Freshening of a Coastal Karst Aquifer Revealed by the Temporal Changes in a Spring Water Composition (La Palme, Southern France)
Abstract
:1. Introduction
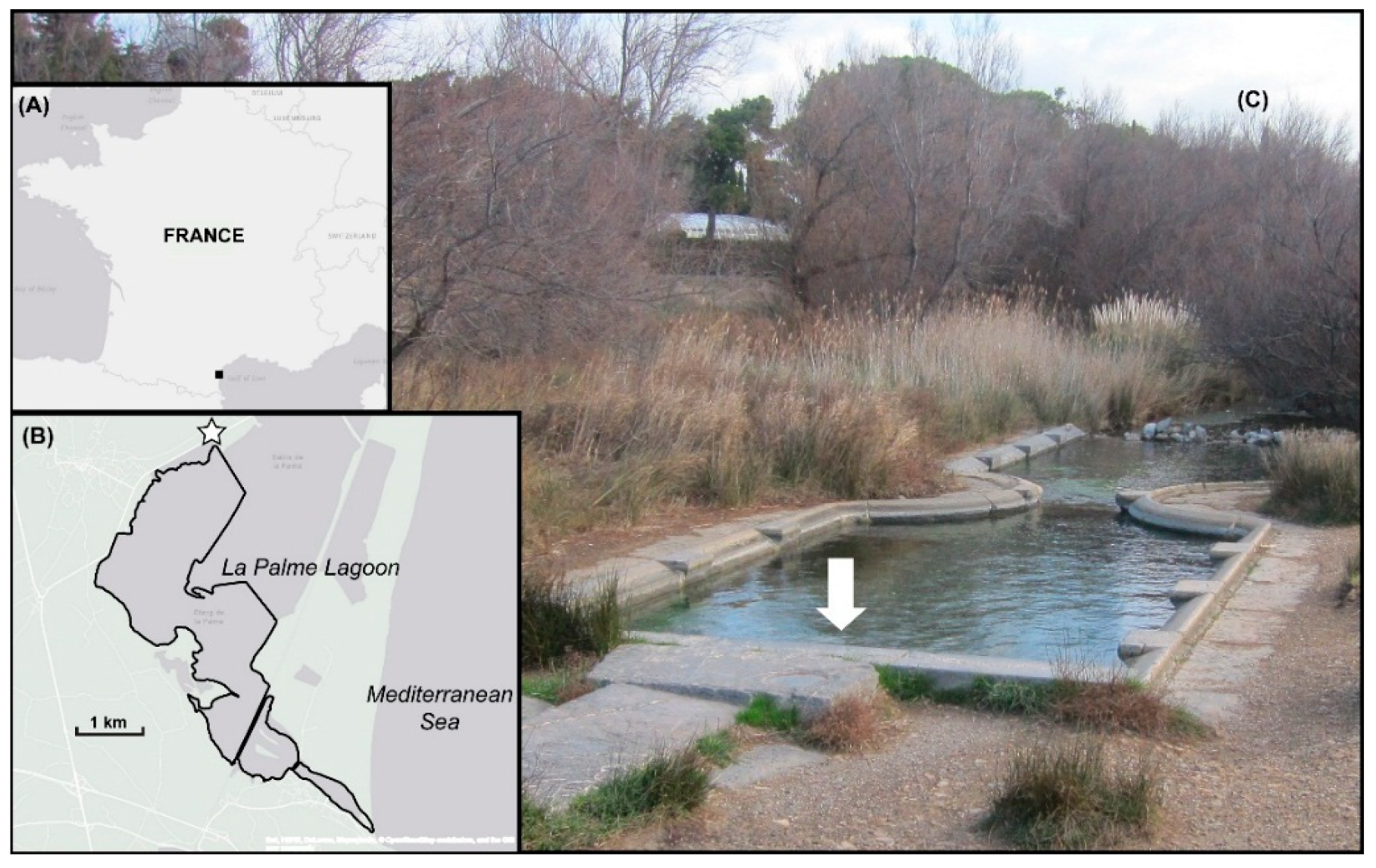
2. Geology of the La Palme Watershed
3. Methods
3.1. In Situ Measurements and Timetable
3.2. Chemical Analyses in the Laboratory
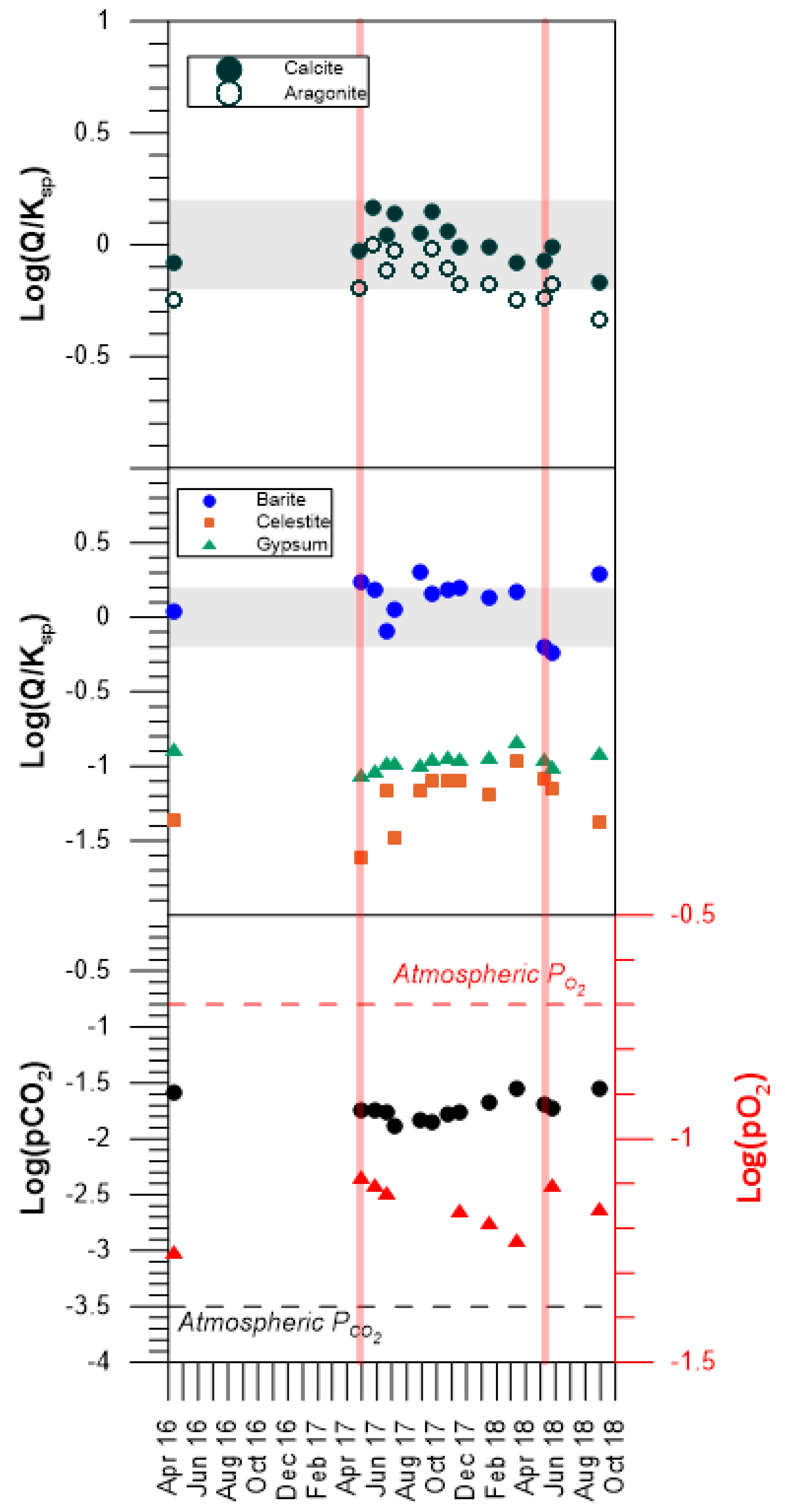
3.3. Thermodynamics: The Calculation of Mineral Saturation States
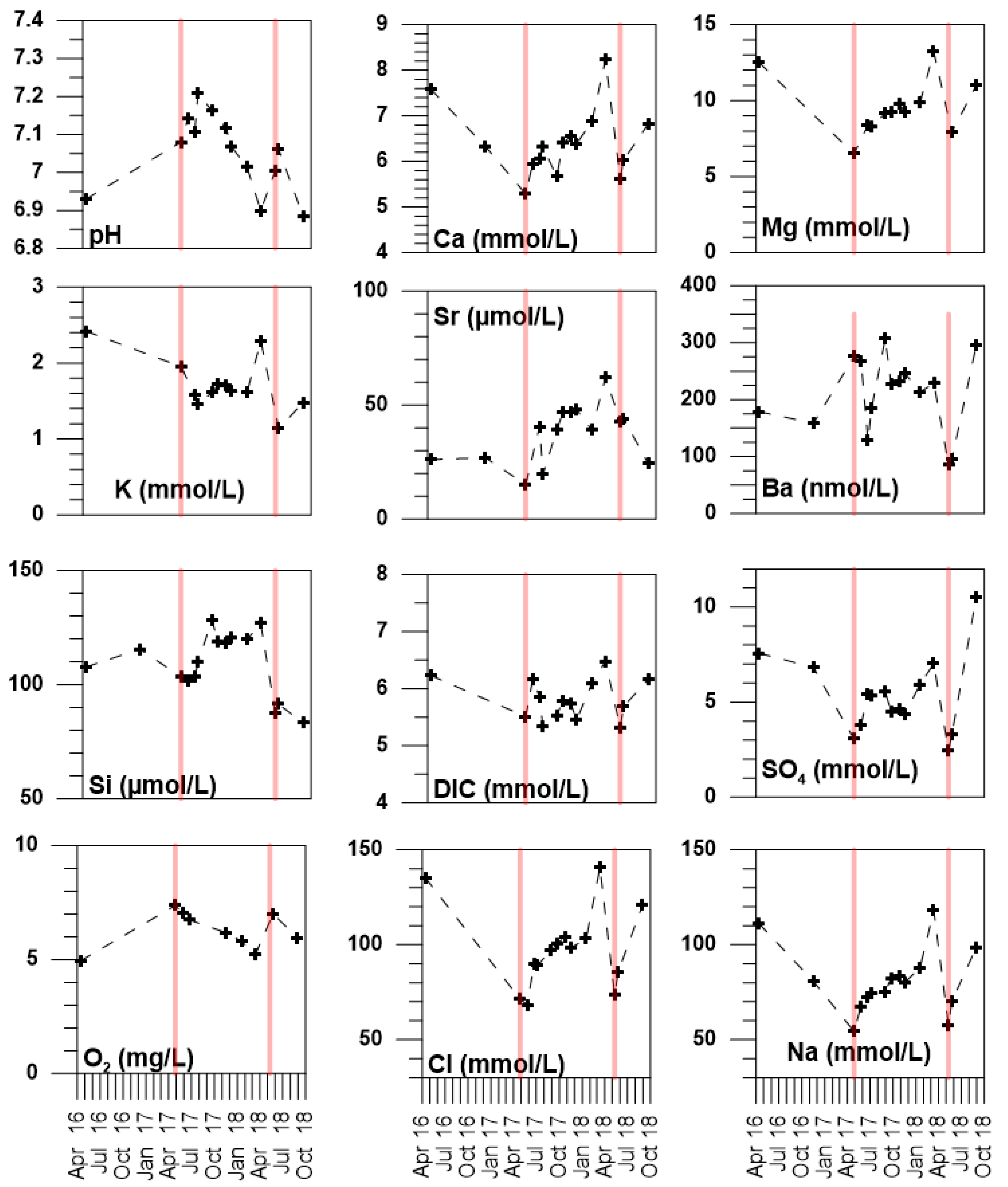
4. Results
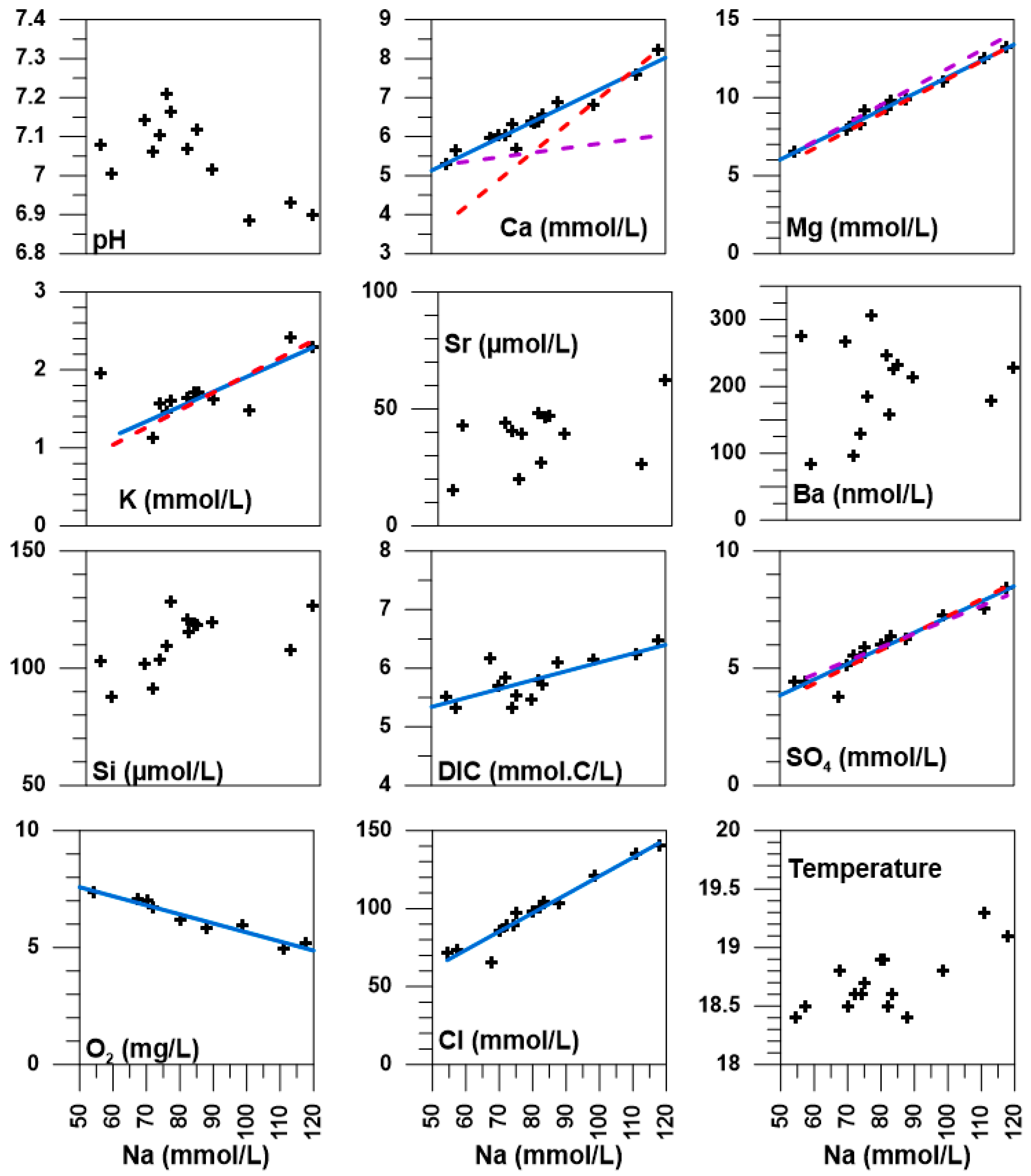
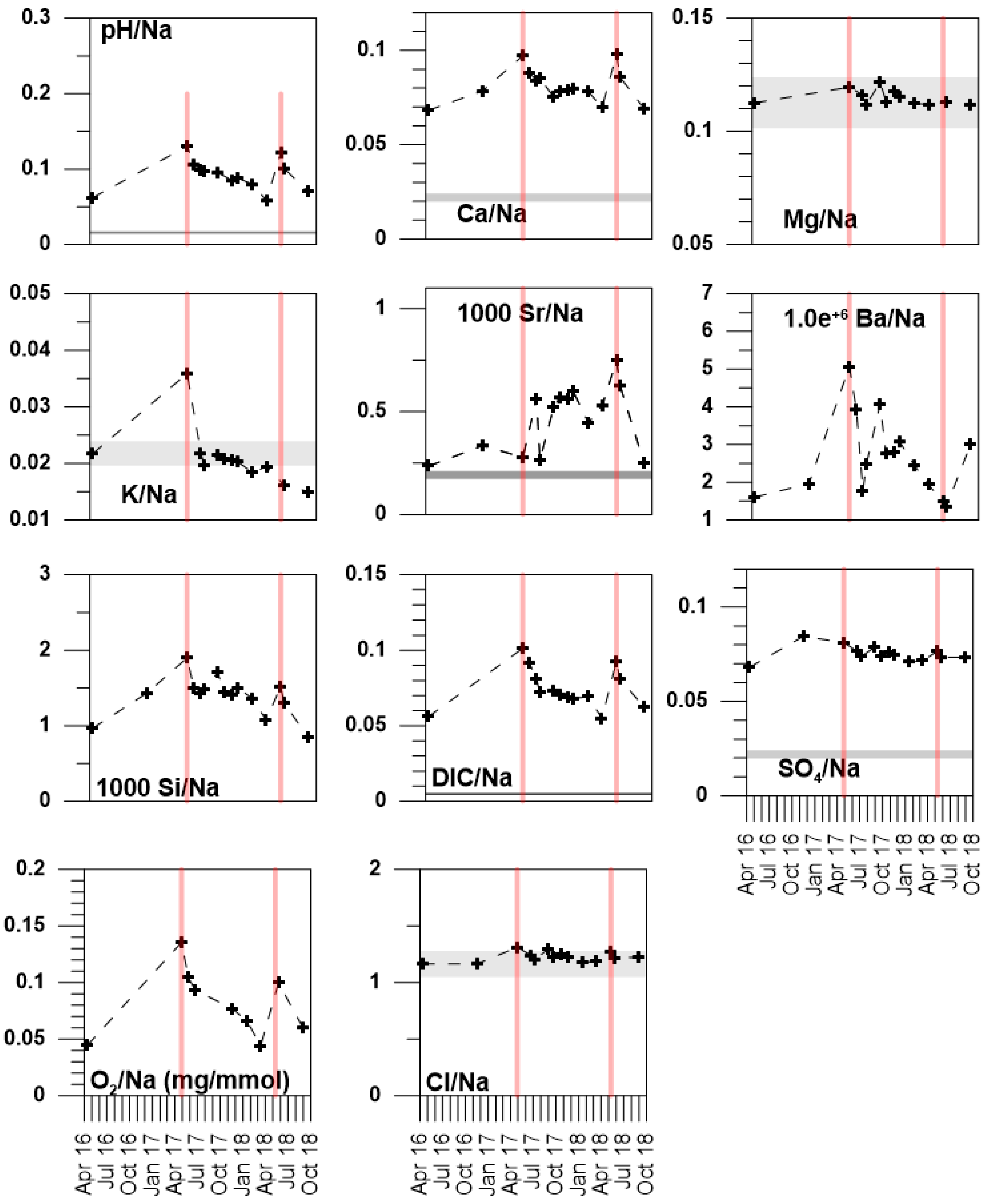
5. Discussion
5.1. Temporal and Spatial Variability
5.2. The Different Water Types in the La Palme Aquifer—Defining the Endmembers
5.2.1. Seawater Intrusion into S1
5.2.2. Rain Infiltration Diluting S2
5.3. Discussion of the Two Hypotheses
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Webb, M.D.; Howard, K.W.F. Modeling the Transient Response of Saline Intrusion to Rising Sea-Levels. Ground Water 2011, 49, 560–569. [Google Scholar] [CrossRef]
- Post, V.E.A.; Werner, A.D. Coastal aquifers: Scientific advances in the face of global environmental challenges. J. Hydrol. 2017, 551, 1–3. [Google Scholar] [CrossRef] [Green Version]
- Fleury, P.; Bakalowicz, M.; de Marsily, G. Submarine springs and coastal karst aquifers: A review. J. Hydrol. 2007, 339, 79–92. [Google Scholar] [CrossRef]
- Newton, A.; Icely, J.; Cristina, S.; Brito, A.; Cardoso, A.C.; Colijn, F.; Riva, S.D.; Gertz, F.; Hansen, J.W.; Holmer, M.; et al. An overview of ecological status, vulnerability and future perspectives of European large shallow, semi-enclosed coastal systems, lagoons and transitional waters. Estuar. Coast. Shelf Sci. 2014, 140, 95–122. [Google Scholar] [CrossRef]
- Moosdorf, N.; Oehler, T. Societal use of fresh submarine groundwater discharge: An overlooked water resource. Earth-Sci. Rev. 2017, 171, 338–348. [Google Scholar] [CrossRef]
- Custodio, E. Coastal aquifers of Europe: An overview. Hydrogeol. J. 2010, 18, 269–280. [Google Scholar] [CrossRef]
- Khaska, M.; La Salle, C.L.G.; Lancelot, J.; ASTER team; Mohamad, A.; Verdoux, P.; Noret, A.; Simler, R. Origin of groundwater salinity (current seawater vs. saline deep water) in a coastal karst aquifer based on Sr and Cl isotopes. Case study of the La Clape massif (southern France). Appl. Geochem. 2013, 37, 212–227. [Google Scholar] [CrossRef]
- Bakalowicz, M. Karst and karst groundwater resources in the Mediterranean. Environ. Earth Sci. 2015, 74, 5–14. [Google Scholar] [CrossRef]
- Todd, D.K.; Mays, L.W. Groundwater Hydrology, 3rd ed.; John Wiley and Sons: Hoboken, NJ, USA, 2005; p. 636. [Google Scholar]
- Schwartz, F.W.; Zhang, H. Fundamentals of Groundwater; John Wiley and Sons: Hoboken, NJ, USA, 2003; p. 582. [Google Scholar]
- Dimova, N.; Ganguli, P.M.; Swarzenski, P.W.; Izbicki, J.A.; O’Leary, D. Hydrogeologic controls on chemical transport at Malibu Lagoon, CA: Implications for land to sea exchange in coastal lagoon systems. J. Hydrol. 2017, 11, 219–233. [Google Scholar] [CrossRef] [Green Version]
- Young, C.; Martin, J.B.; Branyon, J.; Pain, A.; Valle-Levinson, A.; Mariño-Tapia, I.; Vieyra, M.R. Effects of short-term variations in sea level on dissolved oxygen in a coastal karst aquifer, Quintana Roo, Mexico. Limnol. Oceanogr. 2018, 63, 352–362. [Google Scholar] [CrossRef]
- Russak, A.; Sivan, O.; Yechieli, Y. Trace elements (Li, B, Mn and Ba) as sensitive indicators for salinization and freshening events in coastal aquifers. Chem. Geol. 2016, 441, 35–46. [Google Scholar] [CrossRef]
- Mongelli, G.; Monni, S.; Oggiano, G.; Paternoster, M.; Sinisi, R. Tracing groundwater salinization processes in coastal aquifers: A hydrogeochemical and isotopic approach in the Na-Cl brackish waters of northwestern Sardinia, Italy. Hydrol. Earth Syst. Sci. 2013, 17, 2917–2928. [Google Scholar] [CrossRef]
- Hebrard, O.; Pistre, S.; Cheynet, N.; Dazy, J.; Batiot-Guilhe, C.; Seidel, J.-L. Origin of the Languedoc-Roussillon’s chloride rich karstic spring waters. Comptes Rendus Geosci. 2006, 338, 703–710. [Google Scholar] [CrossRef]
- Tamborski, J.; Bejannin, S.; Garcia-Orellana, J.; Souhaut, M.; Charbonnier, C.; Anschutz, P.; Pujo-Pay, M.; Conan, P.; Crispi, O.; Monnin, C.; et al. A comparison between water circulation and terrestrially-driven dissolved silica fluxes to the Mediterranean Sea traced using radium isotopes. Geochimica et Cosmochimica Acta 2018, 238, 496–515. [Google Scholar] [CrossRef] [Green Version]
- Bejannin, S.; van Beek, P.; Thomas, S.; Marc, S.; Joseph, T. Combining airborne thermal infrared images and radium isotopes to study submarine groundwater discharge along the French Mediterranean coastline. J. Hydrol. 2017, 13, 72–90. [Google Scholar] [CrossRef]
- Stieglitz, T.C.; Beek, P.; Souhaut, M.; Cook, P.G. Karstic groundwater discharge and seawater recirculation through sediments in shallow coastal Mediterranean lagoons, determined from water, salt and radon budgets. Mar. Chem. 2013, 156 (Suppl. C), 73–84. [Google Scholar] [CrossRef]
- Tamborski, J.; van Beek, P.; Rodellas, V.; Monnin, C.; Bergsma, E.; Stieglitz, T.; Heilbrun, C.; Cochran, J.K.; Charbonnier, C.; Anschutz, P.; et al. Temporal variability of lagoon-sea water exchange and seawater circulation through a Mediterranean barrier beach. Limnol. Oceanogr. 2019. [Google Scholar] [CrossRef]
- Berger, G.M.; Bertrand-Sarfati, J.; Ovtracht, A.; Héraud, H.; de Bouchony, P.; Tourel, Y.; Got, H.; Le Bail, A.; Blès, J.-L.; Gadel, F.; et al. Carte Geologique de la France a 1/50 000, Feuille Leucate (1079); BRGM: Orleans, France, 1982. [Google Scholar]
- Khaska, M.; La Salle, C.L.G.; Videau, G.; Flinois, J.-S.; Frape, S.; Aster Team; Verdoux, P. Deep water circulation at the northern Pyrenean thrust: Implication of high temperature water-rock interaction process on the mineralization of major spring water in an overthrust area. Chem. Geol. 2015, 419, 114–131. [Google Scholar] [CrossRef]
- Parkhurst, D.L.; Appelo, C.A.J. Description of input and examples for PHREEQC version 3-A computer program for speciation, batch-reaction, one-dimensional transport, and inverse geochemical calculations. In U.S. Geological Survey Techniques and Methods; USGS: Reston, VA, USA, 2013; Book 6, Chapter A43; pp. 1–497. [Google Scholar]
- Giffaut, E.; Grivé, M.; Blanc, P.; Vieillard, P.; Colàs, E.; Gailhanou, H.; Gaboreau, S.; Marty, N.; Madé, B.; Duro, L. Andra thermodynamic database for performance assessment: ThermoChimie. Appl. Geochem. 2014, 49, 225–236. [Google Scholar] [CrossRef]
- Blanc, P.; Bourbon, X.; Lassin, A.; Gaucher, E.C. Chemical model for cement-based materials: Thermodynamic data assessment for phases other than C–S–H. Cem. Concr. Res. 2010, 40, 1360–1374. [Google Scholar] [CrossRef]
- Blanc, P.; Lassin, A.; Piantone, P.; Azaroual, M.; Jacquemet, N.; Fabbri, A.; Gaucher, E.C. Thermoddem: A geochemical database focused on low temperature water/rock interactions and waste materials. Appl. Geochem. 2012, 27, 2107–2116. [Google Scholar] [CrossRef]
- Marion, G.M.; Mironenko, M.V.; Roberts, M.W. FREZCHEM: A geochemical model for cold aqueous solutions. Comput. Geosci. 2010, 36, 10–15. [Google Scholar] [CrossRef]
- Marion, G.M.; Kargel, J.S. Cold Aqueous Planetary Geochemistry with FREZCHEM: From Modeling to the Search for Life at the Limits; Springer: New York, NY, USA, 2008. [Google Scholar]
- Benezeth, P.; Berninger, U.-N.; Bovet, N.; Schott, J.; Oelkers, E.H. Experimental determination of the solubility product of dolomite at 50-253 degrees C. Geochimica et Cosmochimica Acta 2018, 224, 262–275. [Google Scholar] [CrossRef]
- Oehlerich, M.; Mayr, C.; Griesshaber, E.; Lücke, A.; Oeckler, O.M.; Ohlendorf, C.; Schmahl, W.W.; Zolitschka, B. Ikaite precipitation in a lacustrine environment—Implications for palaeoclimatic studies using carbonates from Laguna Potrok Aike (Patagonia, Argentina). Quater. Sci. Rev. 2013, 71, 46–53. [Google Scholar] [CrossRef]
- Monnin, C.; Hoareau, G. Chemical Equilibrium between Aqueous Fluids and Minerals in the Marine Environment, in Ion-partitioning in Ambient Temperature Aqueous Systems; Prieto, M., Stoll, H., Eds.; European Mineralogical Union: Jena, Germany, 2010; pp. 227–257. [Google Scholar]
- Acero, P.; Auqué, L.F.; Galve, J.P.; Gutiérrez, F.; Carbonel, D.; Gimeno, M.J.; Yechieli, Y.; Asta, M.P.; Gómez, J.B. Evaluation of geochemical and hydrogeological processes by geochemical modeling in an area affected by evaporite karstification. J. Hydrol. 2015, 529, 1874–1889. [Google Scholar] [CrossRef]
- Power, I.M.; Kenward, P.A.; Dipple, G.M.; Raudsepp, M. Room Temperature Magnesite Precipitation. Crystal Growth Design 2017, 17, 5652–5659. [Google Scholar] [CrossRef]
- Millero, F.J. Chemical oceanography. In Marine Sciences Series; Kennish, M.K., Ed.; CRC Taylor and Francis: Abingdon-on-Thames, UK, 2006; p. 496. [Google Scholar]
- Gil-Márquez, J.M.; Barberá, J.A.; Andreo, B.; Mudarra, M. Hydrological and geochemical processes constraining groundwater salinity in wetland areas related to evaporitic (karst) systems. A case study from Southern Spain. J. Hydrol. 2017, 544, 538–554. [Google Scholar] [CrossRef]
- Macpherson, G.L. CO2 distribution in groundwater and the impact of groundwater extraction on the global C cycle. Chem. Geol. 2009, 264, 328–336. [Google Scholar] [CrossRef]
- Maas, B.J.; Wicks, C.M. CO2 Outgassing from Spring Waters. Aquat. Geochem. 2017, 23, 53–60. [Google Scholar] [CrossRef]
- Raymond, P.A.; Hartmann, J.; Lauerwald, R.; Sobek, S.; McDonald, C.; Hoover, M.; Butman, D.; Striegl, R.; Mayorga, E.; Humborg, C.; et al. Global carbon dioxide emissions from inland waters. Nature 2013, 503, 355–359. [Google Scholar] [CrossRef] [Green Version]
- Jacquet, S.H.M.; Monnin, C.; Riou, V.; Jullion, L.; Tanhua, T. A high resolution and quasi-zonal transect of dissolved Ba in the Mediterranean Sea. Mar. Chem. 2016, 178, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Anderson, M.P. Heat as a Ground Water Tracer. Groundwater 2005, 43, 951–968. [Google Scholar] [CrossRef]
- Aquilina, L.; Ladouche, B.; Dorfliger, N. Water storage and transfer in the epikarst of karstic systems during high flow periods. J. Hydrol. 2006, 327, 472–485. [Google Scholar] [CrossRef]
- Labat, D.; Mangin, A.; Ababou, R. Rainfall-runoff relations for karstic springs: Multifractal analyses. J. Hydrol. 2002, 256, 176–195. [Google Scholar] [CrossRef]
- Ladouche, B.; Dörfliger, N.; Izac, J.L.; Cubizolles, J.; Le Strat, P.; Du Couëdic, C.; Aunay, B.; Thomson, P. Evaluation des Ressources en eau des Corbières. Phase I—Synthèse de la Caractérisation des Systèmes Karstiques des Corbières Orientales. Rapport Final. Volume 2—Caractérisation Géologique et Hydrogéologique du Système Karstqiue du Synclinal du Bas-Agly; BRGM: Orléans, France, 2004; report BRGM/RP-52919-FR; p. 196. [Google Scholar]
- Lucena-Moya, P.; Gomez-Rodriguez, C.; Pardo, I. Spatio-Temporal Variability in Water Chemistry of Mediterranean Coastal Lagoons and its Management Implications. Wetlands 2012, 32, 1033–1045. [Google Scholar] [CrossRef]
- Petelet-Giraud, E.; Négrel, P.; Aunay, B.; Ladouche, B.; Bailly-Comte, V.; Guerrot, C.; Flehoc, C.; Pezard, P.; Lofi, J.; Dörfliger, N. Coastal groundwater salinization: Focus on the vertical variability in a multi-layered aquifer through a multi-isotope fingerprinting (Roussillon Basin, France). Sci. Total Environ. 2016, 566, 398–415. [Google Scholar] [CrossRef] [PubMed]
- Aquilina, L.; Ladouche, B.; Doerfliger, N.; Seidel, J.L.; Bakalowicz, M.; Dupuy, C.; Le Strat, P. Origin, evolution and residence time of saline thermal fluids (Balaruc springs, southern France): Implications for fluid transfer across the continental shelf. Chem. Geol. 2002, 192, 1–21. [Google Scholar] [CrossRef]
- Werner, A.D.; Bakker, M.; Post, V.E.A.; Vandenbohede, A.; Lu, C.; Ataie-Ashtiani, B.; Simmons, C.T.; Barry, D.A. Seawater intrusion processes, investigation and management: Recent advances and future challenges. Adv. Water Resour. 2013, 51, 3–26. [Google Scholar] [CrossRef]
- Walton Smith, F.G. Handbook of Marine Science; CRC Press: Boca Raton, FL, USA, 1981; p. 627. [Google Scholar]
- Chapelle, F.H. Geochemistry of groundwater. In Surface and Groundwater, Weathering, and Soils; Drever, J.I., Ed.; Elsevier-Pergamon: Oxford, UK, 2003; pp. 425–450. [Google Scholar]
- Martin, W.R.; Sayles, F.L. 7.02—The Recycling of Biogenic Material at the Seafloor. In Treatise on Geochemistry; Holland, H.D., Turekian, K.K., Eds.; Pergamon: Oxford, UK, 2003; pp. 37–65. [Google Scholar]

| Sampling Date | T | pH | ORP | O2 | Salinity | DIC | DOC | Na | K | Ca | Mg | Sr | Ba | B | Si | Cl | SO4 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| °C | mV | mg/L | mmol/L | mmol/L | mmol/L | mmol/L | mmol/L | mmol/L | µmol/L | nmol/L | µmol/L | µmol/L | mmol/L | mmol/L | |||
| 04/13/2016 | 19.3 | 6.930 | 251 | 4.9 | 8.8 | 6.23 | 0.06 | 111.1 | 2.4 | 7.6 | 12.5 | 26.1 | 178.8 | 74.1 | 107.7 | 135.2 | 7.6 |
| 06/15/2016 | 19.6 | 8.9 | |||||||||||||||
| 06/16/2016 | 19.1 | 9.3 | |||||||||||||||
| 11/16/2016 | 18.9 | 133 | 7.4 | 80.7 | 6.3 | 27.0 | 158.4 | 115.5 | 6.8 | ||||||||
| 04/28/2017 | 18.4 | 7.080 | 7.4 | 4.8 | 5.51 | 54.4 | 2.0 | 5.3 | 6.5 | 275.7 | 48.7 | 103.3 | 71.3 | 4.4 | |||
| 05/26/2017 | 18.8 | 7.143 | 7.1 | 5.4 | 6.17 | 67.6 | 6.0 | 266.3 | 101.7 | 3.8 | |||||||
| 06/22/2017 | 18.6 | 7.105 | 138 | 6.7 | 6.0 | 5.85 | 0.09 | 72.1 | 1.6 | 6.0 | 8.4 | 40.6 | 128.3 | 57.9 | 103.4 | 89.6 | 5.5 |
| 07/06/2017 | 18.6 | 7.210 | 116 | 6.1 | 5.33 | 0.08 | 74.1 | 1.5 | 6.3 | 8.3 | 19.7 | 184.2 | 59.1 | 109.7 | 89.1 | 5.5 | |
| 08/30/2017 | 18.7 | 7.162 | 6.5 | 5.53 | 75.2 | 1.6 | 5.7 | 9.2 | 39.1 | 306.6 | 65.2 | 128.3 | 97.2 | 5.9 | |||
| 09/23/2017 | 18.5 | 5.79 | 82.1 | 1.7 | 6.4 | 9.3 | 46.6 | 225.9 | 63.3 | 118.8 | 100.5 | 6.1 | |||||
| 10/25/2017 | 18.6 | 7.117 | 6.9 | 5.73 | 83.4 | 1.7 | 6.6 | 9.8 | 46.8 | 232.0 | 66.8 | 118.2 | 104.2 | 6.3 | |||
| 11/17/2017 | 18.9 | 7.067 | 234 | 6.2 | 6.5 | 5.45 | 80.1 | 1.6 | 6.4 | 9.2 | 48.0 | 246.1 | 65.5 | 120.6 | 98.3 | 6.0 | |
| 01/17/2018 | 18.4 | 7.015 | 150 | 5.8 | 7.0 | 6.09 | 87.9 | 1.6 | 6.9 | 9.9 | 39.1 | 213.6 | 69.8 | 119.6 | 103.2 | 6.2 | |
| 03/13/2018 | 19.1 | 6.900 | 198 | 5.2 | 9.4 | 6.48 | 117.9 | 2.3 | 8.2 | 13.2 | 62.1 | 228.3 | 91.7 | 126.7 | 140.8 | 8.5 | |
| 05/11/2018 | 18.5 | 7.005 | 4.7 | 5.32 | 57.4 | 5.6 | 43.0 | 84.8 | 50.5 | 87.6 | 73.4 | 4.4 | |||||
| 05/24/2018 | 18.5 | 7.061 | 171 | 7.0 | 5.4 | 5.69 | 70.1 | 1.1 | 6.0 | 7.9 | 44.1 | 95.5 | 60.4 | 91.4 | 85.4 | 5.1 | |
| 08/30/2018 | 18.8 | 6.885 | 155 | 6.0 | 8.0 | 6.16 | 98.6 | 1.5 | 6.8 | 11.0 | 24.5 | 296.2 | 81.4 | 83.3 | 120.8 | 7.3 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Monnin, C.; Tamborski, J.; Bejannin, S.; Souhaut, M.; Roques, M.; Olivier, P.; van Beek, P. Freshening of a Coastal Karst Aquifer Revealed by the Temporal Changes in a Spring Water Composition (La Palme, Southern France). Hydrology 2019, 6, 45. https://doi.org/10.3390/hydrology6020045
Monnin C, Tamborski J, Bejannin S, Souhaut M, Roques M, Olivier P, van Beek P. Freshening of a Coastal Karst Aquifer Revealed by the Temporal Changes in a Spring Water Composition (La Palme, Southern France). Hydrology. 2019; 6(2):45. https://doi.org/10.3390/hydrology6020045
Chicago/Turabian StyleMonnin, Christophe, Joseph Tamborski, Simon Bejannin, Marc Souhaut, Manon Roques, Philippe Olivier, and Pieter van Beek. 2019. "Freshening of a Coastal Karst Aquifer Revealed by the Temporal Changes in a Spring Water Composition (La Palme, Southern France)" Hydrology 6, no. 2: 45. https://doi.org/10.3390/hydrology6020045





