/g) | Vmicro (cm3/g) | Median Pore Diameter (Å) |
|---|---|---|---|---|---|
| Na–Bent | 49 | 15 | 34 | 0.0006 | 42.3 |
| Al–PILC | 189 | 157 | 32 | 0.0601 | 5.2 |
| Co/Al–PILC | 116 | 89 | 27 | 0.0345 | 5.3 |
| Run | Codified Values | Experimental Values | Removal (%) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Color | TN | TC | ||||||||
| A | B | C | A | B | C | Y1 (1 h) | Y1 (5 h) | Y2 (5 h) | Y3 (5 h) | |
| 1 | +α | 0 | 0 | 28.11 | 2.85 | 1.25 | 88.85 | 97.99 | 70.52 | 18.47 |
| 2 | 1 | −1 | 1 | 25.00 | 1.20 | 1.50 | 95.38 | 99.24 | 55.20 | 13.97 |
| 3 | −1 | 1 | −1 | 10.00 | 4.50 | 1.00 | 79.23 | 98.51 | 50.75 | 11.10 |
| 4 | 0 | 0 | 0 | 17.50 | 2.85 | 1.25 | 97.67 | 97.94 | 72.02 | 16.42 |
| 5 | −1 | −1 | 1 | 10.00 | 1.20 | 1.50 | 95.12 | 97.58 | 39.46 | 6.028 |
| 6 | 0 | 0 | +α | 17.50 | 2.85 | 1.60 | 96.02 | 99.18 | 71.48 | 10.47 |
| 7 | 0 | 0 | −α | 17.50 | 2.85 | 0.90 | 92.51 | 98.08 | 69.07 | 14.10 |
| 8 | 0 | 0 | 0 | 17.50 | 2.85 | 1.25 | 96.85 | 97.50 | 74.16 | 16.18 |
| 9 | 0 | 0 | 0 | 17.50 | 2.85 | 1.25 | 97.38 | 97.92 | 72.69 | 16.73 |
| 10 | −1 | −1 | −1 | 10.00 | 1.20 | 1.00 | 92.69 | 97.93 | 33.64 | 13.28 |
| 11 | 0 | +α | 0 | 17.50 | 5.18 | 1.25 | 80.70 | 98.08 | 63.17 | 11.97 |
| 12 | 1 | −1 | −1 | 25.00 | 1.20 | 1.00 | 91.48 | 97.80 | 68.51 | 15.34 |
| 13 | 1 | 1 | 1 | 25.00 | 4.50 | 1.50 | 84.54 | 98.55 | 73.78 | 14.23 |
| 14 | −1 | 1 | 1 | 10.00 | 4.50 | 1.50 | 83.58 | 97.77 | 66.26 | 8.93 |
| 15 | 0 | −α | 0 | 17.50 | 0.52 | 1.25 | 95.61 | 98.18 | 38.61 | 11.79 |
| 16 | +α | 0 | 0 | 6.89 | 2.85 | 1.25 | 92.51 | 97.22 | 39.53 | 12.28 |
| 17 | 0 | −α | 0 | 17.50 | 0.52 | 1.25 | 95.75 | 97.86 | 48.25 | 11.94 |
| 18 | +α | 0 | 0 | 28.11 | 2.85 | 1.25 | 87.46 | 97.72 | 73.49 | 18.60 |
| 19 | 1 | 1 | −1 | 25.00 | 4.50 | 1.00 | 80.15 | 98.41 | 70.46 | 13.08 |
| 20 | −α | 0 | 0 | 6.89 | 2.85 | 1.25 | 90.19 | 96.93 | 43.64 | 11.98 |
| 21 | 0 | 0 | +α | 17.50 | 2.85 | 1.60 | 96.57 | 98.08 | 70.07 | 9.96 |
| 22 | 0 | +α | 0 | 17.50 | 5.18 | 1.25 | 80.21 | 98.18 | 62.10 | 12.96 |
| 23 | 0 | 0 | −α | 17.50 | 2.85 | 0.90 | 91.24 | 97.87 | 70.78 | 14.84 |
| 24 | 0 | 0 | 0 | 17.50 | 2.85 | 1.25 | 97.18 | 97.43 | 73.64 | 16.94 |
| Source | Sum of Squares | df | Mean Square | F-Value | p-Value |
|---|---|---|---|---|---|
| Model | 880.20 | 9 | 97.80 | 100.82 | <0.0001 a |
| A—Dye | 4.11 | 1 | 4.11 | 4.23 | 0.0587 b |
| B—H2O2 | 508.87 | 1 | 508.87 | 524.56 | <0.0001 a |
| C—NaHCO3 | 47.51 | 1 | 47.51 | 48.98 | <0.0001 a |
| AB | 1.00 | 1 | 1.00 | 1.03 | 0.3269 b |
| AC | 0.29 | 1 | 0.29 | 0.29 | 0.5963 b |
| BC | 0.73 | 1 | 0.73 | 0.75 | 0.4016 b |
| A2 | 156.17 | 1 | 156.17 | 160.98 | <0.0001 a |
| B2 | 237.63 | 1 | 237.63 | 244.96 | <0.0001 a |
| C2 | 24.93 | 1 | 24.93 | 25.69 | 0.0002 a |
| Residual | 13.58 | 14 | 0.97 | ||
| Lack of fit | 8.48 | 5 | 1.70 | 2.99 | 0.0730 b |
| Pure error | 5.10 | 9 | 0.57 | ||
| Cor total | 893.78 | 23 |
| Source | Sum of Squares | df | Mean Square | F-Value | p-Value |
|---|---|---|---|---|---|
| Model | 4184.74 | 9 | 464.97 | 52.55 | <0.0001 a |
| A—Dye | 1678.56 | 1 | 1678.56 | 189.72 | <0.0001 a |
| B—H2O2 | 881.50 | 1 | 881.50 | 99.63 | <0.0001 a |
| C—NaHCO3 | 11.81 | 1 | 11.81 | 1.33 | 0.2674 b |
| AB | 68.33 | 1 | 68.33 | 7.72 | 0.0148 a |
| AC | 122.62 | 1 | 122.62 | 13.86 | 0.0023 a |
| BC | 86.59 | 1 | 86.59 | 9.79 | 0.0074 a |
| A2 | 574.98 | 1 | 574.98 | 64.99 | <0.0001 a |
| B2 | 929.98 | 1 | 929.98 | 105.11 | <0.0001 a |
| C2 | 0.25 | 1 | 0.25 | 0.028 | 0.8687 b |
| Residual | 123.86 | 14 | 8.85 | ||
| Lack of fit | 58.77 | 5 | 11.75 | 1.62 | 0.2480 b |
| Pure error | 65.10 | 9 | 7.23 | ||
| Cor total | 4308.60 | 23 |
| Source | Sum of Squares | df | Mean Square | F-Value | p-Value |
|---|---|---|---|---|---|
| Model | 207.53 | 9 | 23.06 | 94.57 | <0.0001 a |
| A—Dye | 78.31 | 1 | 78.31 | 321.20 | <0.0001 a |
| B—H2O2 | 0.011 | 1 | 0.011 | 0.045 | 0.8350 b |
| C—NaHCO3 | 29.37 | 1 | 29.37 | 120.45 | <0.0001 a |
| AB | 0.93 | 1 | 0.93 | 3.80 | 0.0716 b |
| AC | 10.58 | 1 | 10.58 | 43.41 | <0.0001 a |
| BC | 7.22 | 1 | 7.22 | 29.63 | <0.0001 a |
| A2 | 2.98 | 1 | 2.98 | 12.21 | 0.0036 a |
| B2 | 52.00 | 1 | 52.00 | 213.29 | <0.0001 a |
| C2 | 47.66 | 1 | 47.66 | 195.49 | <0.0001 a |
| Residual | 3.41 | 14 | 0.24 | ||
| Lack of fit | 2.12 | 5 | 0.42 | 2.94 | 0.0760 b |
| Pure error | 1.30 | 9 | 0.14 | ||
| Cor total | 210.94 | 23 |
| Parameter | Decolorization | TN Removal | TC Removal |
|---|---|---|---|
| R2 | 0.9848 | 0.9713 | 0.9838 |
| Adjusted R2 | 0.9750 | 0.9528 | 0.9734 |
| Predicted R2 | 0.9388 | 0.9019 | 0.9265 |
| Adequate precision | 27.533 | 20.243 | 39.970 |
| Coefficient of variation (CV, %) | 1.08 | 4.85 | 3.69 |
| Variables | Value | Decolorization (%) | TN Removal (%) | TC Removal (%) | |||
|---|---|---|---|---|---|---|---|
| Predic. | Exper. | Predic. | Exper. | Predic. | Exper. | ||
| AR–AC, mg/L | 21.25 | 97.67 | >99.43 | 74.16 | 72.82 | 17.42 | 18.74 |
| H2O2, mM | 2.59 | ||||||
| NaHCO3, mM | 1.25 | ||||||
| AR–AC, mg/L | 8.0 | 91.63 | 95.49 | 26.96 | 28.62 | 22.25 | 23.72 |
| H2O2, mM | 3.0 | ||||||
| NaHCO3, mM | 1.3 | ||||||
| AR–AC, mg/L | 22.0 | 95.26 | 92.52 | 52.04 | 55.17 | 13.83 | 12.58 |
| H2O2, mM | 0.7 | ||||||
| NaHCO3, mM | 1.3 | ||||||
| Pollutant | Catalyst | Conditions | [H2O2] | [NaHCO3] | Results | Ref. |
|---|---|---|---|---|---|---|
| Methylene blue, 50 mg/L | Co/Diatomite | 298 K 300 min | 60 mM | 25 mM | Color removal: 98% COD removal: 70.4% | [24] |
| Methylene blue, 60 mg/L | Co–Mg/AL ternary LDH | 303 K 300 h | 48 mM | 25 mM | Color removal: 100% COD removal ≈50% | [17] |
| Acid Orange 7, 50 µM | CoxOy–N/GAC | 298 K 20 min | 10 mM | 5 mM | Color removal: 94.4% TOC removal: 13% | [25] |
| Acid Orange II, 50 mg/L | S-modified CoFe2O4 | 298 K 30 min | 3 mM | 0.1 g/L | Color removal: 99.0% TOC removal: 39.2% | [14] |
| Sunset Yellow, 20 mg/L | Co/Al–PILC | 298 K 5 h | 14.9 mM | 59.4 mM | Color removal: 99.5% TC removal: 27.9% | [15] |
| Allura Red AC, 21.25 mg/L | Co/Al–PILC | 298 K 5 h | 2.59 mM | 1.25 mM | Color removal: >99.43% TC removal: 18.7% TN removal: 72.8% | This study |
| Tr (min) | Molecular Formula | m/z | Chemical Structure | Chemical Name |
|---|---|---|---|---|
| 1.572 | 156 | 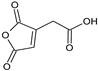 | Aconitic anhydride | |
| 6.885 | 151 |  | Oxime methoxy-phenyl | |
| 12.445 | 150 |  | Carvone | |
| 13.180 | 150 | 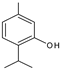 | Thymol | |
| 14.161 | 148 |  | 2-allyl-4-methylphenol | |
| 16.260 | 202 | 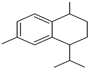 | 4-isopropyl-1,6-dimethyl-1,2,3,4-tetrahydronaphthalene | |
| 17.005 | 194 | 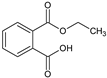 | Phthalic acid monoethyl ester |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marín-González, N.; Giraldo-Loaiza, C.; Macías-Quiroga, I.F.; Rivera-Giraldo, J.D.; Cardona-Castaño, J.A.; Sanabria-González, N.R. Oxidation of Allura Red AC Using the NaHCO3-activated H2O2 System Catalyzed with Cobalt Supported on Al-PILC. ChemEngineering 2024, 8, 14. https://doi.org/10.3390/chemengineering8010014
Marín-González N, Giraldo-Loaiza C, Macías-Quiroga IF, Rivera-Giraldo JD, Cardona-Castaño JA, Sanabria-González NR. Oxidation of Allura Red AC Using the NaHCO3-activated H2O2 System Catalyzed with Cobalt Supported on Al-PILC. ChemEngineering. 2024; 8(1):14. https://doi.org/10.3390/chemengineering8010014
Chicago/Turabian StyleMarín-González, Natalia, Camila Giraldo-Loaiza, Iván F. Macías-Quiroga, Juan D. Rivera-Giraldo, Julio A. Cardona-Castaño, and Nancy R. Sanabria-González. 2024. "Oxidation of Allura Red AC Using the NaHCO3-activated H2O2 System Catalyzed with Cobalt Supported on Al-PILC" ChemEngineering 8, no. 1: 14. https://doi.org/10.3390/chemengineering8010014





