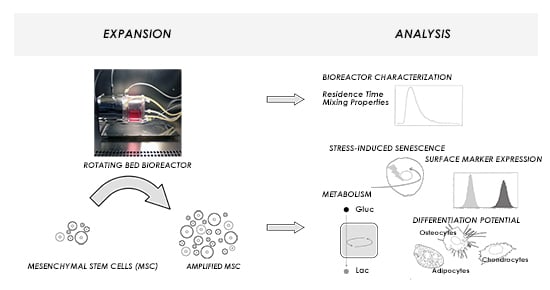
Characterization and Application of a Disposable Rotating Bed Bioreactor for Mesenchymal Stem Cell Expansion
Abstract
:1. Introduction

2. Experimental Section
2.1. Z®RP Platform
2.2. Residence Time Distribution
2.3. Cumulative Residence Time Function
2.4. Cell Culture
2.5. Expansion of UC-MSC in the Z®RP 2000 H Bioreactor
2.6. Cell Number, Population Doublings and Doubling Time
2.7. Cell Differentiation
2.8. Histological Stainings
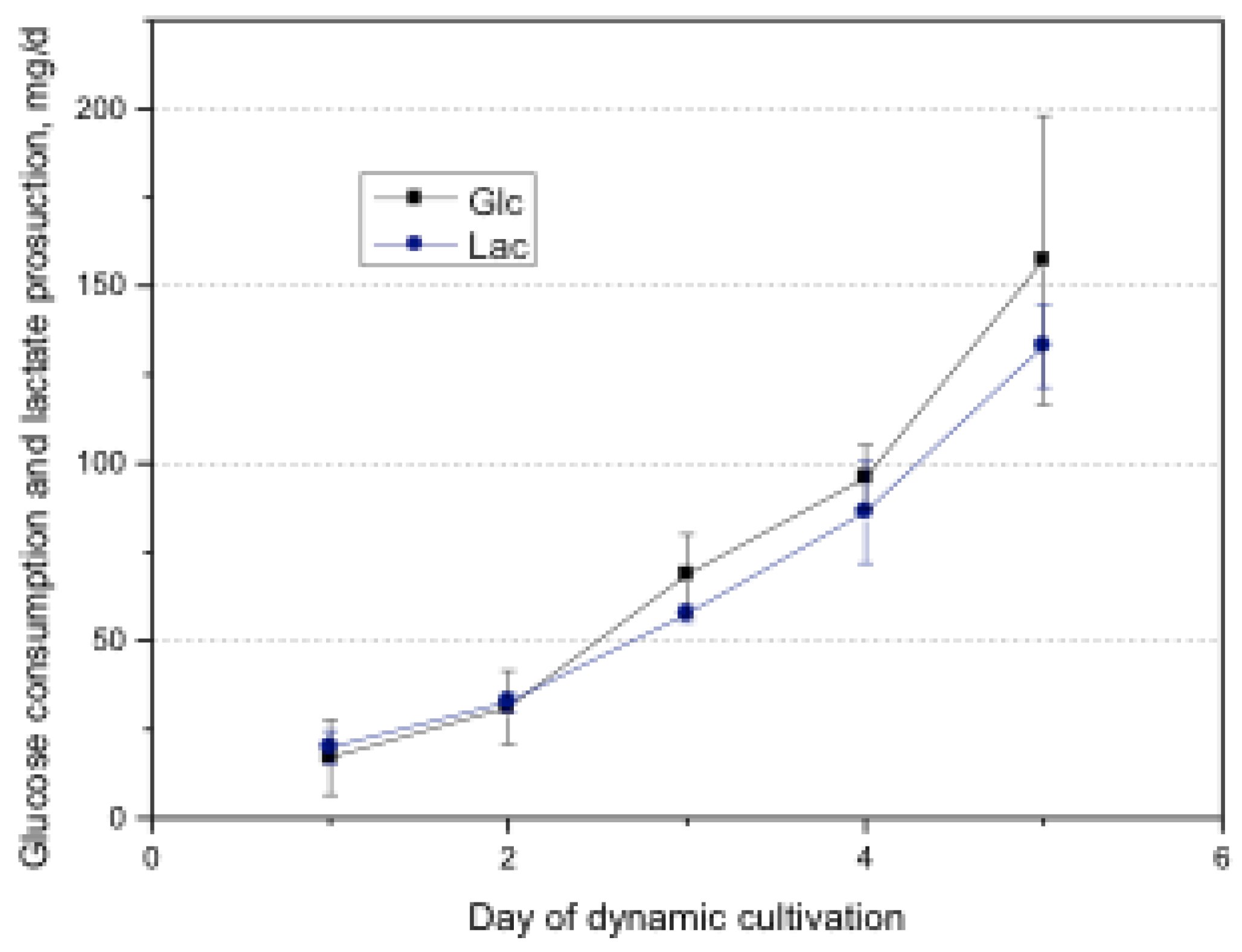
2.9. Glucose and Consumption and Lactate Production
2.10. Senescence-Associated β-Galactosidase Staining
2.11. Flow Cytometric Analysis of Surface Antigen Expression
3. Results and Discussion
3.1. Residence Time
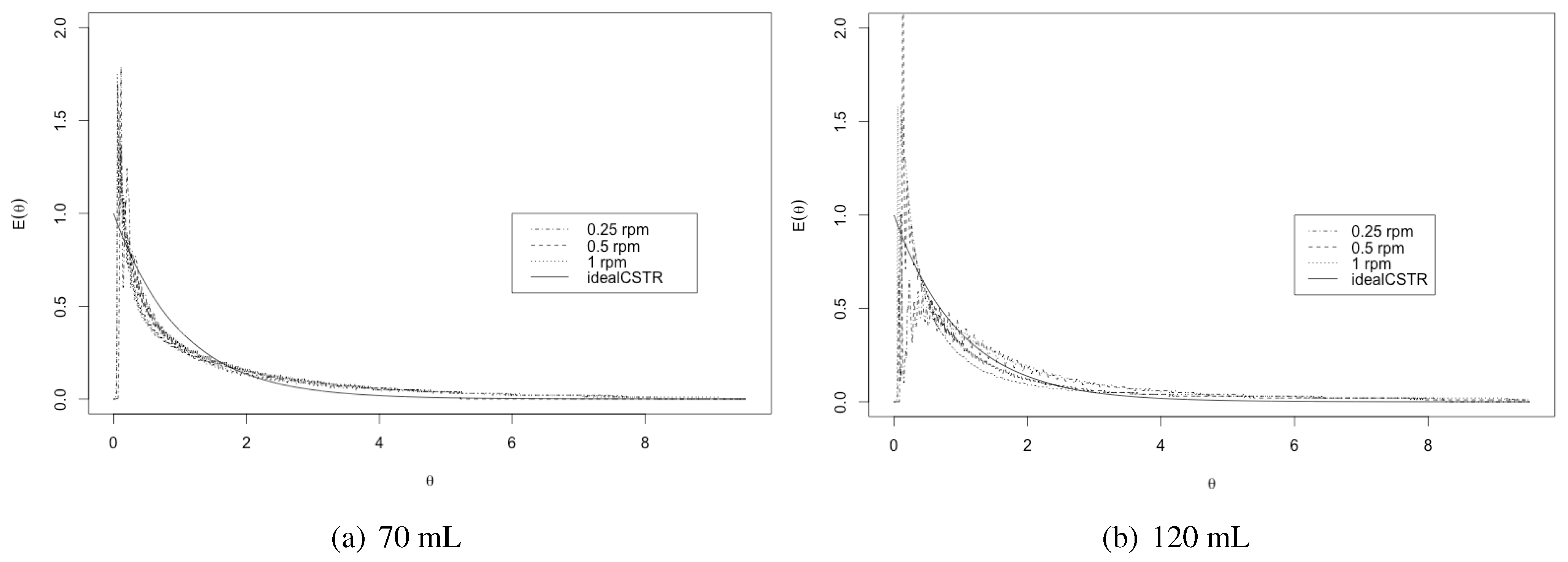
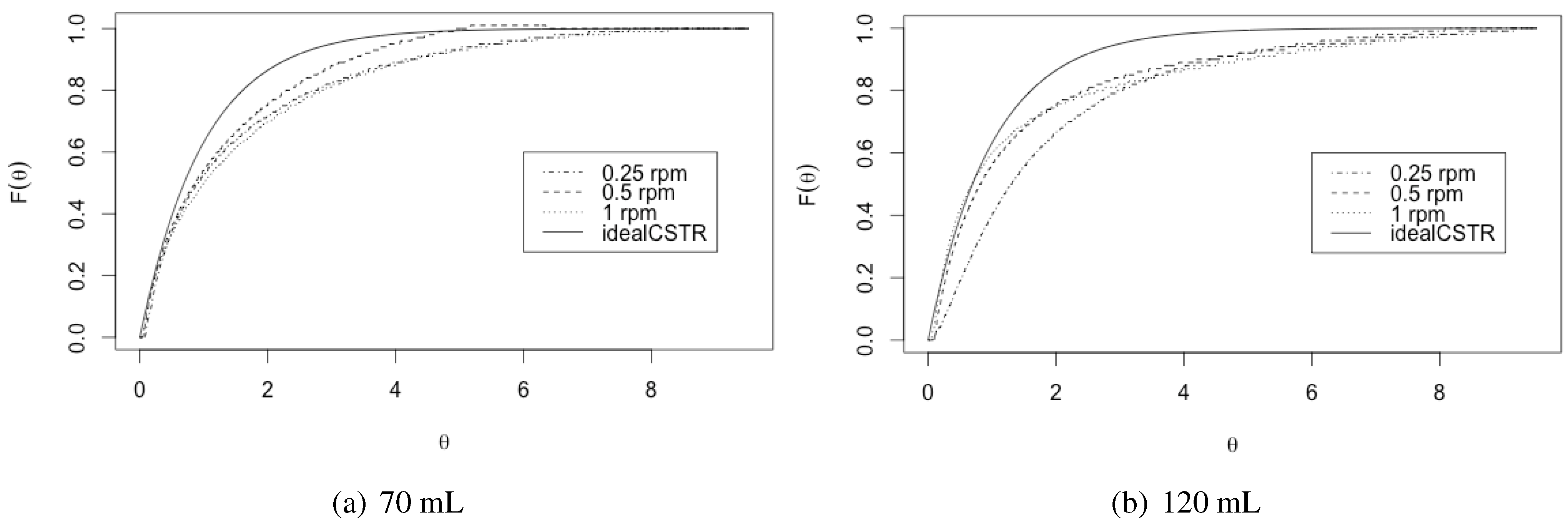

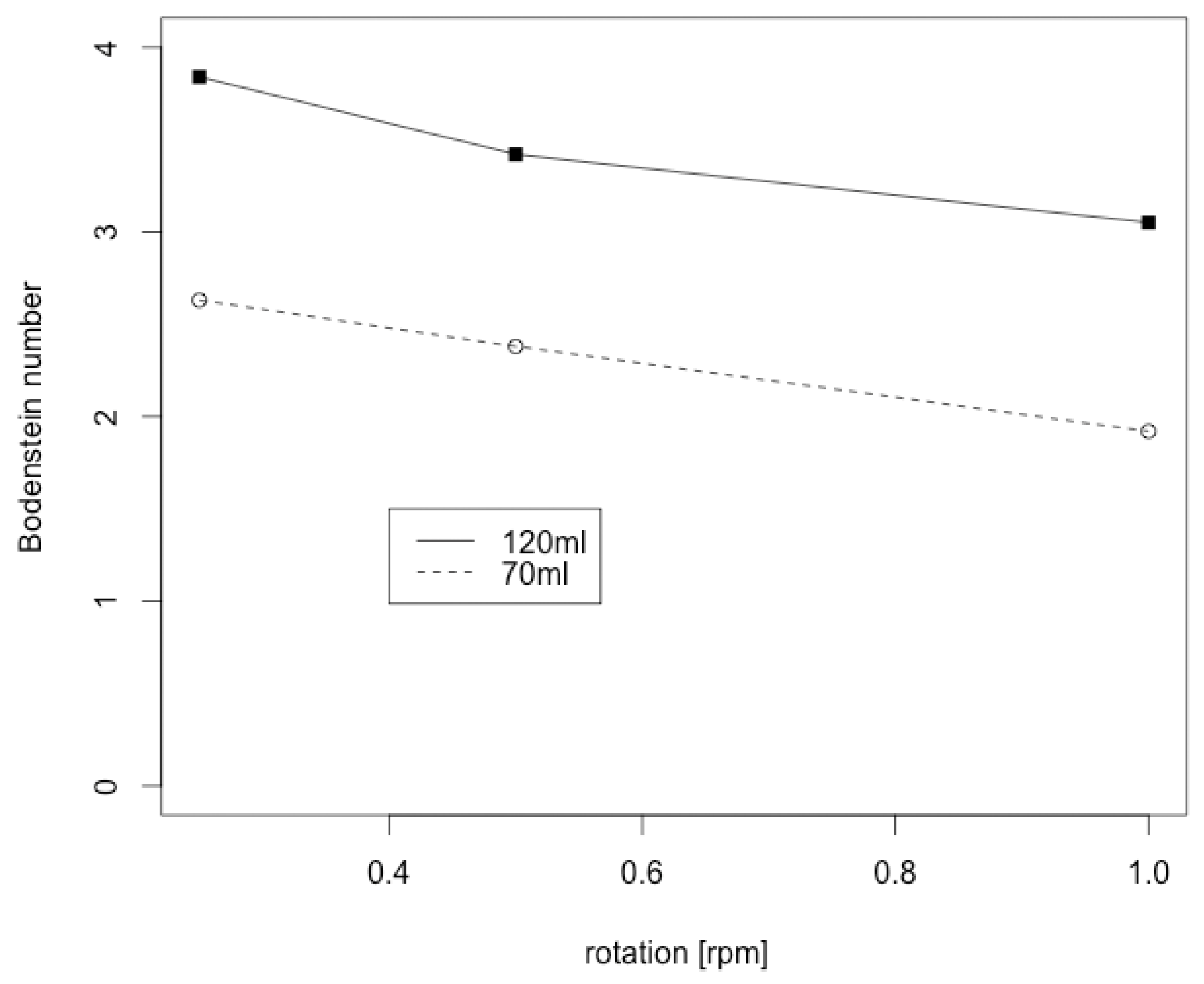
3.2. Bodenstein Number
3.3. Cell Expansion in the Z®RP 2000 H Bioreactor
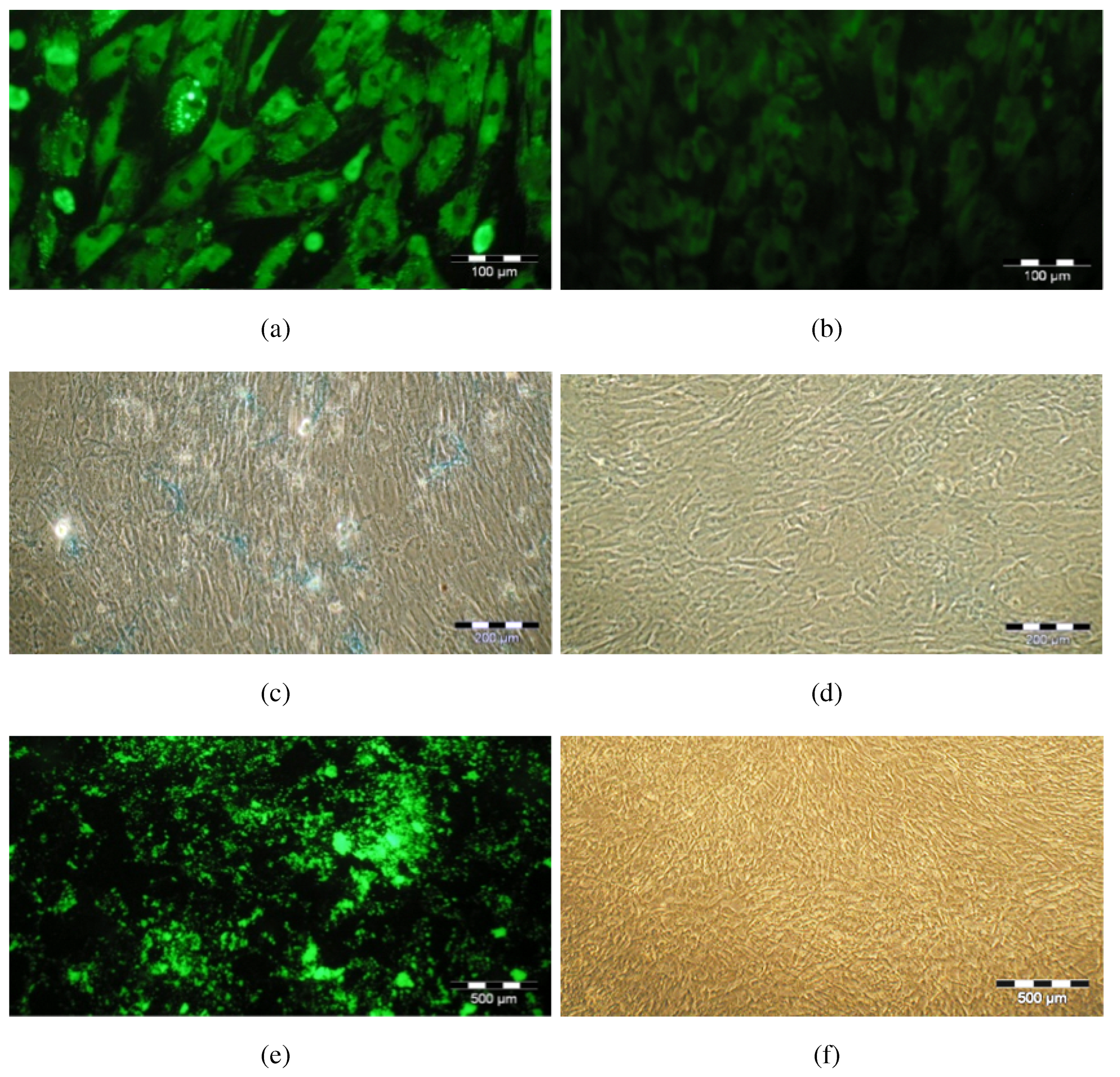
3.4. Cellular Senescence after Expansion in Z®RP 2000 H Bioreactor
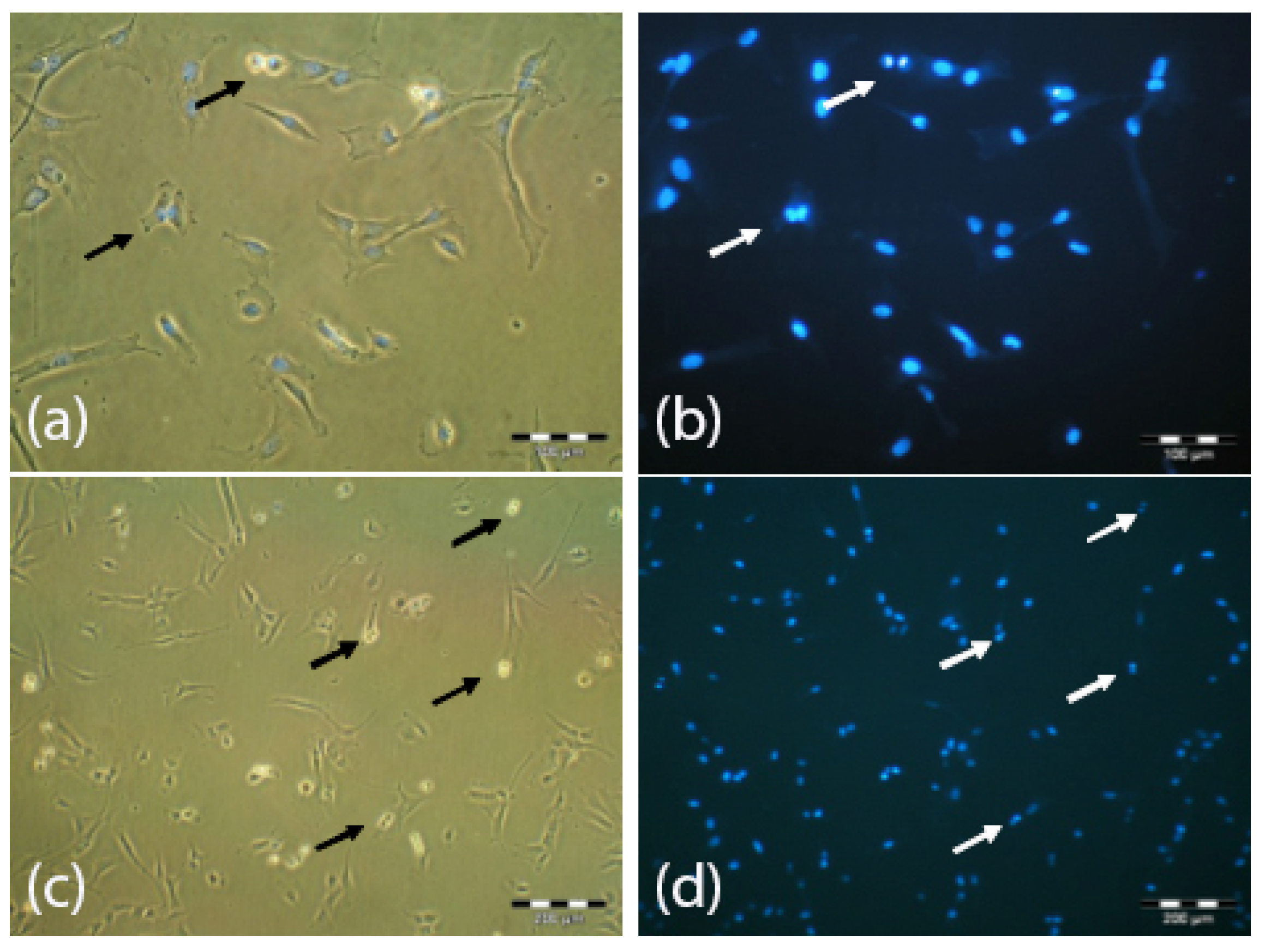
3.5. Surface Immunophenotype Characterization of UC-MSC after Expansion in the Z®RP 2000 H Bioreactor
| Marker | Expression |
|---|---|
| CD31 | 0.0% |
| CD34 | 0.3% |
| CD44 | 98.3% |
| CD45 | 0.3% |
| CD73 | 99.9% |
| CD90 | 98.1% |
| CD105 | 99.8% |
4. Conclusions
Author Contributions
Conflicts of Interest
References
- ClinicalTrials.gov. Avaliable online: http://www.clinicaltrials.gov (accessed on 11 September 2014).
- Raviv, L.; Karnieli, O. Cell therapies—The challenges and possible solutions for transferring cell therapy from the bench to the industry. Drug Dev. Deliv. 2014, 3. [Google Scholar]
- Ramot, Y.; Meiron, M.; Toren, A.; Steiner, M.; Nyska, A. Safety and biodistribution profile of placental-derived mesenchymal stromal cells (PLX-PAD) following intramuscular delivery. Toxicol. Pathol. 2009, 37, 606–616. [Google Scholar] [CrossRef] [PubMed]
- Nold, P.; Brendel, C.; Neubauer, A.; Bein, G.; Hackstein, H. Good manufacturing practice-compliant animal-free expansion of human bone marrow derived mesenchymal stroma cells in a closed hollow-fiber-based bioreactor. Biochem. Biophys. Res. Commun. 2013, 430, 325–330. [Google Scholar] [CrossRef] [PubMed]
- Asnaghi, M.A.; Jungebluth, P.; Raimondi, M.T.; Dickinson, S.C.; Rees, L.E.; Go, T.; Cogan, T.A.; Dodson, A.; Parnigotto, P.P.; Hollander, A.P.; et al. A double-chamber rotating bioreactor for the development of tissue-engineered hollow organs: From concept to clinical trial. Biomaterials 2009, 30, 5260–5269. [Google Scholar] [CrossRef] [PubMed]
- Martin, I.; Wendt, D.; Heberer, M. The role of bioreactors in tissue engineering. Trends Biotechnol. 2004, 22, 89–86. [Google Scholar] [CrossRef] [PubMed]
- Ratcliffe, A.; Niklason, L.E. Bioreactors and bioprocessing for tissue engineering. Ann. N. Y. Acad. Sci. 2002, 961, 210–215. [Google Scholar] [CrossRef] [PubMed]
- Hansmann, J.; Groeber, F.; Kahlig, A.; Kleinhans, C.; Walles, H. Bioreactors in tissue engineering—Principles, applications and commercial constraints. Biotechnol. J. 2013, 8, 298–307. [Google Scholar] [CrossRef] [PubMed]
- Glindkamp, A.; Riechers, D.; Rehbock, C.; Hitzmann, B.; Scheper, T.; Reardon, K.F. Sensors in disposable bioreactors status and trends. Adv. Biochem. Eng. Biotechnol. 2009, 115, 145–169. [Google Scholar] [PubMed]
- Behr, L.; Joeris, K.; Burnett, M.; Scheper, T. A novel in situ probe for oxygen uptake rate measurement in mammalian cell cultures. Biotechnol. Prog. 2012, 28, 581–586. [Google Scholar] [CrossRef] [PubMed]
- Babitzky, A.; Lindner, P.; Scheper, T. Cell assessment by at-line microscopy. Methods Mol. Biol. 2014, 1104, 343–353. [Google Scholar] [PubMed]
- Committee for Advanced Therapies. Reflection paper on stem cell-based medicinal products. EMA/CAT/571134/2009 2011, 1, 1–14. [Google Scholar]
- Naughton, G.K. From lab bench to market: Critical issues in tissue engineering. Ann. N. Y. Acad. Sci. 2002, 961, 372–385. [Google Scholar] [CrossRef] [PubMed]
- Reichardt, A.; Polchow, P.; Shakibaei, M.; Henrich, W.; Hetzer, R.; Lueders, C. Large scale expansion of human umbilical cord cells in a rotating bed system bioreactor for cardiovascular tissue engineering applications. Open Biomed. Eng. J. 2013, 7, 50–61. [Google Scholar] [PubMed]
- Bianco, P.; Riminucci, M.; Gronthos, S.; Robey, P.G. Bone marrow stromal stem cells: Nature, biology, and potential applications. Stem Cells 2001, 19, 180–192. [Google Scholar] [CrossRef] [PubMed]
- Crisan, M.; Yap, S.; Casteilla, L.; Chen, C.-W.; Corselli, M.; Park, T.-S.; Andriolo, G.; Sun, B.; Zheng, B.; Zhang, L.; et al. A perivascular origin for mesenchymal stem cells in multiple human organs. Cell Stem Cell 2008, 3, 301–313. [Google Scholar] [CrossRef] [PubMed]
- Yeatts, A.B.; Fisher, J.B. Bone tissue engineering bioreactors: Dynamic culture and the influence of shear stress. Bone 2011, 48, 171–181. [Google Scholar] [CrossRef] [PubMed]
- Majore, I.; Moretti, P.; Hass, R.; Kasper, C. Identification of subpopulations in mesenchymal stem cell-like cultures from human umbilical cord. Cell Commun. Signal. 2009, 7, 1–6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moretti, P. Establishment of Recombinant Cell Lines and Characterization of Primary Cells for Stem Cell Technology Applications. Ph.D. Thesis, Institute for Technical Chemistry, Leibniz University of Hanover, Hanover, Germany, 2010. [Google Scholar]
- Hagen, J. Chemische Reaktionstechnik; VCH: Weinheim, Germany, 1992. [Google Scholar]
- Toussaint, O.; Dumont, P.; Remacle, J.; Dierick, J.-F.; Pascal, T.; Frippiat, C.; Magalhaes, J.P.; Zdanov, S.; Chainiaux, F. Stress-induced premature senescence or stress-induced senescence-like phenotype: One in vivo reality, two possible definitions? Sci. World J. 2002, 2, 230–247. [Google Scholar] [CrossRef] [PubMed]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Neumann, A.; Lavrentieva, A.; Heilkenbrinker, A.; Loenne, M.; Kasper, C. Characterization and Application of a Disposable Rotating Bed Bioreactor for Mesenchymal Stem Cell Expansion. Bioengineering 2014, 1, 231-245. https://doi.org/10.3390/bioengineering1040231
Neumann A, Lavrentieva A, Heilkenbrinker A, Loenne M, Kasper C. Characterization and Application of a Disposable Rotating Bed Bioreactor for Mesenchymal Stem Cell Expansion. Bioengineering. 2014; 1(4):231-245. https://doi.org/10.3390/bioengineering1040231
Chicago/Turabian StyleNeumann, Anne, Antonina Lavrentieva, Alexandra Heilkenbrinker, Maren Loenne, and Cornelia Kasper. 2014. "Characterization and Application of a Disposable Rotating Bed Bioreactor for Mesenchymal Stem Cell Expansion" Bioengineering 1, no. 4: 231-245. https://doi.org/10.3390/bioengineering1040231
APA StyleNeumann, A., Lavrentieva, A., Heilkenbrinker, A., Loenne, M., & Kasper, C. (2014). Characterization and Application of a Disposable Rotating Bed Bioreactor for Mesenchymal Stem Cell Expansion. Bioengineering, 1(4), 231-245. https://doi.org/10.3390/bioengineering1040231