Bioactive Restorative Materials Applied over Coronal Dentine—A Bibliometric and Critical Review
Abstract
:1. Introduction
2. Materials and Methods
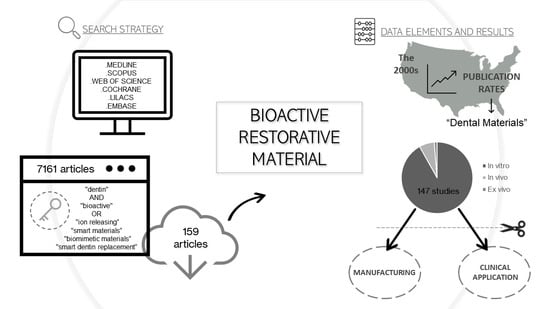
2.1. Information Sources and Search Strategy
2.2. Eligibility Criteria
2.3. Screening and Selection of Articles
2.4. Data Elements and Collection Method for Study Features
2.5. Data Elements and Collection Method for Bibliometric Analysis
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Schwendicke, F.; Göstemeyer, G.; Blunck, U.; Paris, S.; Hsu, L.Y.; Tu, Y.K. Directly Placed Restorative Materials: Review and Network Meta-analysis. J. Dent. Res. 2016, 95, 613–622. [Google Scholar] [CrossRef]
- Di Foggia, M.; Prati, C.; Gandolfi, M.G.; Taddei, P. An in vitro study on dentin demineralization and remineralization: Collagen rearrangements and influence on the enucleated phase. J. Inorg. Biochem. 2019, 193, 84–93. [Google Scholar] [CrossRef]
- Sauro, S.; Pashley, D.H. Strategies to stabilise dentine-bonded interfaces through remineralising operative approaches: State of the art. Int. J. Adhes. Adhes. 2016, 69, 39–57. [Google Scholar] [CrossRef]
- Alaohali, A.; Brauer, D.S.; Gentleman, E.; Sharpe, P.T. A modified glass ionomer cement to mediate dentine repair. Dent. Mater. 2021, 37, 1307–1315. [Google Scholar] [CrossRef]
- Schumacher, G.E.; Antonucci, J.M.; O’Donnell, J.N.; Skrtic, D. The use of amorphous calcium phosphate composites as bioactive basing materials: Their effect on the strength of the composite/adhesive/dentin bond. J. Am. Dent. Assoc. 2007, 138, 1476–1484. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.S.; Syed, M.R. A review of bioceramics-based dental restorative materials. Dent. Mater. J. 2019, 38, 163–176. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vallittu, P.K.; Boccaccini, A.R.; Hupa, L.; Watts, D.C. Bioactive dental materials-Do they exist and what does bioactivity mean? Dent. Mater. 2018, 34, 693–694. [Google Scholar] [CrossRef] [PubMed]
- Ferracane, J.L.; Giannobile, W.V. Novel biomaterials and technologies for the dental, oral, and craniofacial structures. J. Dent. Res. 2014, 93, 1185–1186. [Google Scholar] [CrossRef] [Green Version]
- Giannini, M.; Sauro, S. “Bioactivity” in Restorative Dentistry: Standing for the Use of Innovative Materials to Improve the Longevity of Restorations in Routine Dental Practice. J. Adhes. Dent. 2021, 23, 176–178. [Google Scholar] [CrossRef]
- Maas, M.S.; Alania, Y.; Natale, L.C.; Rodrigues, M.C.; Watts, D.C.; Braga, R.R. Trends in restorative composites research: What is in the future? Braz. Oral. Res. 2017, 31 (Suppl. S1), e55. [Google Scholar] [CrossRef] [Green Version]
- Pires, P.M.; Neves, A.A.; Makeeva, I.M.; Schwendicke, F.; Faus-Matoses, V.; Yoshihara, K.; Banerjee, A.; Sauro, S. Contemporary restorative ion-releasing materials: Current status, interfacial properties and operative approaches. Br. Dent. J. 2020, 229, 450–458. [Google Scholar] [CrossRef]
- Banerjee, A.; Frencken, J.E.; Schwendicke, F.; Innes, N.P.T. Contemporary operative caries management: Consensus recommendations on minimally invasive caries removal. Br. Dent. J. 2017, 223, 215–222. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schmalz, G.; Galler, K.M. Biocompatibility of biomaterials—Lessons learned and considerations for the design of novel materials. Dent. Mater. 2017, 33, 382–393. [Google Scholar] [CrossRef]
- Iftikhar, S.; Jahanzeb, N.; Saleem, M.; Ur Rehman, S.; Matinlinna, J.P.; Khan, A.S. The trends of dental biomaterials research and future directions: A mapping review. Saudi Dent. J. 2021, 33, 229–238. [Google Scholar] [CrossRef]
- Owens, B. Bioactivity, Biocompatibility and Biomimetic Properties for Dental Materials: Clarifying the Confusion? Mod. Approaches Dent. Oral Health Care 2018, 2. [Google Scholar] [CrossRef]
- Atmeh, A.R.; Chong, E.Z.; Richard, G.; Festy, F.; Watson, T.F. Dentin-cement interfacial interaction: Calcium silicates and polyalkenoates. J. Dent. Res. 2012, 91, 454–459. [Google Scholar] [CrossRef] [PubMed]
- Watson, T.F.; Atmeh, A.R.; Sajini, S.; Cook, R.J.; Festy, F. Present and future of glass-ionomers and calcium-silicate cements as bioactive materials in dentistry: Biophotonics-based interfacial analyses in health and disease. Dent. Mater. 2014, 30, 50–61. [Google Scholar] [CrossRef] [Green Version]
- Tahmasebi, E.; Alam, M.; Yazdanian, M.; Tebyanian, H.; Yazdanian, A.; Seifalian, A.; Mosaddad, S. Current biocompatible materials in oral regeneration: A comprehensive overview of composite materials. J. Mater. Res. Technol. 2020, 9, 11731–11755. [Google Scholar] [CrossRef]
- Yazdanian, M.; Rahmani, A.; Tahmasebi, E.; Tebyanian, H.; Yazdanian, A.; Mosaddad, S.A. Current and Advanced Nanomaterials in Dentistry as Regeneration Agents: An Update. Mini Rev. Med. Chem. 2021, 21, 899–918. [Google Scholar] [CrossRef]
- Kidd, E.A.; Fejerskov, O. What constitutes dental caries? Histopathology of carious enamel and dentin related to the action of cariogenic biofilms. J. Dent. Res. 2004, 83, C35–C38. [Google Scholar] [CrossRef] [PubMed]
- Schwendicke, F.; Splieth, C.; Breschi, L.; Banerjee, A.; Fontana, M.; Paris, S.; Burrow, M.F.; Crombie, F.; Page, L.F.; Gatón-Hernández, P.; et al. When to intervene in the caries process? An expert Delphi consensus statement. Clin. Oral. Investig. 2019, 23, 3691–3703. [Google Scholar] [CrossRef]
- Atmeh, A.R.; Chong, E.Z.; Richard, G.; Boyde, A.; Festy, F.; Watson, T.F. Calcium silicate cement-induced remineralisation of totally demineralised dentine in comparison with glass ionomer cement: Tetracycline labelling and two-photon fluorescence microscopy. J. Microsc. 2015, 257, 151–160. [Google Scholar] [CrossRef]
- Erhardt, M.C.; Toledano, M.; Osorio, R.; Pimenta, L.A. Histomorphologic characterization and bond strength evaluation of caries-affected dentin/resin interfaces: Effects of long-term water exposure. Dent. Mater. 2008, 24, 786–798. [Google Scholar] [CrossRef]
- Nakajima, M.; Sano, H.; Urabe, I.; Tagami, J.; Pashley, D.H. Bond strengths of single-bottle dentin adhesives to caries-affected dentin. Oper. Dent. 2000, 25, 2–10. [Google Scholar] [PubMed]
- Meraji, N.; Nekoofar, M.H.; Yazdi, K.A.; Sharifian, M.R.; Fakhari, N.; Camilleri, J. Bonding to caries affected dentine. Dent. Mater. 2018, 34, e236–e245. [Google Scholar] [CrossRef]
- Moron, B.M.; Comar, L.P.; Wiegand, A.; Buchalla, W.; Yu, H.; Buzalaf, M.A.; Magalhães, A.C. Different protocols to produce artificial dentine carious lesions in vitro and in situ: Hardness and mineral content correlation. Caries Res. 2013, 47, 162–170. [Google Scholar] [CrossRef] [Green Version]
- Skucha-Nowak, M.; Gibas, M.; Tanasiewicz, M.; Twardawa, H.; Szklarski, T. Natural and Controlled Demineralization for Study Purposes in Minimally Invasive Dentistry. Adv. Clin. Exp. Med. 2015, 24, 891–898. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maske, T.T.; Brauner, K.V.; Nakanishi, L.; Arthur, R.A.; van de Sande, F.H.; Cenci, M.S. An in vitro dynamic microcosm biofilm model for caries lesion development and antimicrobial dose-response studies. Biofouling 2016, 32, 339–348. [Google Scholar] [CrossRef]
- Pires, P.M.; Santos, T.P.D.; Fonseca-Gonçalves, A.; Pithon, M.M.; Lopes, R.T.; Neves, A.A. A dual energy micro-CT methodology for visualization and quantification of biofilm formation and dentin demineralization. Arch. Oral. Biol. 2018, 85, 10–15. [Google Scholar] [CrossRef]
- Schwendicke, F.; Al-Abdi, A.; Pascual Moscardó, A.; Ferrando Cascales, A.; Sauro, S. Remineralization effects of conventional and experimental ion-releasing materials in chemically or bacterially-induced dentin caries lesions. Dent. Mater. 2019, 35, 772–779. [Google Scholar] [CrossRef] [PubMed]
- Andersen, J.; Belmont, J.; Cho, C.T. Journal impact factor in the era of expanding literature. J. Microbiol. Immunol. Infect. 2006, 39, 436–443. [Google Scholar]
- Bornmann, L.; Daniel, H.D. What do citation counts measure? A review of studies on citing behavior. J. Doc. 2008, 64, 45–80. [Google Scholar] [CrossRef] [Green Version]
- Giacaman, R.; Perez, V.A.; Carrera, A. Mineralisation processes in hard tissues: Teeth. In Biomineralisation and Biomaterials: Fundamentals and Applications; Aparicio, C., Ginebra, M.P., Eds.; Woodhead Publishing: Waltham, UK, 2016; pp. 147–185. [Google Scholar]
- Eliaz, N.; Metoki, N. Calcium Phosphate Bioceramics: A review of their history, structure, properties, coating technologies and biomedical applications. Materials 2017, 10, 334. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khvostenko, D.; Hilton, T.J.; Ferracane, J.L.; Mitchell, J.C.; Kruzic, J.J. Bioactive glass fillers reduce bacterial penetration into marginal gaps for composite restorations. Dent. Mater. 2016, 32, 73–81. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tezvergil-Mutluay, A.; Seseogullari-Dirihan, R.; Feitosa, V.P.; Cama, G.; Brauer, D.S.; Sauro, S. Effects of Composites Containing Bioactive Glasses on Demineralized Dentin. J. Dent. Res. 2017, 96, 999–1005. [Google Scholar] [CrossRef] [PubMed]






| Database | Strategy |
|---|---|
| PubMed | ((dentin[MeSH Terms]) OR (dentin*[Title/Abstract])) AND ((((((((((((((((bioactiv*[Title/Abstract]) OR (biomater*[Title/Abstract])) OR (“ion releasing”[Title/Abstract])) OR (“ions releasing”[Title/Abstract])) OR (ion releasing material[Title/Abstract])) OR (ions releasing mater*[Title/Abstract])) OR (“Smart Materials”[Mesh])) OR (“Smart Materials”[Title/Abstract])) OR (“Smart Material”[Title/Abstract])) OR (“Biomimetic Materials”[Mesh])) OR (“biomimetic mater*”[Title/Abstract])) OR (Biomimetics[Mesh])) OR (Biomimetic*[tiab])) OR (“Smart Dentin Replacement”[Supplementary Concept])) OR (“Smart Dentin Replacement”[tiab])) OR (Dentin* Replacement[Title/Abstract])) |
| Scopus | (TITLE-ABS-KEY (dentin) OR TITLE-ABS-KEY (dentin*) AND TITLE-ABS-KEY (bioactiv*) OR TITLE-ABS-KEY (biomater*) OR TITLE-ABS-KEY (“ion releasing”) OR TITLE-ABS-KEY (“ions releasing”) OR TITLE-ABS-KEY (“ion releasing material”) OR TITLE-ABS-KEY (ions AND releasing AND mater*) OR TITLE-ABS-KEY (“smart materials”) OR TITLE-ABS-KEY (“smart material”) OR TITLE-ABS-KEY (“biomimetic materials”) OR TITLE-ABS-KEY (biomimetic AND mater*) OR TITLE-ABS-KEY (biomimetics) OR TITLE-ABS-KEY (biomimetic*) OR TITLE-ABS-KEY (“smart dentin replacement”) OR TITLE-ABS-KEY (dentin*replacement)) |
| Web of Science | TS = (dentin) OR TS = (dentin*) AND TS = (bioactiv*) OR TS = (biomater*) OR TS = (“ion relasing”) OR TS = (“ions releasing”) OR TS = (“ion releasing material”) OR TS = (ions releasing mater*) OR TS = (“smart materials”) OR TS = (“smart material”) OR TS = (“biomimetic materials”) OR TS = (biomimetic mater*) OR TS = (biomimetics) OR TS = (biomimetic*) OR TS = (“smart dentin replacement”) OR TS = (dentin*replacement) |
| Cochrane Library | ID Search Hits 1 MeSH descriptor: [Dentin] explode all trees 1260 2 (dentin*):ti,ab,kw 4468 3 (bioactiv*):ti,ab,kw 2166 4 (biomater*):ti,ab,kw 573 5 (“ion releasing”):ti,ab,kw 10 6 (“ions releasing”):ti,ab,kw 0 7 (“ion releasing material”):ti,ab,kw 2 8 (ions releasing mater*):ti,ab,kw 5 9 MeSH descriptor: [Smart Materials] explode all trees 1 10 (“Smart Material”):ti,ab,kw 1 11 MeSH descriptor: [Biomimetic Materials] explode all trees 1340 12 (biomimetic mater*):ti,ab,kw 54 13 MeSH descriptor: [Biomimetics] explode all trees 11 14 (Biomimetic*):ti,ab,kw 124 15 (“Smart Dentin Replacement”):ti,ab,kw 7 16 (Dentin* Replacement):ti,ab,kw 71 17 1 OR 2 4468 18 3 OR 4 OR 5 OR 6 OR 7 OR 8 OR 9 OR 10 OR 11 OR 12 OR 13 OR 14 OR 15 OR 16 4208 19 17 AND #8 198 |
|
VHL
(BBO/LILACS) | (mh: dentin OR dentin*) AND (bioactive* OR biomater* OR “ion releasing” OR “ions releasing” OR “ion releasing material” OR ions releasing mater* OR mh: “smart materials” OR “smart material” OR mh: “biomimetic materials” OR biomimetric mater* OR mh: biomimetics OR biomimetic* OR “smart dentin replacement” OR dentin*replacement) AND (db:(“LILACS”)) |
| EMBASE | dentin:ti,ab,kw OR dentin*:ti,ab,kw AND bioactiv*:ti,ab,kw OR biomaterial:ti,ab,kw OR biomater*:ti,ab,kw OR ‘ion releasing’:ti,ab,kw OR ‘ions releasing’:ti,ab,kw OR ‘ion releasing material’:ti,ab,kw OR ‘ions releasing mater*’:ti,ab,kw OR ‘smart materials’:ti,ab,kw OR ‘smart material’:ti,ab,kw OR ‘biomimetic materials’:ti,ab,kw OR ‘biomimetic material’:ti,ab,kw OR biomimetics:ti,ab,kw OR biomimetic*:ti,ab,kw OR ‘smart dentin replacement’:ti,ab,kw OR ‘dentin* replacement’:ti,ab,kw |
| Study | Country | First Author | Year | Journal | Material | Times Cited |
|---|---|---|---|---|---|---|
| Hydroxyapatite nanorods as novel fillers for improving the properties of dental adhesives: Synthesis and application | Iran | Sadat-Shojai, M | 2010 | Dent Mater | Adhesive | 181 |
| Mechanical properties and biochemical activity of remineralizing resin-based Ca-PO4 cements | USA | Dickens, S.H | 2003 | Dent Mater | Cement | 155 |
| Novel dental adhesives containing nanoparticles of silver and amorphous calcium phosphate | USA | Melo, MA | 2013 | Dent Mater | Adhesive | 132 |
| Toughness, bonding and fluoride-release properties of hydroxyapatite-added glass ionomer cement | Japan | Lucas, ME | 2003 | Biomater | Cement | 99 |
| Novel dental adhesive containing antibacterial agents and calcium phosphate nanoparticles | USA | Melo, MA | 2013 | J Biomed Mater Res B Appl Biomater | Adhesive | 90 |
| Therapeutic effects of novel resin bonding systems containing bioactive glasses on mineral-depleted areas within the bonded-dentine interface | UK | Sauro, S | 2012 | J Mater Sci Mater Med | Composite | 90 |
| Biomimetic remineralization of human dentin using promising innovative calcium-silicate hybrid “smart” materials | Italy | Gandolfi, MG | 2011 | Dent Mater | Composite | 84 |
| Anti-biofilm dentin primer with quaternary ammonium and silver nanoparticles | USA | Cheng, L | 2012 | J Dent Res | Adhesive | 80 |
| Antibacterial activity and ion release of bonding agent containing amorphous calcium phosphate nanoparticles | China | Chen, C | 2014 | Dent Mater | Adhesive | 76 |
| Remineralization of artificial dentinal caries lesions by biomimetically modified mineral trioxide aggregate | China | Qi, YP | 2012 | Acta Biomater | Cement | 75 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pires, P.M.; Rosa, T.d.C.; Ribeiro-Lages, M.B.; Duarte, M.L.; Cople Maia, L.; Neves, A.d.A.; Sauro, S. Bioactive Restorative Materials Applied over Coronal Dentine—A Bibliometric and Critical Review. Bioengineering 2023, 10, 731. https://doi.org/10.3390/bioengineering10060731
Pires PM, Rosa TdC, Ribeiro-Lages MB, Duarte ML, Cople Maia L, Neves AdA, Sauro S. Bioactive Restorative Materials Applied over Coronal Dentine—A Bibliometric and Critical Review. Bioengineering. 2023; 10(6):731. https://doi.org/10.3390/bioengineering10060731
Chicago/Turabian StylePires, Paula Maciel, Thamirys da Costa Rosa, Mariana Batista Ribeiro-Lages, Maysa Lannes Duarte, Lucianne Cople Maia, Aline de Almeida Neves, and Salvatore Sauro. 2023. "Bioactive Restorative Materials Applied over Coronal Dentine—A Bibliometric and Critical Review" Bioengineering 10, no. 6: 731. https://doi.org/10.3390/bioengineering10060731