Selenium Bio-Nanocomposite Based on Alteromonas macleodii Mo169 Exopolysaccharide: Synthesis, Characterization, and In Vitro Antioxidant Activity
Abstract
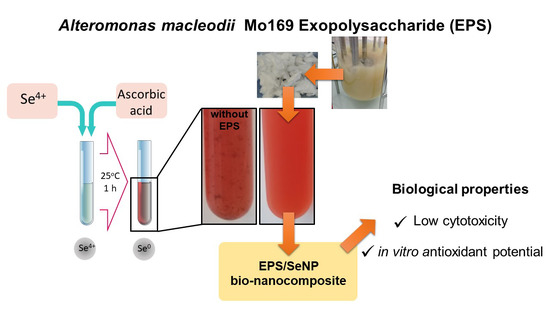
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of the Bio-Nanocomposites
2.3. Characterization of the Bio-Nanocomposites
2.4. Biological Assays
2.4.1. Cell Cultures
2.4.2. Cytotoxicity Assays
2.4.3. Cellular Antioxidant Activity
2.5. Data Analysis
3. Results and Discussion
3.1. NPs Synthesis
3.2. NPs Characterization
3.2.1. Composition
3.2.2. Colloidal Stability
3.2.3. SeNPs Morphology
3.2.4. SeNPs and Bio-Nanocomposite’s Size
3.2.5. EPS-NPs Interaction
3.3. Biological Assays
3.3.1. Assessment of Cytotoxicity
3.3.2. Evaluation of Cellular Antioxidant Activity
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, J.; Shen, B.; Nie, S.; Duan, Z.; Chen, K. A Combination of Selenium and Polysaccharides: Promising Therapeutic Potential. Carbohydr. Polym. 2019, 206, 163–173. [Google Scholar] [CrossRef] [PubMed]
- Khurana, A.; Tekula, S.; Saifi, M.A.; Venkatesh, P.; Godugu, C. Therapeutic Applications of Selenium Nanoparticles. Biomed. Pharmacother. 2019, 111, 802–812. [Google Scholar] [CrossRef]
- Qiu, W.-Y.; Wang, Y.-Y.; Wang, M.; Yan, J.-K. Construction, Stability, and Enhanced Antioxidant Activity of Pectin-Decorated Selenium Nanoparticles. Colloids Surf. B Biointerfaces 2018, 170, 692–700. [Google Scholar] [CrossRef]
- Tang, L.; Luo, X.; Wang, M.; Wang, Z.; Guo, J.; Kong, F.; Bi, Y. Synthesis, Characterization, in Vitro Antioxidant and Hypoglycemic Activities of Selenium Nanoparticles Decorated with Polysaccharides of Gracilaria lemaneiformis. Int. J. Biol. Macromol. 2021, 193, 923–932. [Google Scholar] [CrossRef]
- Pires, R.; Costa, M.; Silva, J.; Pedras, B.; Concórdio-Reis, P.; Lapa, N.; Ventura, M. Se-Enrichment of Chlorella Vulgaris Grown under Different Trophic States for Food Supplementation. Algal Res. 2022, 68, 102876. [Google Scholar] [CrossRef]
- Jiang, H.; Wang, R.; Zhou, F.; Wu, Y.; Li, S.; Huo, G.; Ye, J.; Hua, C.; Wang, Z. Preparation, Physicochemical Characterization, and Cytotoxicity of Selenium Nanoparticles Stabilized by Oudemansiella Raphanipies Polysaccharide. Int. J. Biol. Macromol. 2022, 211, 35–46. [Google Scholar] [CrossRef]
- Xiao, Y.; Zhang, X.; Huang, Q. Protective Effects of Cordyceps Sinensis Exopolysaccharide-selenium Nanoparticles on H2O2-Induced Oxidative Stress in HepG2 Cells. Int. J. Biol. Macromol. 2022, 213, 339–351. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Huang, Q.; Zheng, Z.; Guan, H.; Liu, S. Construction of a Cordyceps Sinensis Exopolysaccharide-Conjugated Selenium Nanoparticles and Enhancement of Their Antioxidant Activities. Int. J. Biol. Macromol. 2017, 99, 483–491. [Google Scholar] [CrossRef]
- Tang, S.; Wang, T.; Jiang, M.; Huang, C.; Lai, C.; Fan, Y.; Yong, Q. Construction of Arabinogalactans/Selenium Nanoparticles Composites for Enhancement of the Antitumor Activity. Int. J. Biol. Macromol. 2019, 128, 444–451. [Google Scholar] [CrossRef]
- Cao, B.; Zhang, Q.; Guo, J.; Guo, R.; Fan, X.; Bi, Y. Synthesis and Evaluation of Grateloupia livida Polysaccharides-Functionalized Selenium Nanoparticles. Int. J. Biol. Macromol. 2021, 191, 832–839. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.; Fu, Y.; Yang, F.; Yang, Y.; Liu, T.; Zheng, W.; Zeng, L.; Chen, T. Gracilaria lemaneiformis Polysaccharide as Integrin-Targeting Surface Decorator of Selenium Nanoparticles to Achieve Enhanced Anticancer Efficacy. ACS Appl. Mater. Interfaces 2014, 6, 13738–13748. [Google Scholar] [CrossRef] [PubMed]
- Casillo, A.; Lanzetta, R.; Parrilli, M.; Corsaro, M. Exopolysaccharides from Marine and Marine Extremophilic Bacteria: Structures, Properties, Ecological Roles and Applications. Mar. Drugs 2018, 16, 69. [Google Scholar] [CrossRef] [PubMed]
- Shen, S.; Chen, X.; Shen, Z.; Chen, H. Marine Polysaccharides for Wound Dressings Application: An Overview. Pharmaceutics 2021, 13, 1666. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Gao, X.; Chen, Z.; Chen, Y.; Chen, H. Preparation, Characterization and Application of Polysaccharide-Based Metallic Nanoparticles: A Review. Polymers 2017, 9, 689. [Google Scholar] [CrossRef]
- Concórdio-Reis, P.; Alves, V.D.; Moppert, X.; Guézennec, J.; Freitas, F.; Reis, M.A.M. Characterization and Biotechnological Potential of Extracellular Polysaccharides Synthesized by Alteromonas Strains Isolated from French Polynesia Marine Environments. Mar. Drugs 2021, 19, 522. [Google Scholar] [CrossRef] [PubMed]
- Concórdio-Reis, P.; Ferreira, S.S.; Alves, V.D.; Mopper, X.; Guézennec, J.; Coimbra, M.A.; Reis, M.A.M.; Freitas, F. Rheological Characterization of the Exopolysaccharide Produced by Alteromonas Macleodii Mo 169. Int. J. Biol. Macromol. 2022, 227, 619–629. [Google Scholar] [CrossRef]
- Yan, J.-K.; Qiu, W.-Y.; Wang, Y.-Y.; Wang, W.-H.; Yang, Y.; Zhang, H.-N. Fabrication and Stabilization of Biocompatible Selenium Nanoparticles by Carboxylic Curdlans with Various Molecular Properties. Carbohydr. Polym. 2018, 179, 19–27. [Google Scholar] [CrossRef]
- Concórdio-Reis, P.; Pereira, C.V.; Batista, M.P.; Sevrin, C.; Grandfils, C.; Marques, A.C.; Fortunato, E.; Gaspar, F.B.; Matias, A.A.; Freitas, F.; et al. Silver Nanocomposites Based on the Bacterial Fucose-Rich Polysaccharide Secreted by Enterobacter A47 for Wound Dressing Applications: Synthesis, Characterization and in vitro Bioactivity. Int. J. Biol. Macromol. 2020, 163, 959–969. [Google Scholar] [CrossRef]
- Matos, M.S.; Romero-Díez, R.; Álvarez, A.; Bronze, M.R.; Rodríguez-Rojo, S.; Mato, R.B.; Cocero, M.J.; Matias, A.A. Polyphenol-Rich Extracts Obtained from Winemaking Waste Streams as Natural Ingredients with Cosmeceutical Potential. Antioxidants 2019, 8, 355. [Google Scholar] [CrossRef]
- Serra, A.T.; Matias, A.A.; Frade, R.F.M.; Duarte, R.O.; Feliciano, R.P.; Bronze, M.R.; Figueira, M.E.; de Carvalho, A.; Duarte, C.M.M. Characterization of Traditional and Exotic Apple Varieties from Portugal. Part 2—Antioxidant and Antiproliferative Activities. J. Funct. Foods 2010, 2, 46–53. [Google Scholar] [CrossRef]
- Cai, W.; Hu, T.; Bakry, A.M.; Zheng, Z.; Xiao, Y.; Huang, Q. Effect of Ultrasound on Size, Morphology, Stability and Antioxidant Activity of Selenium Nanoparticles Dispersed by a Hyperbranched Polysaccharide from Lignosus rhinocerotis. Ultrason. Sonochem. 2018, 42, 823–831. [Google Scholar] [CrossRef] [PubMed]
- Zeng, S.; Ke, Y.; Liu, Y.; Shen, Y.; Zhang, L.; Li, C.; Liu, A.; Shen, L.; Hu, X.; Wu, H.; et al. Synthesis and Antidiabetic Properties of Chitosan-Stabilized Selenium Nanoparticles. Colloids Surf. B Biointerfaces 2018, 170, 115–121. [Google Scholar] [CrossRef]
- Jia, X.; Liu, Q.; Zou, S.; Xu, X.; Zhang, L. Construction of Selenium Nanoparticles/β-Glucan Composites for Enhancement of the Antitumor Activity. Carbohydr. Polym. 2015, 117, 434–442. [Google Scholar] [CrossRef] [PubMed]
- Kong, H.; Yang, J.; Zhang, Y.; Fang, Y.; Nishinari, K.; Phillips, G.O. Synthesis and Antioxidant Properties of Gum Arabic-Stabilized Selenium Nanoparticles. Int. J. Biol. Macromol. 2014, 65, 155–162. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Yang, X.; Zhang, J.; Liang, L.; Miao, F.; Ji, T.; Ye, Z.; Chu, M.; Ren, J.; Xu, X. Synthesis, Stability and Anti-Fatigue Activity of Selenium Nanoparticles Stabilized by Lycium Barbarum Polysaccharides. Int. J. Biol. Macromol. 2021, 179, 418–428. [Google Scholar] [CrossRef]
- Liu, Y.; Zeng, S.; Liu, Y.; Wu, W.; Shen, Y.; Zhang, L.; Li, C.; Chen, H.; Liu, A.; Shen, L.; et al. Synthesis and Antidiabetic Activity of Selenium Nanoparticles in the Presence of Polysaccharides from Catathelasma ventricosum. Int. J. Biol. Macromol. 2018, 114, 632–639. [Google Scholar] [CrossRef]
- Singh, S.; Sran, K.S.; Pinnaka, A.K.; Roy Choudhury, A. Purification, Characterization and Functional Properties of Exopolysaccharide from a Novel Halophilic Natronotalea sambharensis Sp. Nov. Int. J. Biol. Macromol. 2019, 136, 547–558. [Google Scholar] [CrossRef]
- Huang, B.; Zhang, J.; Hou, J.; Chen, C. Free Radical Scavenging Efficiency of Nano-Se in vitro. Free Radic. Biol. Med. 2003, 35, 805–813. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Wang, H.; Bao, Y.; Zhang, L. Nano Red Elemental Selenium Has No Size Effect in the Induction of Seleno-Enzymes in Both Cultured Cells and Mice. Life Sci. 2004, 75, 237–244. [Google Scholar] [CrossRef]
- Akturk, O. Colloidal Stability and Biological Activity Evaluation of Microbial Exopolysaccharide Levan-Capped Gold Nanoparticles. Colloids Surf. B Biointerfaces 2020, 192, 111061. [Google Scholar] [CrossRef]
- Chen, X.; Yan, J.-K.; Wu, J.-Y. Characterization and Antibacterial Activity of Silver Nanoparticles Prepared with a Fungal Exopolysaccharide in Water. Food Hydrocoll. 2016, 53, 69–74. [Google Scholar] [CrossRef]
- Chen, X.; Zhao, X.; Gao, Y.; Yin, J.; Bai, M.; Wang, F. Green Synthesis of Gold Nanoparticles Using Carrageenan Oligosaccharide and Their In Vitro Antitumor Activity. Mar. Drugs 2018, 16, 277. [Google Scholar] [CrossRef]
- Pooja, D.; Panyaram, S.; Kulhari, H.; Rachamalla, S.S.; Sistla, R. Xanthan Gum Stabilized Gold Nanoparticles: Characterization, Biocompatibility, Stability and Cytotoxicity. Carbohydr. Polym. 2014, 110, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Scala, A.; Piperno, A.; Hada, A.; Astilean, S.; Vulpoi, A.; Ginestra, G.; Marino, A.; Nostro, A.; Zammuto, V.; Gugliandolo, C. Marine Bacterial Exopolymers-Mediated Green Synthesis of Noble Metal Nanoparticles with Antimicrobial Properties. Polymers 2019, 11, 1157. [Google Scholar] [CrossRef] [PubMed]
- Navarro Gallón, S.M.; Alpaslan, E.; Wang, M.; Larese-Casanova, P.; Londoño, M.E.; Atehortúa, L.; Pavón, J.J.; Webster, T.J. Characterization and Study of the Antibacterial Mechanisms of Silver Nanoparticles Prepared with Microalgal Exopolysaccharides. Mater. Sci. Eng. C 2019, 99, 685–695. [Google Scholar] [CrossRef]
- Freitas, F.; Torres, C.A.V.; Araújo, D.; Farinha, I.; Pereira, J.R.; Concórdio-Reis, P.; Reis, M.A.M. Advanced Microbial Polysaccharides. In Biopolymers for Biomedical and Biotechnological Applications; Rehm, B., Moradali, M.F., Eds.; Wiley: Hoboken, NJ, USA, 2021; pp. 19–62. ISBN 978-3-527-81831-0. [Google Scholar]
- Cheng, Y.; Xiao, X.; Li, X.; Song, D.; Lu, Z.; Wang, F.; Wang, Y. Characterization, Antioxidant Property and Cytoprotection of Exopolysaccharide-Capped Elemental Selenium Particles Synthesized by Bacillus paralicheniformis SR14. Carbohydr. Polym. 2017, 178, 18–26. [Google Scholar] [CrossRef] [PubMed]





 ) and the EPS/SeNPs bio-nanocomposite (
) and the EPS/SeNPs bio-nanocomposite (  ) on CCD-10795k (A) and HaCaT (B) cell lines after 24 h incubation. Control experiments (
) on CCD-10795k (A) and HaCaT (B) cell lines after 24 h incubation. Control experiments (  ) were performed by incubating the cells with only culture medium. Statistically significant differences comparing samples with the control were calculated according to the t-test (***, p ≤ 0.001, **** p ≤ 0.0001).
) were performed by incubating the cells with only culture medium. Statistically significant differences comparing samples with the control were calculated according to the t-test (***, p ≤ 0.001, **** p ≤ 0.0001).
 ) and the EPS/SeNPs bio-nanocomposite (
) and the EPS/SeNPs bio-nanocomposite (  ) on CCD-10795k (A) and HaCaT (B) cell lines after 24 h incubation. Control experiments (
) on CCD-10795k (A) and HaCaT (B) cell lines after 24 h incubation. Control experiments (  ) were performed by incubating the cells with only culture medium. Statistically significant differences comparing samples with the control were calculated according to the t-test (***, p ≤ 0.001, **** p ≤ 0.0001).
) were performed by incubating the cells with only culture medium. Statistically significant differences comparing samples with the control were calculated according to the t-test (***, p ≤ 0.001, **** p ≤ 0.0001).
 ), and the EPS/SeNPs bio-nanocomposite (
), and the EPS/SeNPs bio-nanocomposite (  ) on the inhibition of AAPH-induced ROS production in HaCaT cells. Control experiments (
) on the inhibition of AAPH-induced ROS production in HaCaT cells. Control experiments (  ) were performed by incubating the cells only with AAPH. Statistically significant differences comparing samples with the negative control were calculated according to the t-test (***, p ≤ 0.001, **** p ≤ 0.0001).
) were performed by incubating the cells only with AAPH. Statistically significant differences comparing samples with the negative control were calculated according to the t-test (***, p ≤ 0.001, **** p ≤ 0.0001).
 ), and the EPS/SeNPs bio-nanocomposite (
), and the EPS/SeNPs bio-nanocomposite (  ) on the inhibition of AAPH-induced ROS production in HaCaT cells. Control experiments (
) on the inhibition of AAPH-induced ROS production in HaCaT cells. Control experiments (  ) were performed by incubating the cells only with AAPH. Statistically significant differences comparing samples with the negative control were calculated according to the t-test (***, p ≤ 0.001, **** p ≤ 0.0001).
) were performed by incubating the cells only with AAPH. Statistically significant differences comparing samples with the negative control were calculated according to the t-test (***, p ≤ 0.001, **** p ≤ 0.0001).
| Polysaccharide | Size (nm) | Reference | |
|---|---|---|---|
| TEM | DLS | ||
| Alteromonas macleodii Mo 169 EPS | 22–76/32 | 297 | This study |
| Cordyceps sinensis Cs-HK1 EPS | 50 | n.a. | [8] |
| Gracilaria lemaneiformis PS | 83.6 | 93–138 | [4] |
| Grateloupia livida PS | 100 | 115 | [10] |
| Larix principis-rupprechtii PS | n.a. | 94–173 | [9] |
| Lycium barbarum PS | n.a. | 105 | [25] |
| Oudemansiella raphanipies PS | 60 | n.a. | [6] |
| Carboxylated curdlan | 56–65 | 118–243 | [17] |
| Gum Arabic | 34.9 | 145–170 | [24] |
| Lectinan | 33–52 | 100 | [23] |
| Pectin | 41 | n.a. | [3] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Concórdio-Reis, P.; Macedo, A.C.; Cardeira, M.; Moppert, X.; Guézennec, J.; Sevrin, C.; Grandfils, C.; Serra, A.T.; Freitas, F. Selenium Bio-Nanocomposite Based on Alteromonas macleodii Mo169 Exopolysaccharide: Synthesis, Characterization, and In Vitro Antioxidant Activity. Bioengineering 2023, 10, 193. https://doi.org/10.3390/bioengineering10020193
Concórdio-Reis P, Macedo AC, Cardeira M, Moppert X, Guézennec J, Sevrin C, Grandfils C, Serra AT, Freitas F. Selenium Bio-Nanocomposite Based on Alteromonas macleodii Mo169 Exopolysaccharide: Synthesis, Characterization, and In Vitro Antioxidant Activity. Bioengineering. 2023; 10(2):193. https://doi.org/10.3390/bioengineering10020193
Chicago/Turabian StyleConcórdio-Reis, Patrícia, Ana Catarina Macedo, Martim Cardeira, Xavier Moppert, Jean Guézennec, Chantal Sevrin, Christian Grandfils, Ana Teresa Serra, and Filomena Freitas. 2023. "Selenium Bio-Nanocomposite Based on Alteromonas macleodii Mo169 Exopolysaccharide: Synthesis, Characterization, and In Vitro Antioxidant Activity" Bioengineering 10, no. 2: 193. https://doi.org/10.3390/bioengineering10020193