Catalysts Based on Iron Oxides for Wastewater Purification from Phenolic Compounds: Synthesis, Physicochemical Analysis, Determination of Catalytic Activity
Abstract
:1. Introduction
2. Materials and Methods
3. Results
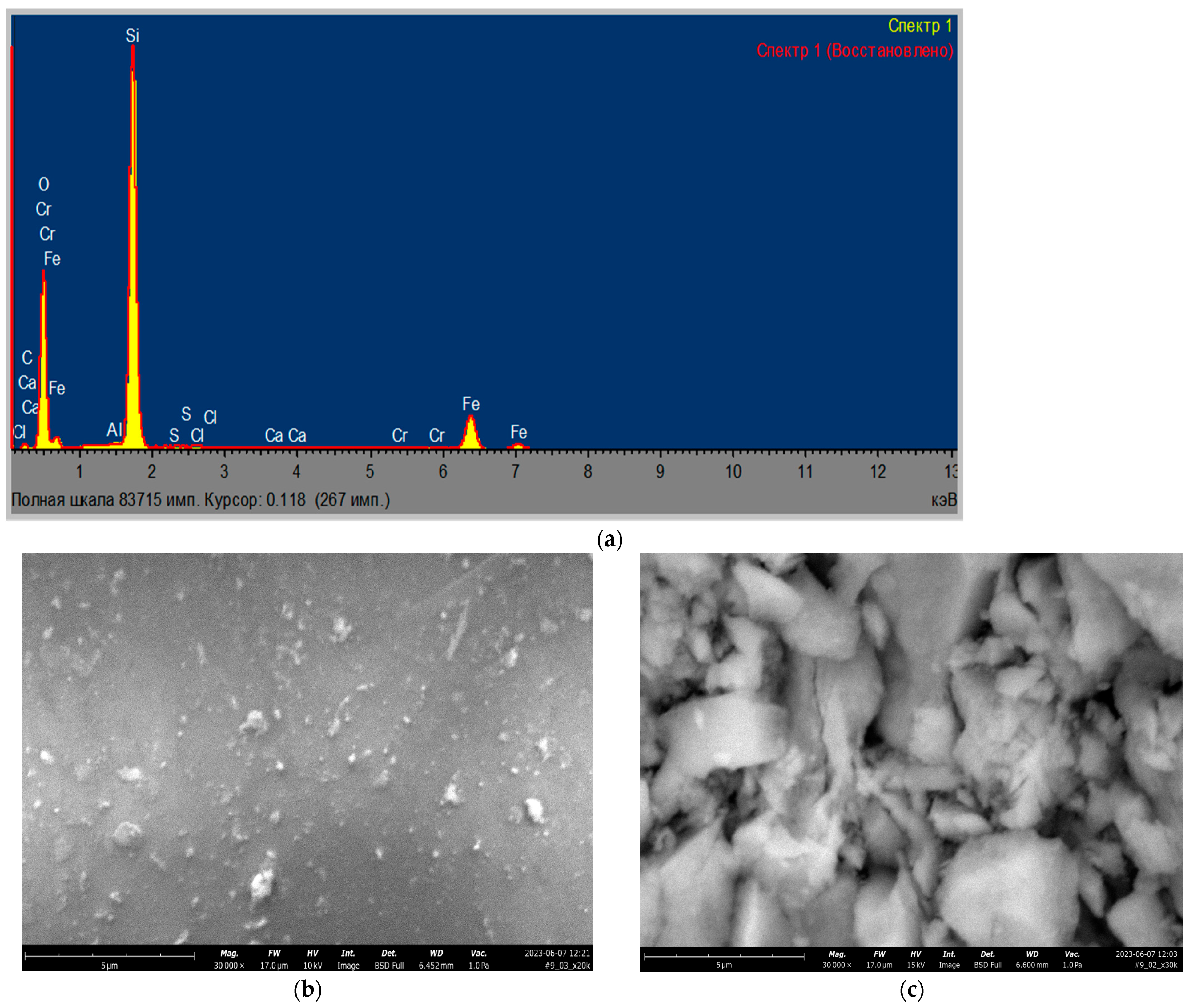
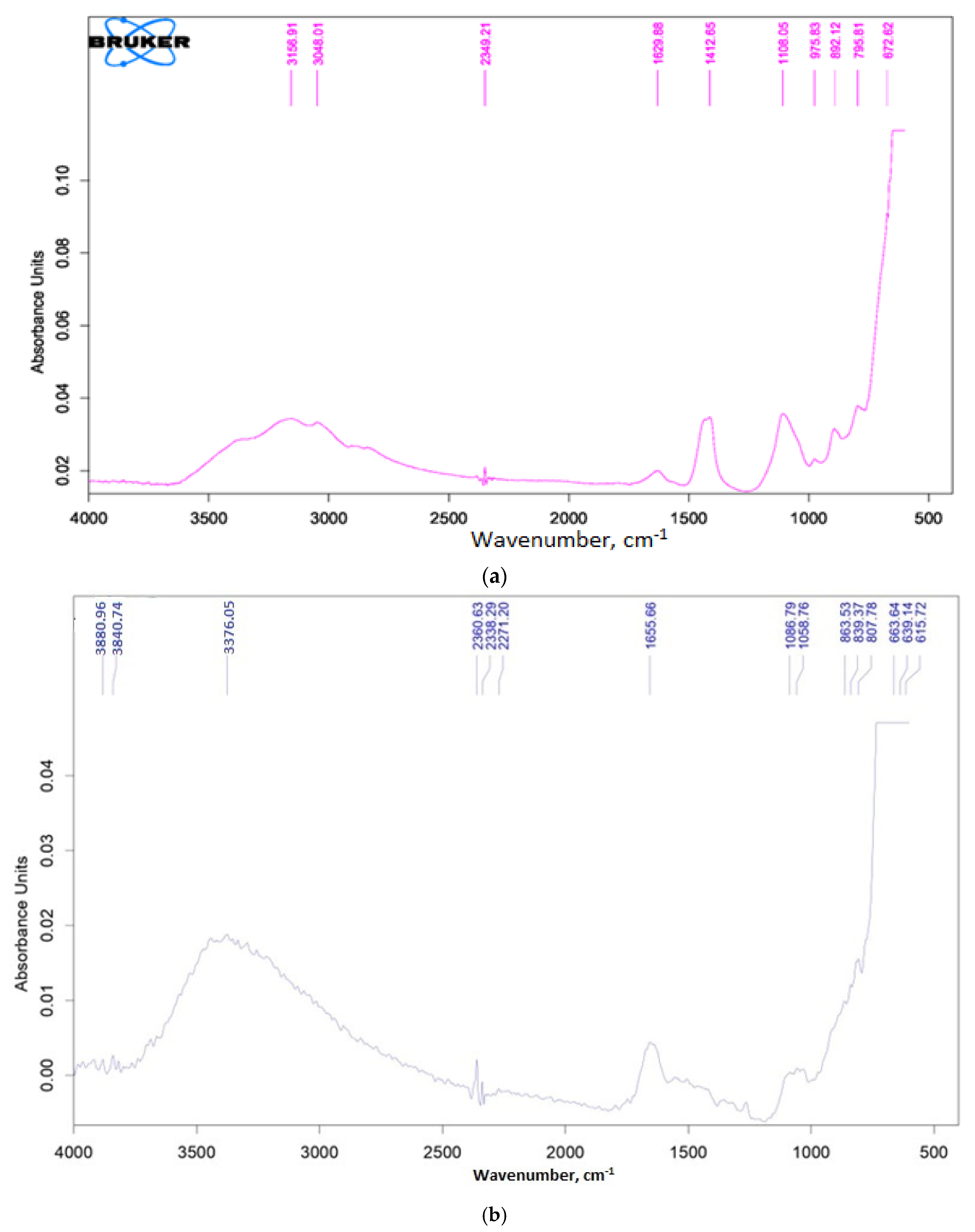
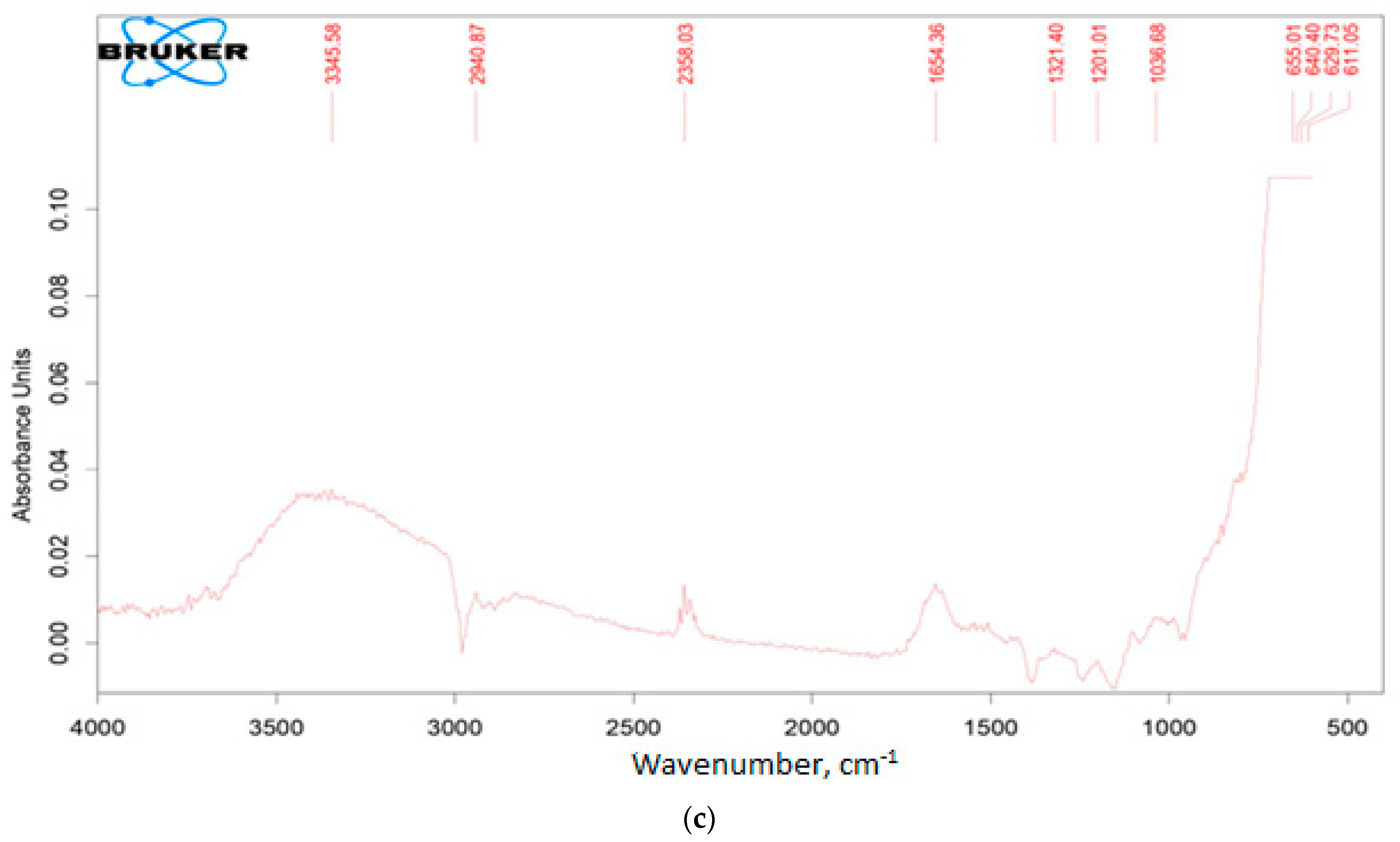
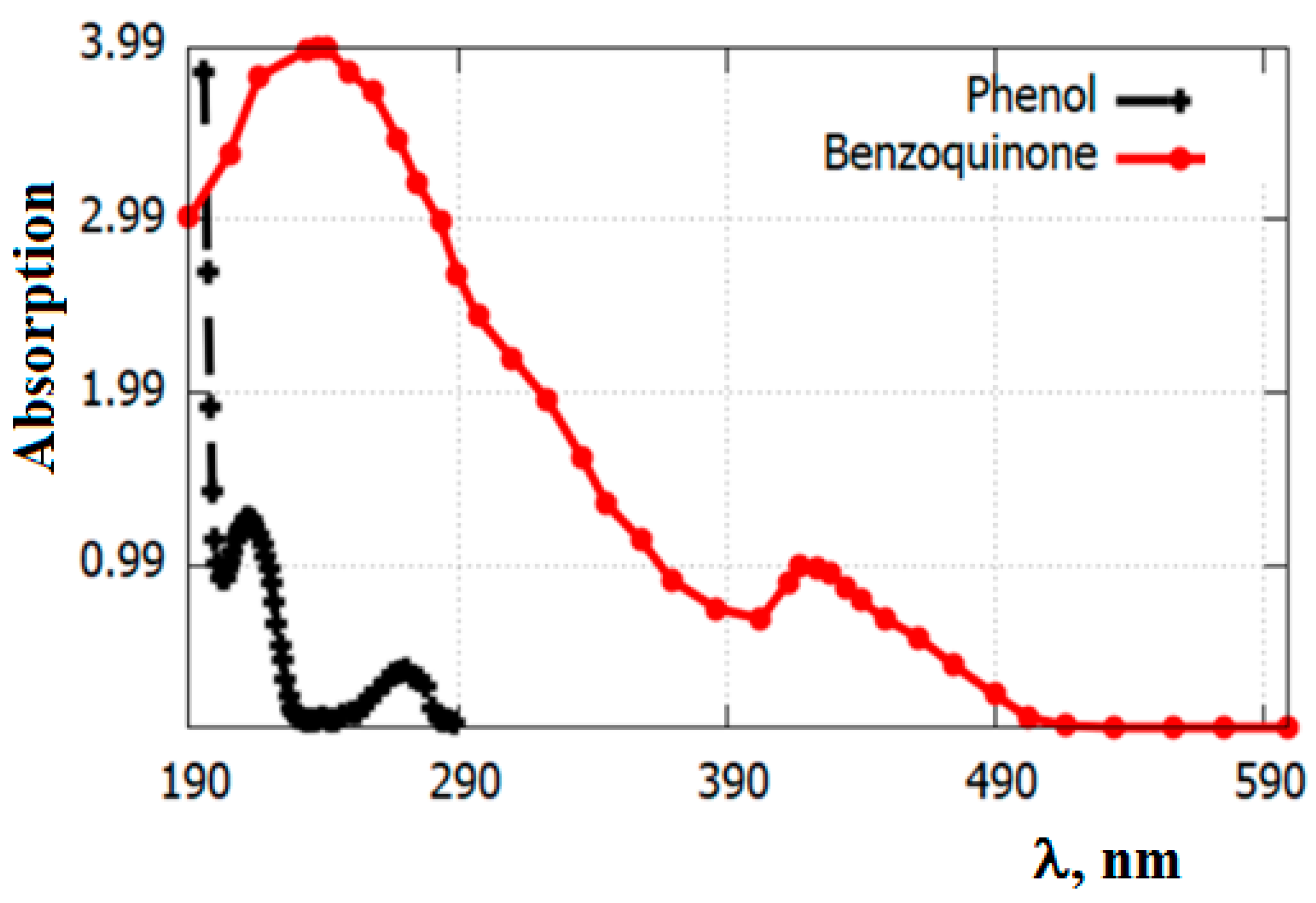
3.1. Catalysts Characterization
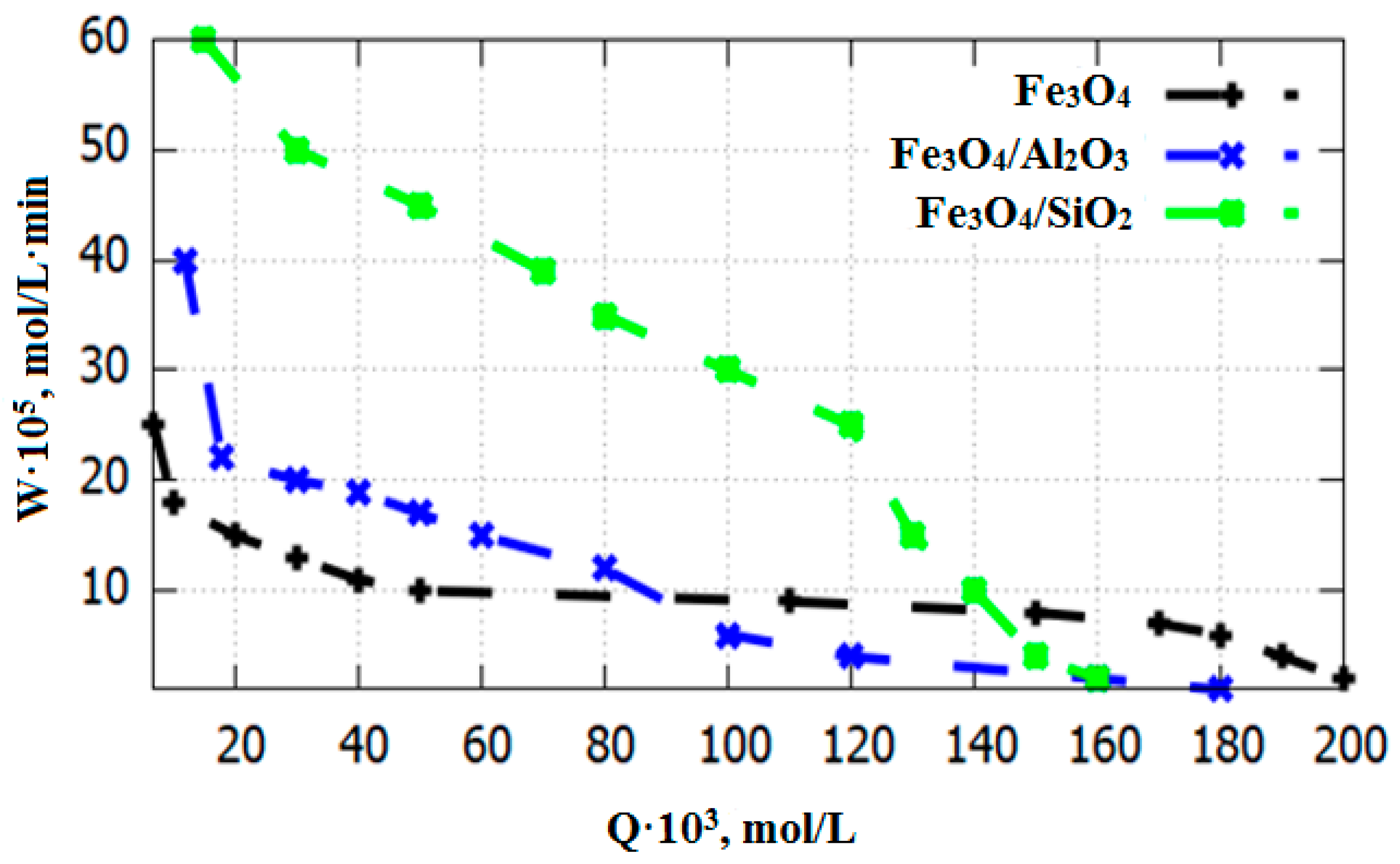
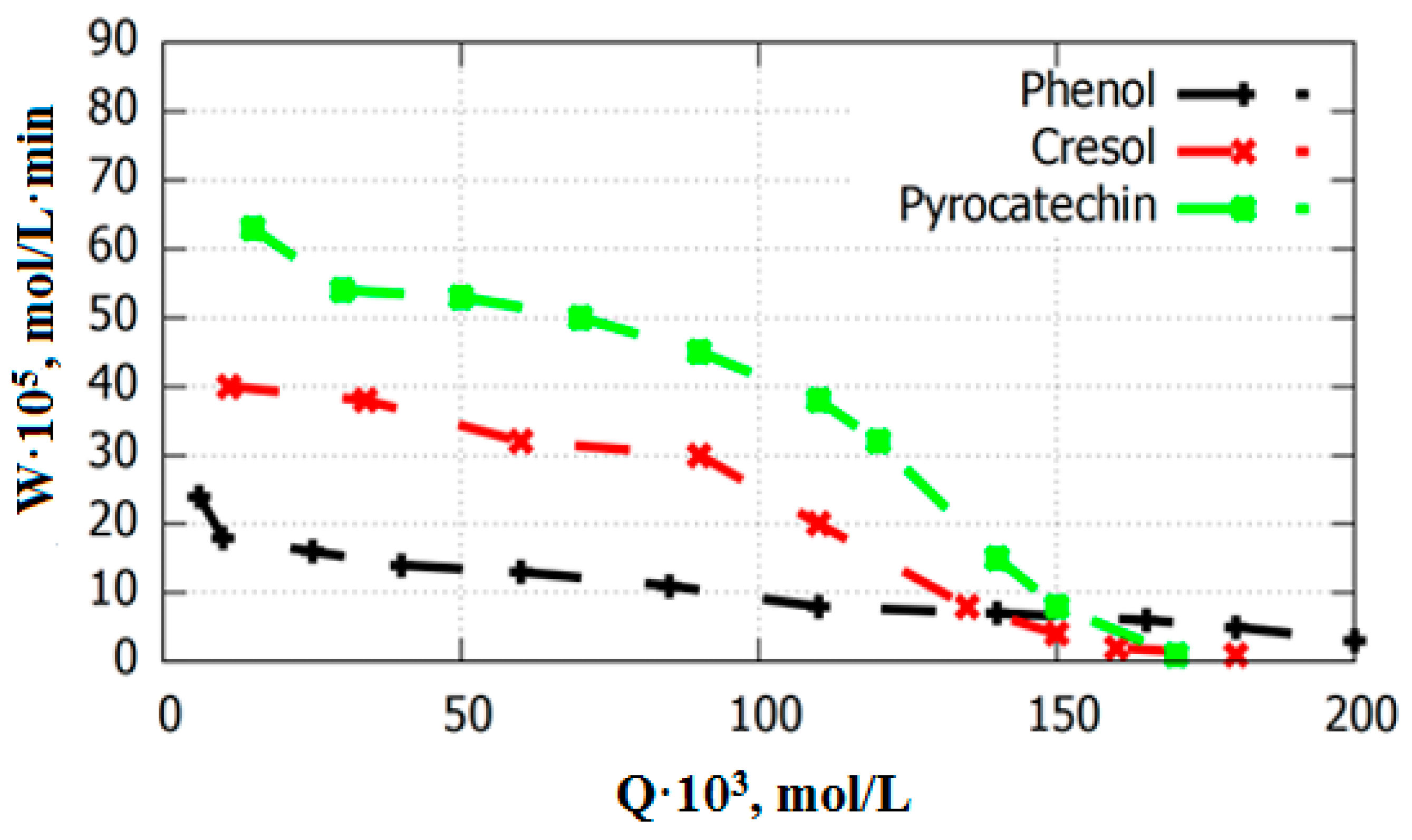
3.2. Catalysts Testing in the Oxidation Reaction
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Bierkens, M.F.P. Global hydrology 2015: State, trends, and directions. Water Resour. Res. 2015, 51, 4923–4947. [Google Scholar] [CrossRef]
- Pedro-Monzonís, M.; Solera, A.; Ferrer, J.; Estrela, T.; Paredes-Arquiola, J. A review of water scarcity and drought indexes in water resources planning and management. J. Hydrol. 2015, 527, 482–493. [Google Scholar] [CrossRef]
- Carr, G.; Blöschl, G.; Loucks, D.P. Evaluating participation in water resource management: A review. Water Resour. Res. 2012, 48, W11401. [Google Scholar] [CrossRef]
- Mousazadeh, M.; Khademi, N.; Kabdaşlı, I.; Rezaei, S.; Hajalifard, Z.; Moosakhani, Z.; Hashim, K. Domestic greywater treatment using electrocoagulation-electrooxidation process: Optimisation and experimental approaches. Sci. Rep. 2023, 13, 15852. [Google Scholar] [CrossRef]
- Haryanto, A.A.B.; Ramelan, A.H.; Budiastuti, M.T.S. Comprehensive study of chromium heavy metal adsorption from textile wastewater using natural composite adsorbent. Rasayan J. Chem. 2022, 15, 2527–2533. [Google Scholar] [CrossRef]
- Kouacou, K.F.J.; Ouattara, A.; Assémian, A.S.; Yao, K.B. Life cycle assessment of wastewater treatment in a refinery with focus on the desalting process. Asian J. Water Environ. Pollut. 2023, 20, 1–8. [Google Scholar] [CrossRef]
- Khatri, N.; Tyagi, S. Influences of natural and anthropogenic factors on surface and groundwater quality in rural and urban areas. Front. Life Sci. 2015, 8, 23–39. [Google Scholar] [CrossRef]
- Dossumova, B.T.; Sassykova, L.R.; Shakiyeva, T.V.; Muktaly, D.; Baizhomartov, B.; Kanapiyeva, F.M.; Hideki Kurokawa, H. Natural waters and industrial wastewater, wastewater with phenol-containing compounds, methods of water purification. Rasayan J. Chem. 2023, 16, 1591–1598. [Google Scholar] [CrossRef]
- Cosgrove, W.J.; Loucks, D.P. Water management: Current and future challenges and research directions. Water Resour. Res. 2015, 51, 4823–4839. [Google Scholar] [CrossRef]
- Ejraei, A.; Aroon, M.A.; Saravani, A.Z. Wastewater treatment using a hybrid system combining adsorption, photocatalytic degradation and membrane filtration processes. J. Water Process. Eng. 2019, 28, 45–53. [Google Scholar] [CrossRef]
- Taghipour, S.; Hosseini, S.M.; Ataie-Ashtiani, B. Engineering nanomaterials for water and wastewater treatment: Review of classifications, properties and applications. New J. Chem. 2019, 43, 7902–7927. [Google Scholar] [CrossRef]
- Crini, G.; Lichtfouse, E. Advantages and disadvantages of techniques used for wastewater treatment. Environ. Chem. Lett. 2019, 17, 145–155. [Google Scholar] [CrossRef]
- Sharma, P.; Gaur, V.K.; Gupta, S.; Varjani, S.; Pandey, A.; Gnansounou, E.; You, S.; Ngo, H.H.; Wong, J.W.C. Trends in mitigation of industrial waste: Global health hazards, environmental implications and waste derived economy for environmental sustainability. Sci. Total. Environ. 2022, 811, 152357. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Malik, M.Z.; Khan, A.; Ali, N.; Malik, S.; Bilal, M. Environmental impacts of hazardous waste, and management strategies to reconcile circular economy and eco-sustainability. Sci. Total. Environ. 2022, 807, 150856. [Google Scholar] [CrossRef]
- Henze, M. Wastewater Treatment: Biological and Chemical Processes, 3rd ed.; Springer: Berlin, Germany, 2002; pp. 87–112. [Google Scholar]
- Sonune, A.; Ghate, R. Developments in wastewater treatment methods. Desalination 2004, 167, 55–63. [Google Scholar] [CrossRef]
- Whitesides, G. 2030. Technology That Will Change the World. By Rutger van Santen, Djan Khoe und Bram Vermeer. Angew. Chem. Int. Ed. 2012, 124, 879–880. [Google Scholar] [CrossRef]
- Busca, G.; Berardinelli, S.; Resini, C.; Arrighi, L. Technologies for the removal of phenol from fluid streams: A short review of recent developments. J. Hazard. Mater. 2008, 160, 265–288. [Google Scholar] [CrossRef]
- Liotta, L.F.; Gruttadauria, M.; Carlo, G.D.; Perrini, G.; Librando, V. Heterogeneous catalytic degradation of phenolic substrates: Catalysts activity. J. Hazard. Mater. 2009, 162, 588–606. [Google Scholar] [CrossRef]
- Tashmukhambetova, Z.K.; Sassykova, L.R.; Aubakirov, Y.A.; Dangaliyeva, A.K.; Kanatbayeva, M.A.; Rustem, A.E. New catalysts for toluene oxidation technology in the liquid phase. Mater. Today Proc. 2020, 31, 529–531. [Google Scholar] [CrossRef]
- Tian, X.; Zhang, H.; Hu, C.; Yan, Y. Preparation of microfiber composite nitrogen doped carbon nanotube membranes and their degradation properties of phenol in the structured fixed bed. J. Environ. Chem. Eng. 2023, 11, 109255. [Google Scholar] [CrossRef]
- Tomita, K.; Oshima, Y. Stability of manganese oxide in catalytic supercritical water oxidation of phenol. Ind. Eng. Chem. Res. 2004, 43, 7740–7743. [Google Scholar] [CrossRef]
- Droste, R.L.; Gehr, R.L. Theory and Practice of Water and Wastewater Treatment; John Wiley & Sons: Hoboken, NJ, USA, 2018; pp. 218–278. [Google Scholar]
- Krajnc, M.; Levec, J. Oxidation of phenol over a transition-metal oxide catalyst in supercritical water. Ind. Eng. Chem. Res. 1997, 36, 3439–3445. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, S.; Guo, Y.; Xu, D.; Gong, Y.; Tang, X.; Ma, H. Oxidative degradation of lurgi coal-gasification wastewater with Mn2O3, Co2O3, and CuO catalysts in supercritical water. Ind. Eng. Chem. Res. 2012, 51, 16573–16579. [Google Scholar] [CrossRef]
- Nihemaiti, M.; Miklos, D.B.; Hübner, U.; Linden, K.G.; Drewes, J.E.; Croué, J.P. Removal of trace organic chemicals in wastewater effluent by UV/H2O2 and UV/PDS. Water Res. 2018, 145, 487–497. [Google Scholar] [CrossRef] [PubMed]
- Silva, C.L.; Garlapalli, R.K.; Trembly, J.P. Removal of phenol from oil/gas produced water using supercritical water treatment with TiO2 supported MnO2 catalyst. J. Environ. Chem. Eng. 2017, 5, 488–493. [Google Scholar] [CrossRef]
- Baloyi, J.; Ntho, T.; Moma, J. Synthesis and application of pillared clay heterogeneous catalysts for wastewater treatment: A review. RSC Adv. 2018, 8, 5197–5211. [Google Scholar] [CrossRef] [PubMed]
- Calleja, G.; Melero, J.A.; Martínez, F.; Molina, R. Activity and resistance of iron-containing amorphous, zeolitic and mesostructured materials for wet peroxide oxidation of phenol. Water Res. 2005, 39, 1741–1750. [Google Scholar] [CrossRef]
- Maurya, M.R.; Titinchi, S.J.J.; Chand, S. Oxidation of phenol with H2O2 catalysed by Cr(III), Fe(III) or Bi(III) N,N′-bis(salicylidene)diethylenetriamine (H2saldien) complexes encapsulated in zeolite-Y. J. Mol. Catal. A Chem. 2003, 193, 165–176. [Google Scholar] [CrossRef]
- Barrault, J. Catalytic wet peroxide oxidation over mixed (Al–Fe) pillared clays. Appl. Catal. B 2000, 27, L225–L230. [Google Scholar] [CrossRef]
- Baabu, P.R.S.; Kumar, H.K.; Gumpu, M.B.; Babu, K.J.; Kulandaisamy, A.J.; Rayappan, J.B.B. Iron Oxide Nanoparticles: A Review on the Province of Its Compounds, Properties and Biological Applications. Materials 2023, 16, 59. [Google Scholar] [CrossRef]
- Wanna, W.H.; Janmanchi, D.; Thiyagarajan, N.; Ramu, R.; Tsai, Y.F.; Yu, S.S.F. Selective oxidation of simple aromatics catalyzed by nano-biomimetic metal oxide catalysts: A mini review. Front. Chem. 2020, 8, 589178. [Google Scholar] [CrossRef] [PubMed]
- Arefieva, O.D.; Vasilyeva, M.S.; Kuryavy, V.G.; Ustinov, A.Y.; Zemnukhova, L.A.; Gushchina, D.D. Oxidative destruction of phenol on Fe/SiO2 catalysts. Water Sci. Technol. 2020, 81, 2189–2201. [Google Scholar] [CrossRef] [PubMed]
- Guélou, E.; Barrault, J.; Fournier, J.; Tatibouët, J.-M. Active iron species in the catalytic wet peroxide oxidation of phenol over pillared clays containing iron. Appl. Catal. B 2003, 44, 1–8. [Google Scholar] [CrossRef]
- Abid, M.F.; Alwan, G.M.; Abdul-Ridha, L.A. Study on catalytic wet air oxidation process for phenol degradation in synthetic wastewater using trickle bed reactor. Arab. J. Sci. Eng. 2016, 41, 2659–2670. [Google Scholar] [CrossRef]
- Nimz, M.; Lietz, G.; Völter, J.; Lázár, K.; Guczi, L. Direct conversion of syngas to aromatics on FePd/SiO2 catalyst. Catal. Lett. 1988, 1, 93–98. [Google Scholar] [CrossRef]
- Iorio, E.D.; Colombo, C.; Cheng, Z.; Capitani, G.; Mele, D.; Ventruti, G.; Angelico, R. Characterization of magnetite nanoparticles synthetized from Fe(II)/nitrate solutions for arsenic removal from water. J. Environ. Chem. Eng. 2019, 7, 102986. [Google Scholar] [CrossRef]
- Sassykova, L.; Shakiyeva, T.; Dossumova, B.; Ilmuratova, M.S.; Muktaly, D.; Zhaxibayeva, Z.M.; Sassykova, A.; Baizhomartov, B. Catalysts, magnetic composites for removal of phenol-containing compounds from wastewater. Rasayan J. Chem. 2023, 16, 1605–1612. [Google Scholar] [CrossRef]
- Datsko, T.; Zelentsov, V.; Dvornikov, D. Advanced Nanotechnology-Based Approaches to Waste Water Purification from Organic Pollutants. IFMBE Proc. 2024, 91, 134–146. [Google Scholar]
- Khabibullin, V.R.; Stepanov, G.V. Effect of a low-frequency magnetic field on the release of heat by magnetic nanoparticles of different shapes. Russ. J. Phys. Chem. A 2020, 94, 439–444. [Google Scholar] [CrossRef]
- Ayati, A.; Ahmadpour, A.; Bamoharram, F.F.; Tanhaei, B.; Mänttäri, M.; Sillanpää, M. A review on catalytic applications of Au/TiO2 nanoparticles in the removal of water pollutant. Chemosphere 2014, 107, 163–174. [Google Scholar] [CrossRef]
- Jung, H.; Kim, J.W.; Choi, H.; Lee, J.H.; Hur, H.G. Synthesis of nanosized biogenic magnetite and comparison of its catalytic activity in ozonation. Appl. Catal. B Environ. 2008, 83, 208–213. [Google Scholar] [CrossRef]
- Bai, Y.; Wang, L.; Lu, S.; Zhang, A.; Qu, W.; He, K.; Hu, N. Research on ultrafast catalytic degradation of heterocyclic compounds by the novel high entropy catalyst. J. Environ. Chem. Eng. 2023, 11, 110703. [Google Scholar] [CrossRef]
- Bukharkina, T.V.; Digurov, N.G.; Mil’K, S.B.; Shelud’Ko, A.B. Development of the Process of p-Nitroacetophenone and Benzoic Acid Manufacture by the Liquid-Phase Oxidation of Aromatic Compounds. Org. Process. Res. Dev. 1999, 3, 404–408. [Google Scholar] [CrossRef]
- Pestunova, O.P.; Ogorodnikova, O.L.; Parmon, V.N. Studies on the Phenol Wet Peroxide Oxidation in the Presence of Solid Catalysts. Chem. Sustain. Dev. 2003, 11, 227–232. [Google Scholar]
- Timofeeva, M.; Khankhasaeva, S.; Chesalov, Y.; Tsybulya, S.; Panchenko, V.; Dashinamzhilova, E. Synthesis of Fe, Al-pillared clays starting from the Al, Fe-polymeric precursor: Effect of synthesis parameters on textural and catalytic properties. Appl. Catal. B Environ. 2009, 88, 127–134. [Google Scholar] [CrossRef]
- Brussino, P.; Gross, M.S.; Ulla, M.A.; Banús, E.D. Copper and iron-based monolithic catalysts for phenol Catalytic Wet Peroxide Oxidation (CWPO): Support and iron effects on the catalytic performance. J. Environ. Chem. Eng. 2023, 11, 110858. [Google Scholar] [CrossRef]
- Catrinescu, C.; Teodosiu, C.; Macoveanu, M.; Miehe-Brendlé, J.; Dred, R.L. Catalytic wet peroxide oxidation of phenol over Fe-exchanged pillared beidellite. Water Res. 2003, 37, 1154–1160. [Google Scholar] [CrossRef]
- Zambrzycki, C.; Shao, R.; Misra, A.; Streb, C.; Herr, U.; Güttel, R. Iron based core-shell structures as versatile materials: Magnetic support and solid catalyst. Catalysts 2021, 11, 72. [Google Scholar] [CrossRef]
- Sassykova, L.R.; Dossumova, B.T.; Ilmuratova, M.S.; Shakiyeva, T.V.; Baizhomartov, B.B.; Sassykova, A.R.; Zhaxibayeva, Z.M.; Abildin, T.S. Development of nanostructured catalysts for catalytic oxidative water purification from organic impurities, including phenolic compounds. Chim. Techno Acta. 2023, 10, 202310309. [Google Scholar] [CrossRef]
- Dossumova, B.T.; Shakiyeva, T.V.; Muktaly, D.; Sassykova, L.R.; Baizhomartov, B.B.; Subramanian, S. Synthesis, Characterization of magnetic composites and testing of their activity in liquid-phase oxidation of phenol with oxygen. Chem. Eng. 2022, 6, 68. [Google Scholar] [CrossRef]
- Huang, H.; Zhang, H.; Yan, Y. Preparation of novel catalyst-free Fe3C nanocrystals encapsulated NCNT structured catalyst for continuous catalytic wet peroxide oxidation of phenol. J. Hazard. Mater. 2021, 407, 124371. [Google Scholar] [CrossRef] [PubMed]
- Ovejero, G.; Sotelo, J.L.; Martínez, F.; Gordo, L. Novel heterogeneous catalysts in the wet peroxide oxidation of phenol. Water Sci. Technol. 2001, 44, 153–160. [Google Scholar] [CrossRef] [PubMed]
- Habibi, D.; Faraji, A.R.; Arshadi, M.; Veisi, H.; Gil, A. Manganese nanocatalyst and N-hydroxyphthalimide as an efficient catalytic system for selective oxidation of ethylbenzene, cyclohexene and oximes under aerobic condition. J. Mol. Catal. A Chem. 2014, 382, 41–54. [Google Scholar] [CrossRef]
- Sapir, L.; Stanley, C.B.; Harries, D. Properties of Polyvinylpyrrolidone in a Deep Eutectic Solvent. J. Phys. Chem. A 2016, 120, 3253–3259. [Google Scholar] [CrossRef] [PubMed]
- Prijic, S.; Sersa, G. Magnetic nanoparticles as targeted delivery systems in oncology. Radiol. Oncol. 2011, 45, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Balcı, S.; Tomul, F. Catalytic wet peroxide oxidation of phenol through mesoporous silica-pillared clays supported iron and/or titanium incorporated catalysts. J. Environ. Mana. 2023, 326 Pt B, 116835. [Google Scholar] [CrossRef]
- Huth, S.; Lausier, J.; Gersting, S.W.; Rudolph, C.; Plank, C.; Welsch, U.; Rosenecker, J. Insights into the mechanism of magnetofection using PEI-based magnetofectins for gene transfer. J. Genet. Med. 2004, 6, 923–936. [Google Scholar] [CrossRef] [PubMed]
- Sassykova, L.; Kubekova, S.N.; Batyrbayeva, A.; Azhigulova, R.N.; Zhaxibayeva, Z.M.; Kozhaisakova, M.A.; Zhusupova, L.A.; Sendilvelan, S.; Ponomarenko, O. Hydrogenation of aromatic nitro compounds to amines on nickel and iron-containing catalysts. Rasayan J. Chem. 2021, 14, 1223–1229. [Google Scholar] [CrossRef]
- Xenariou, S.; Griesenbach, U.; Ferrari, S.; Dean, P.; Scheule, R.K.; Cheng, S.H.; Geddes, D.M.; Plank, C.; Alton, E.W.F.W. Using magnetic forces to enhance non-viral gene transfer to airway epithelium in vivo. Gene Ther. 2006, 13, 1545–1552. [Google Scholar] [CrossRef]
- Nakamoto, K. Infrared and Raman Spectra of Inorganic and Coordination Compounds: Part A: Theory and Application in Inorganic Chemistry; John Willey& Sons: Hoboken, NJ, USA, 2008; pp. 240–295. [Google Scholar]
- Petranovska, A.L.; Fedorenko, O.M.; Horbyk, P.P.; Chuyko, O.O.; Chehun, V.F.; Dubrovin, I.V.; Semko, L.S.; Storozhuk, L.P.; Abramov, M.V.; Revo, S.L. Development and properties of magnetically sensitive nanocomposites for directed transport of medicinal products. Nanosistemi Nanomater. Nanotehnol. 2005, 3, 812–823. [Google Scholar]
- Ryberg, R. Infrared spectroscopy of adsorbed molecules: Some experimental aspects. Le J. de Phys. Colloq. 1983, 44, C10-421–C10-427. [Google Scholar] [CrossRef]
- Semko, L.S.; Storozhuk, L.P.; Khutornoi, S.V.; Abramov, N.V.; Gorbik, P.P. Template synthesis, structure, and properties of magnetically controlled, large surface area Fe3O4/TiO2 adsorbents. Inorg. Mater. 2015, 51, 430–435. [Google Scholar] [CrossRef]
- Paukshtis, E.A.; Yurchenko, E.N. Study of the Acid–Base Properties of Heterogeneous Catalysts by Infrared Spectroscopy. Russ. Chem. Rev. 1983, 52, 242–258. [Google Scholar] [CrossRef]
- Tsyganenko, A.A.; Smirnov, K.S.; Rzhevskij, A.M.; Mardilovich, P.P. Infrared spectroscopic evidence for the structural OH groups of spinel alumina modifications. Mater. Chem. Phys. 1990, 26, 35–46. [Google Scholar] [CrossRef]
- Davydov, A. Molecular Spectroscopy of Oxide Catalyst Surfaces; John Wiley & Sons: Hoboken, NJ, USA, 2003; 690p, ISBN 978-0-471-98731-4. [Google Scholar]
- Aluker, N.L.; Lavrentieva, A.L.; Suzdaltseva, Y.M. Direct optical research methods in the analytics of phenol. Opt. Spectrosc. 2020, 128, 422–428. [Google Scholar] [CrossRef]
- Lázár, K.; Nimz, M.; Lietz, G.; Guczi, L. Formation of PdFe alloys on silica supported catalysts. Hyperfine Interact. 1988, 41, 657–660. [Google Scholar] [CrossRef]
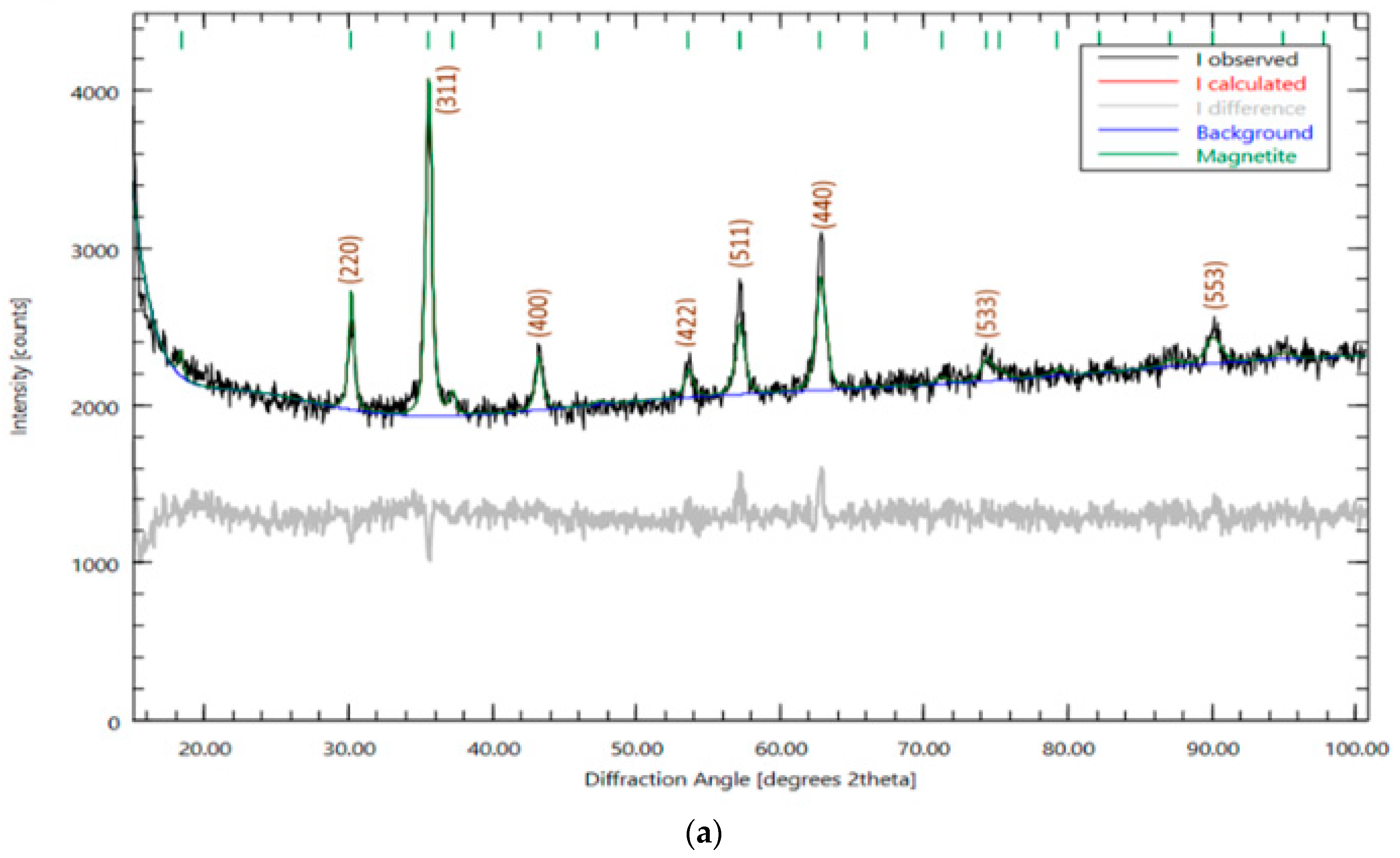
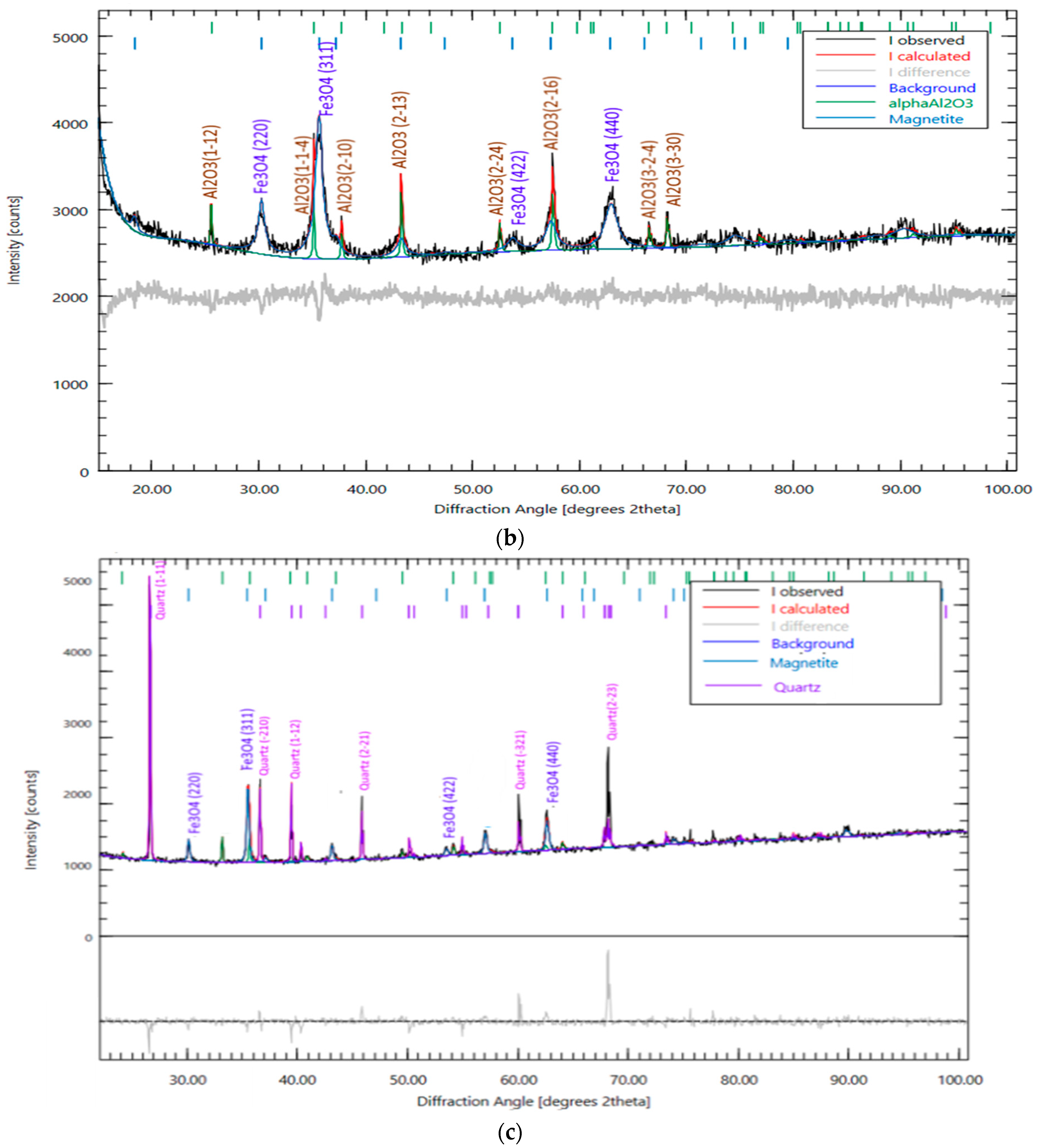

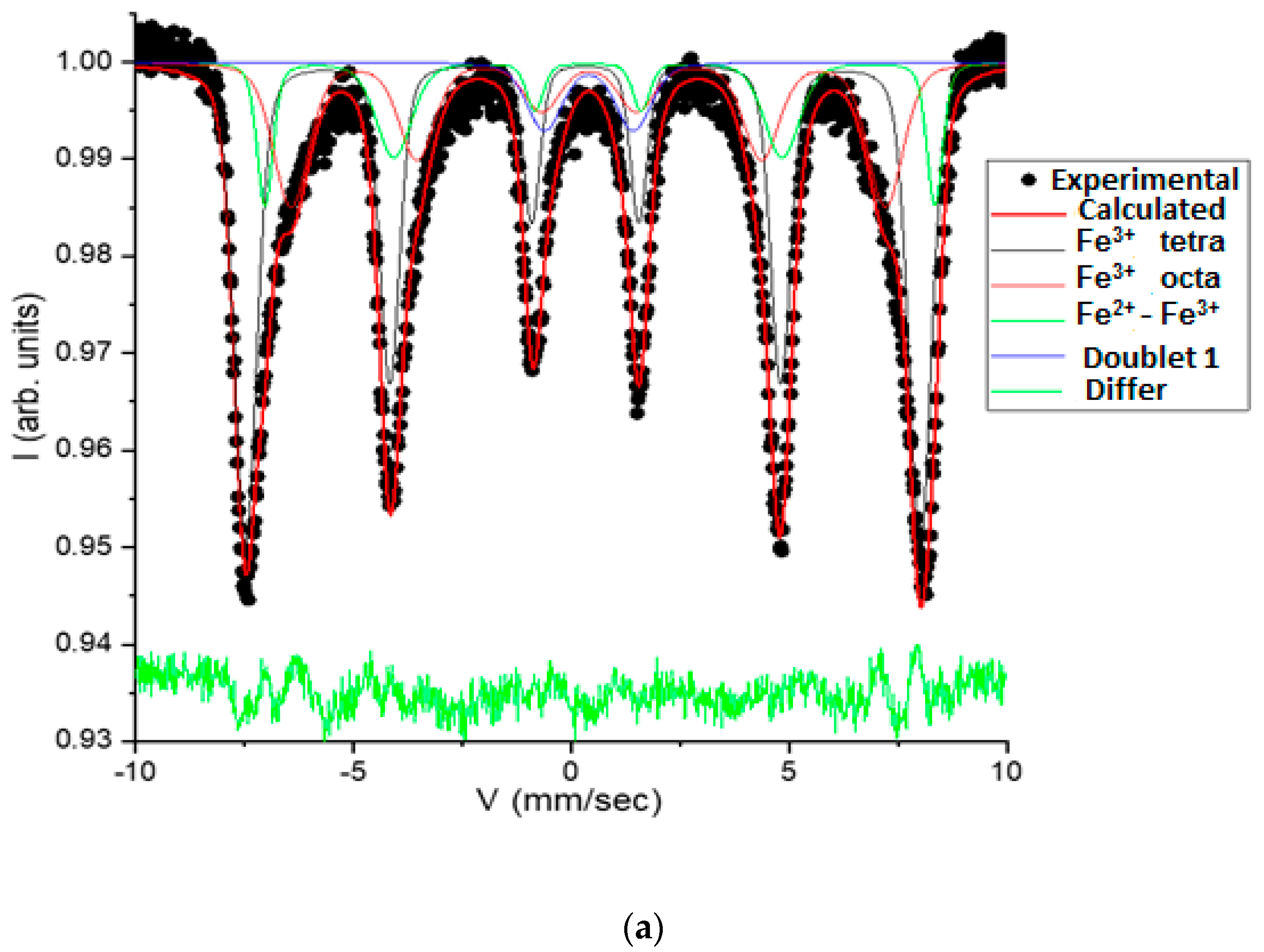

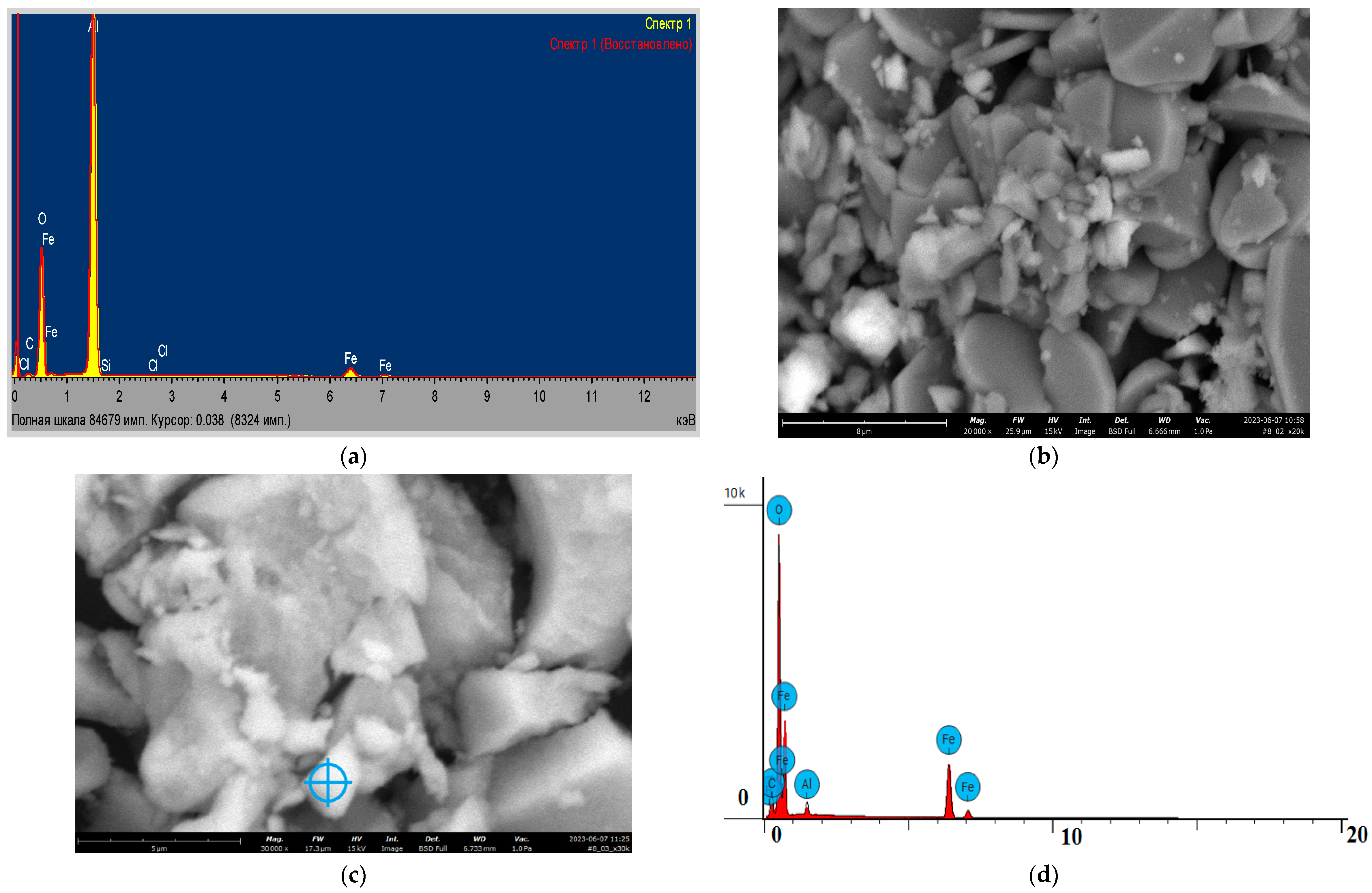


| No | Name | Is, MM/c | Qs, mm/s | H, keV | Srelative,% | G, mm/s |
|---|---|---|---|---|---|---|
| 1-C | Fe3+_tetrahedron | 0.2898 | −0.0545 | 480.95 | 51.29 | 0.6221 |
| 2-C | Fe3+_octahedron | 0.3966 | −0.0200 | 423.85 | 25.99 | 0.9699 |
| 3-C | Fe2+_Fe3+_octahedron | 0.5192 | 0.2737 | 477.61 | 16.57 | 0.9699 |
| 4-D | Doublet_1 | 0.4062 | 2.0478 | - | 6.15 | 0.9700 |
| No | Name | Is, MM/c | Qs, mm/s | H, keV | Srelative,% | G, mm/s |
|---|---|---|---|---|---|---|
| 1-C | Fe3+_ tetrahedron | 0.2870 | 0.2215 | 400.32 | 17.20 | 0.9699 |
| 2-C | Fe3+_Fe2+_ octahedron | 0.6700 | −0.2000 | 444.31 | 12.14 | 0.9700 |
| 3-C | Fe3+_ octahedron | 0.3127 | 0.3193 | 351.52 | 15.64 | 0.9698 |
| 4-C | Fe3+_ tetrahedron | 0.1500 | 0.0481 | 245.82 | 18.08 | 0.6017 |
| 5-C | Fe2+_Fe3+_ octahedron | 0.6053 | −0.1704 | 303.71 | 12.79 | 0.7540 |
| 6-C | Fe2+_Fe3+_ octahedron | 0.8727 | 0.0017 | 228.28 | 19.40 | 0.5934 |
| 7-D | Doublet_1 | 0.3690 | 1.0407 | 4.76 | 0.9699 |
| No | Name | Is, MM/c | Qs, mm/s | H, keV | Srelative,% | G, mm/s |
|---|---|---|---|---|---|---|
| 1-C | Fe3+_ tetrahedron | 0.2966 | 0.3630 | 383.50 | 12.17 | 0.8444 |
| 2-C | Fe3+_Fe2+_ octahedron | 0.5962 | −0.2000 | 431.43 | 14.16 | 0.9700 |
| 3-C | Fe3+_ octahedron | 0.2926 | 0.3958 | 337.47 | 14.52 | 0.9698 |
| 4-C | Fe3+_ tetrahedron | 0.1500 | −0.0295 | 237.17 | 20.37 | 0.6390 |
| 5-C | Fe2+_Fe3+_ octahedron | 0.6405 | −0.2000 | 291.97 | 9.26 | 0.6401 |
| 6-C | Fe2+_Fe3+_ octahedron | 0.8502 | −0.0190 | 219.76 | 22.56 | 0.6205 |
| 7-D | Doublet_1 | 0.3690 | 1.0407 | - | 6.95 | 0.9699 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dossumova, B.T.; Sassykova, L.R.; Shakiyeva, T.V.; Muktaly, D.; Batyrbayeva, A.A.; Kozhaisakova, M.A. Catalysts Based on Iron Oxides for Wastewater Purification from Phenolic Compounds: Synthesis, Physicochemical Analysis, Determination of Catalytic Activity. ChemEngineering 2024, 8, 8. https://doi.org/10.3390/chemengineering8010008
Dossumova BT, Sassykova LR, Shakiyeva TV, Muktaly D, Batyrbayeva AA, Kozhaisakova MA. Catalysts Based on Iron Oxides for Wastewater Purification from Phenolic Compounds: Synthesis, Physicochemical Analysis, Determination of Catalytic Activity. ChemEngineering. 2024; 8(1):8. https://doi.org/10.3390/chemengineering8010008
Chicago/Turabian StyleDossumova, Binara T., Larissa R. Sassykova, Tatyana V. Shakiyeva, Dinara Muktaly, Aigul A. Batyrbayeva, and Madina A. Kozhaisakova. 2024. "Catalysts Based on Iron Oxides for Wastewater Purification from Phenolic Compounds: Synthesis, Physicochemical Analysis, Determination of Catalytic Activity" ChemEngineering 8, no. 1: 8. https://doi.org/10.3390/chemengineering8010008






