Epicardial Pulsed Field Ablation of Ganglionated Plexi: Computational and Pre-Clinical Evaluation of a Bipolar Sub-Xiphoid Catheter for the Treatment of Atrial Fibrillation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ablation System Designs
2.2. Electric Field Modeling
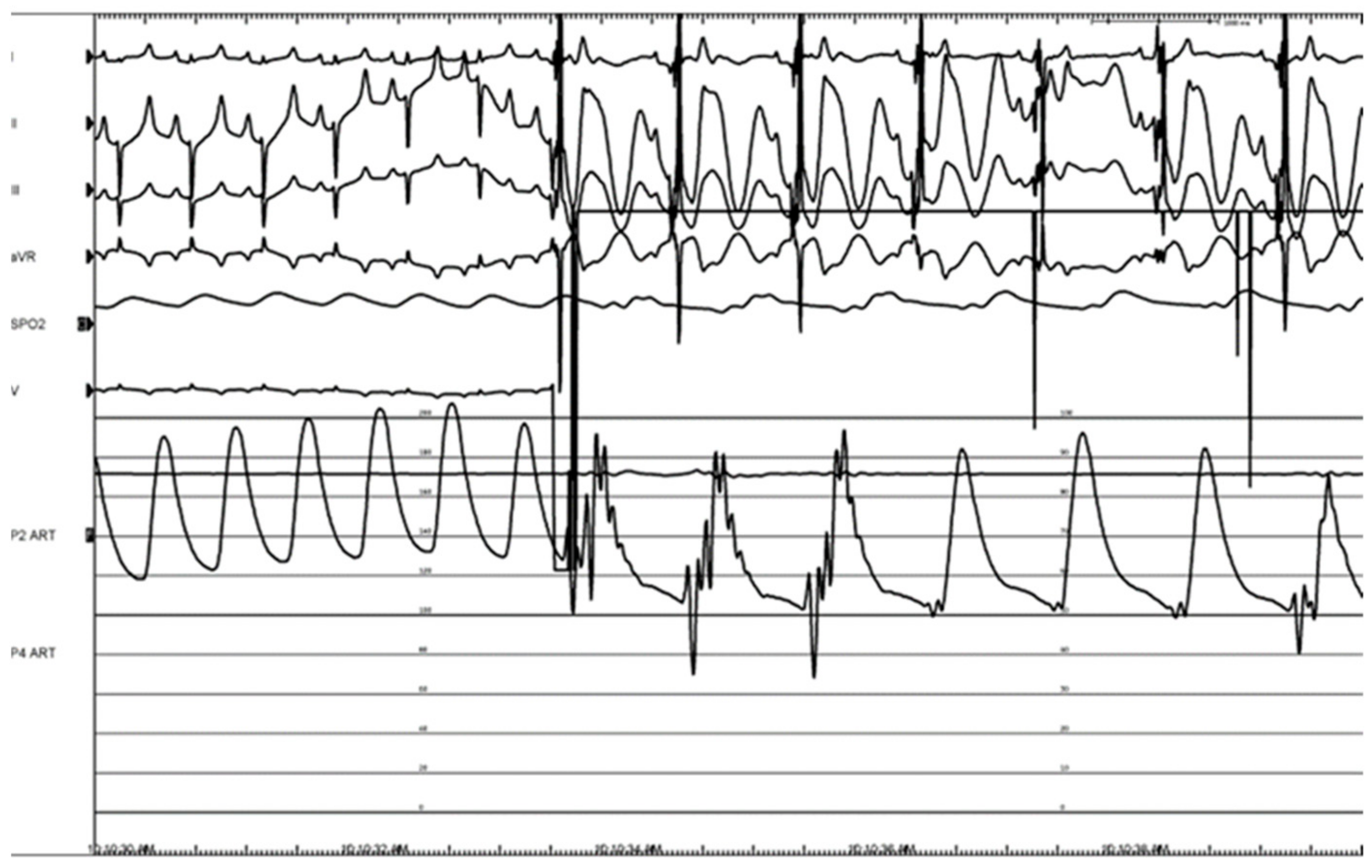
2.3. Canine Study—Ablation Efficacy
2.4. Histological Evaluation
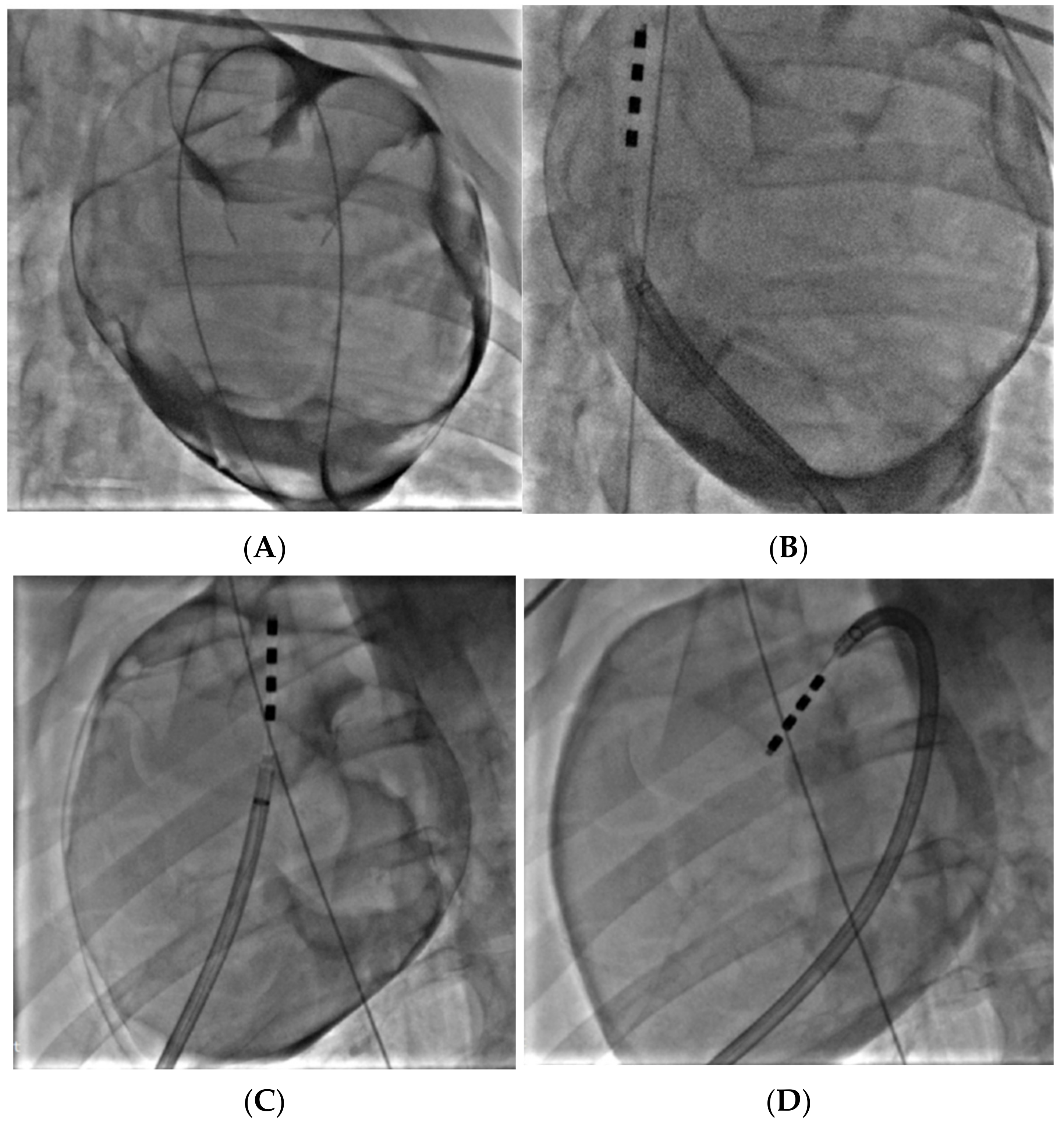
2.5. Porcine Study—Catheter Access and Navigation
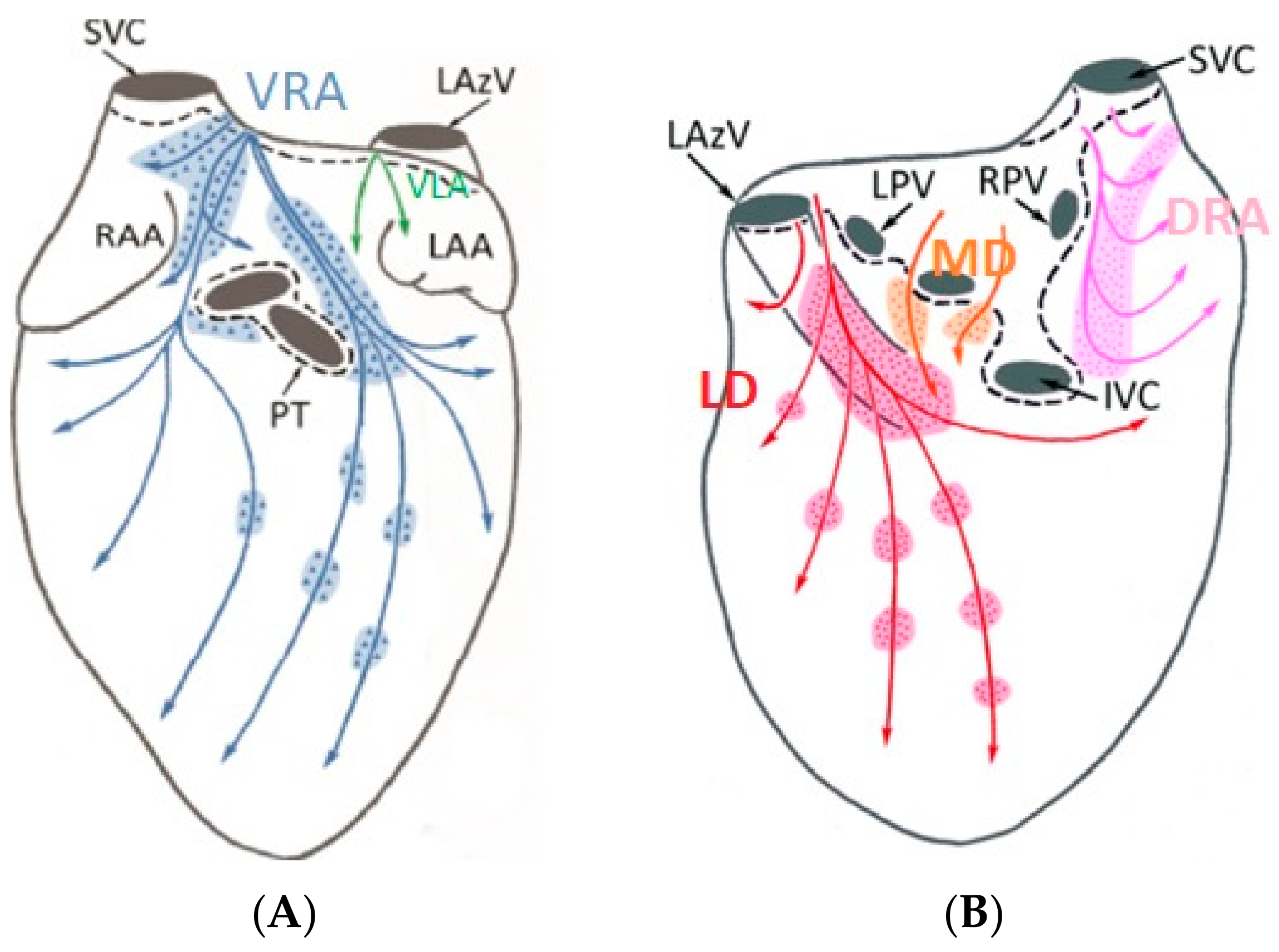
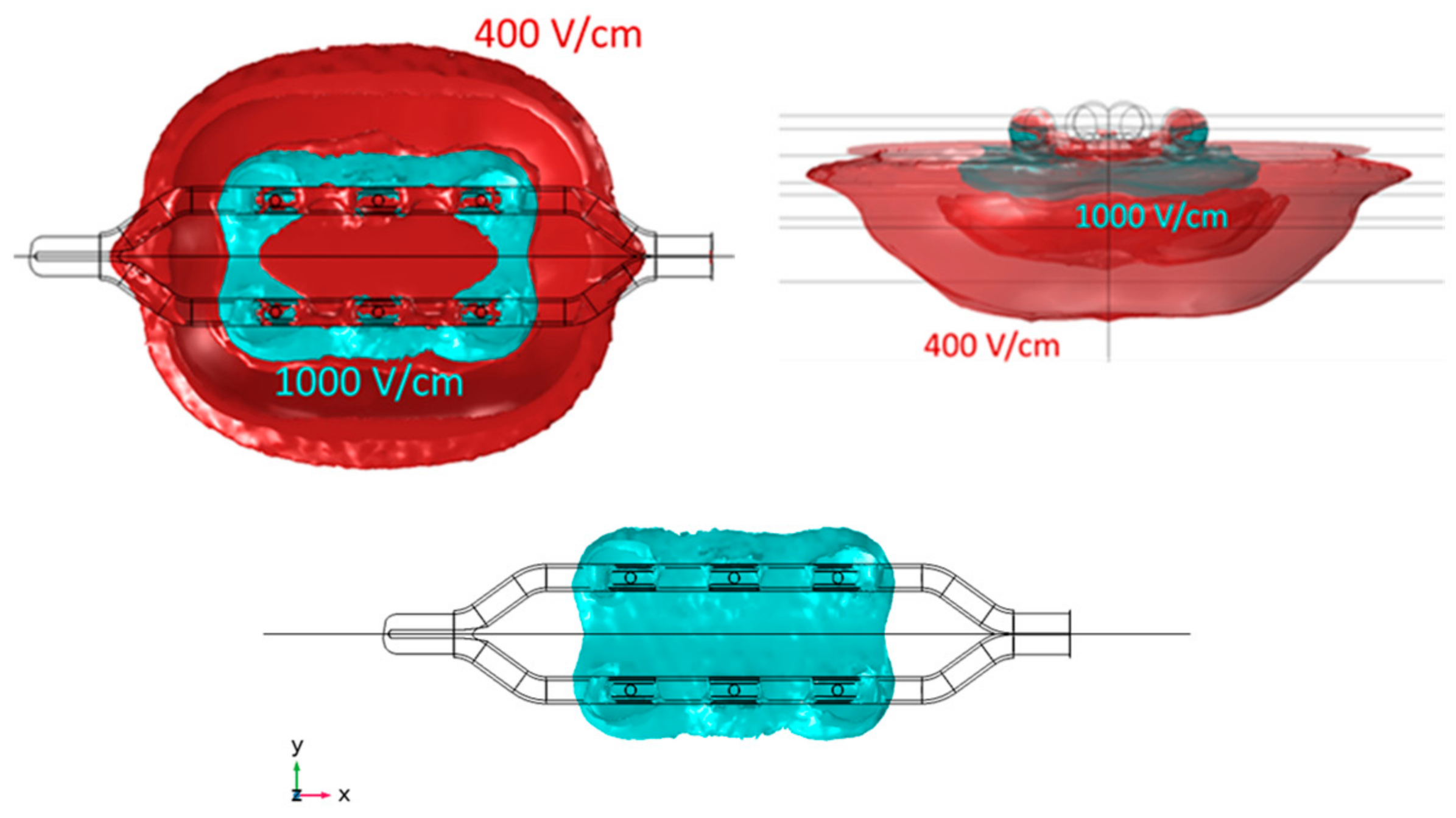
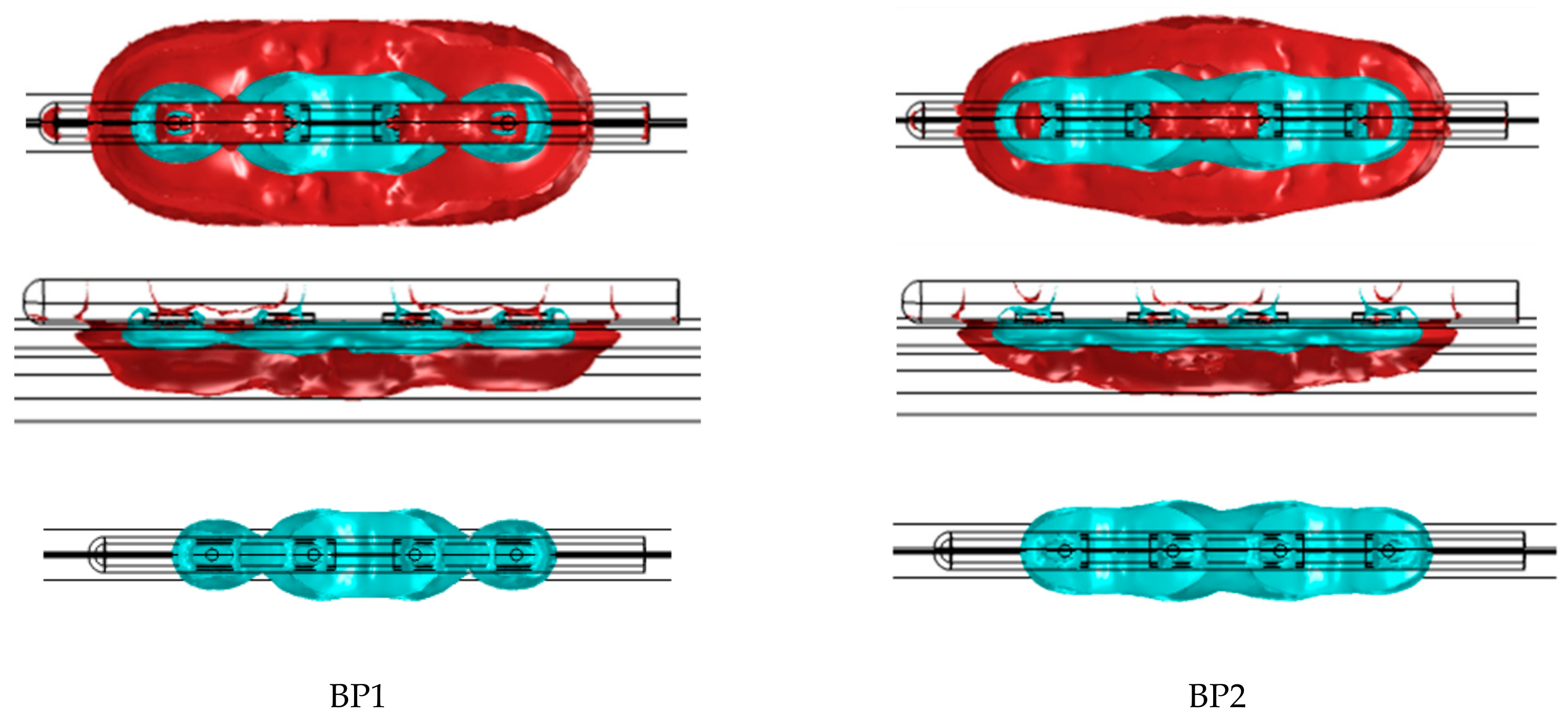
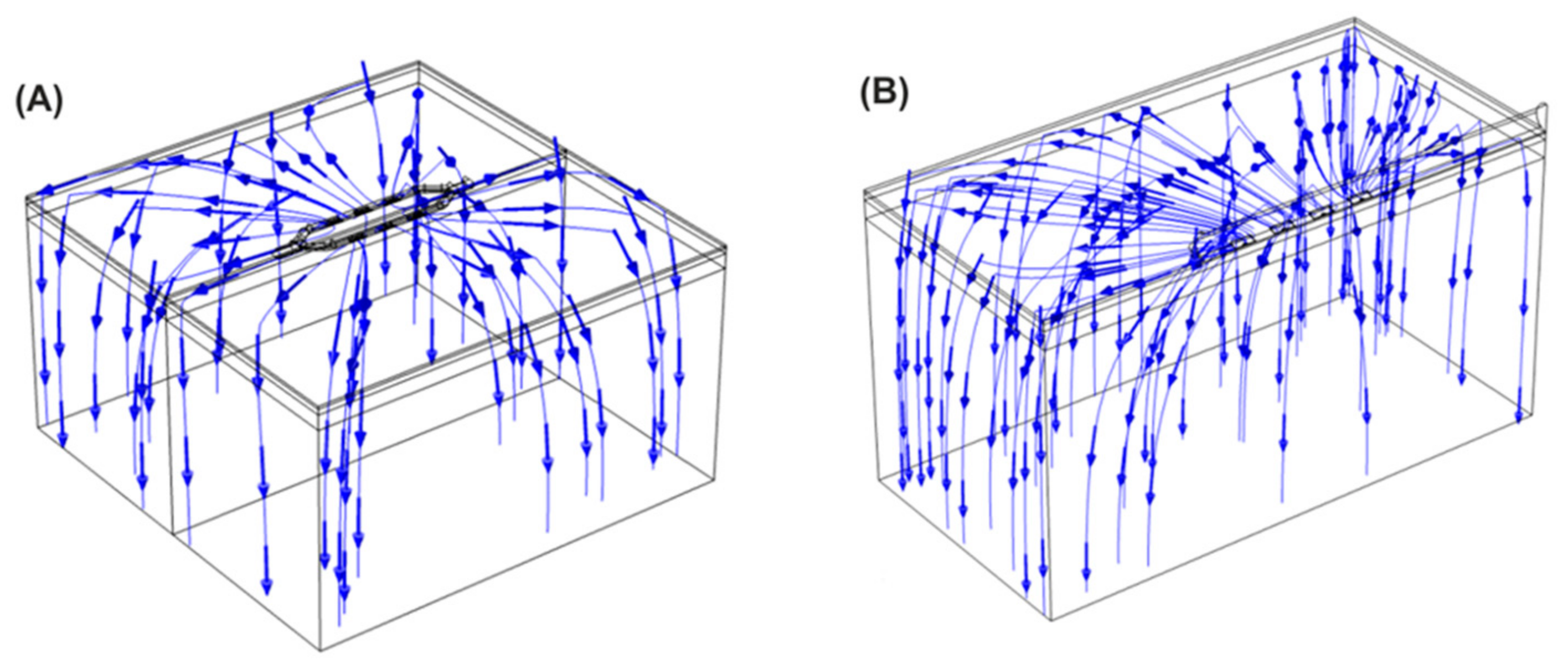
3. Results
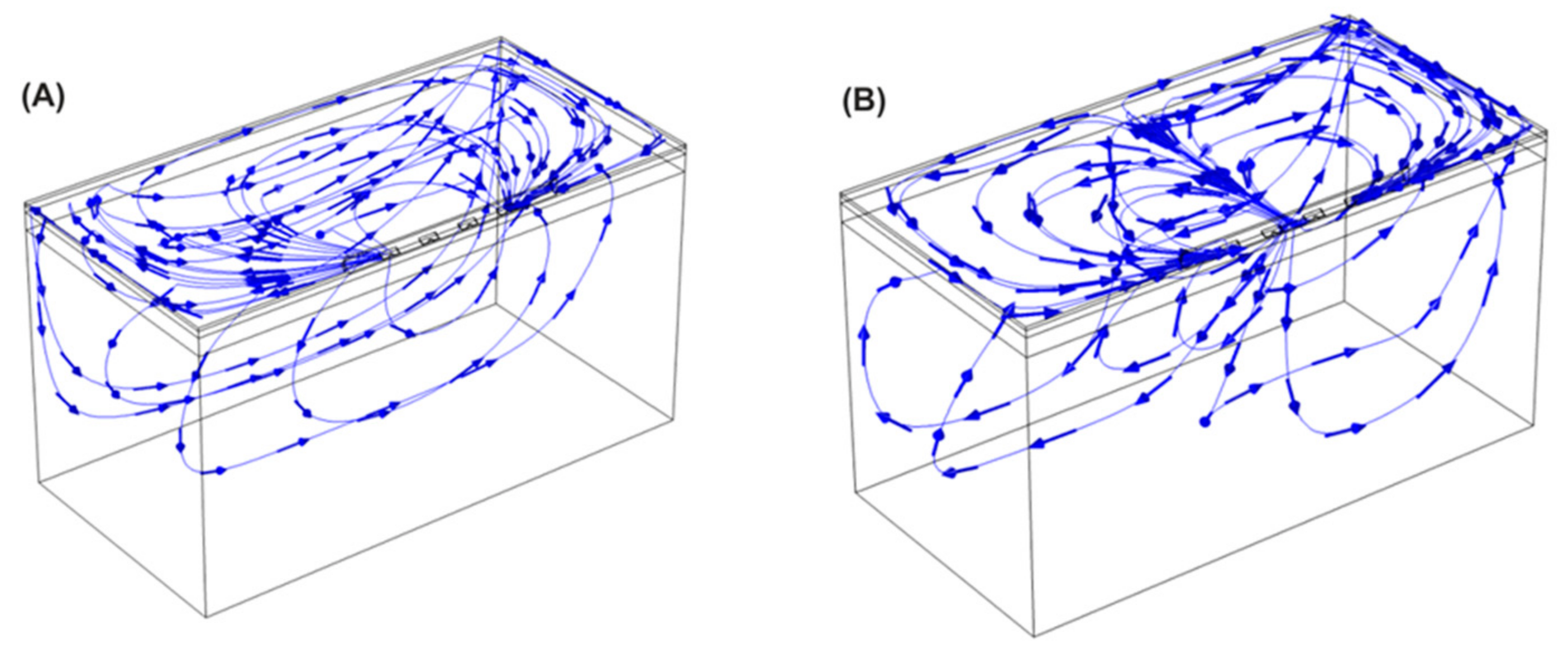
3.1. Electric Field Models
3.2. Canine Study—Ablation Efficacy
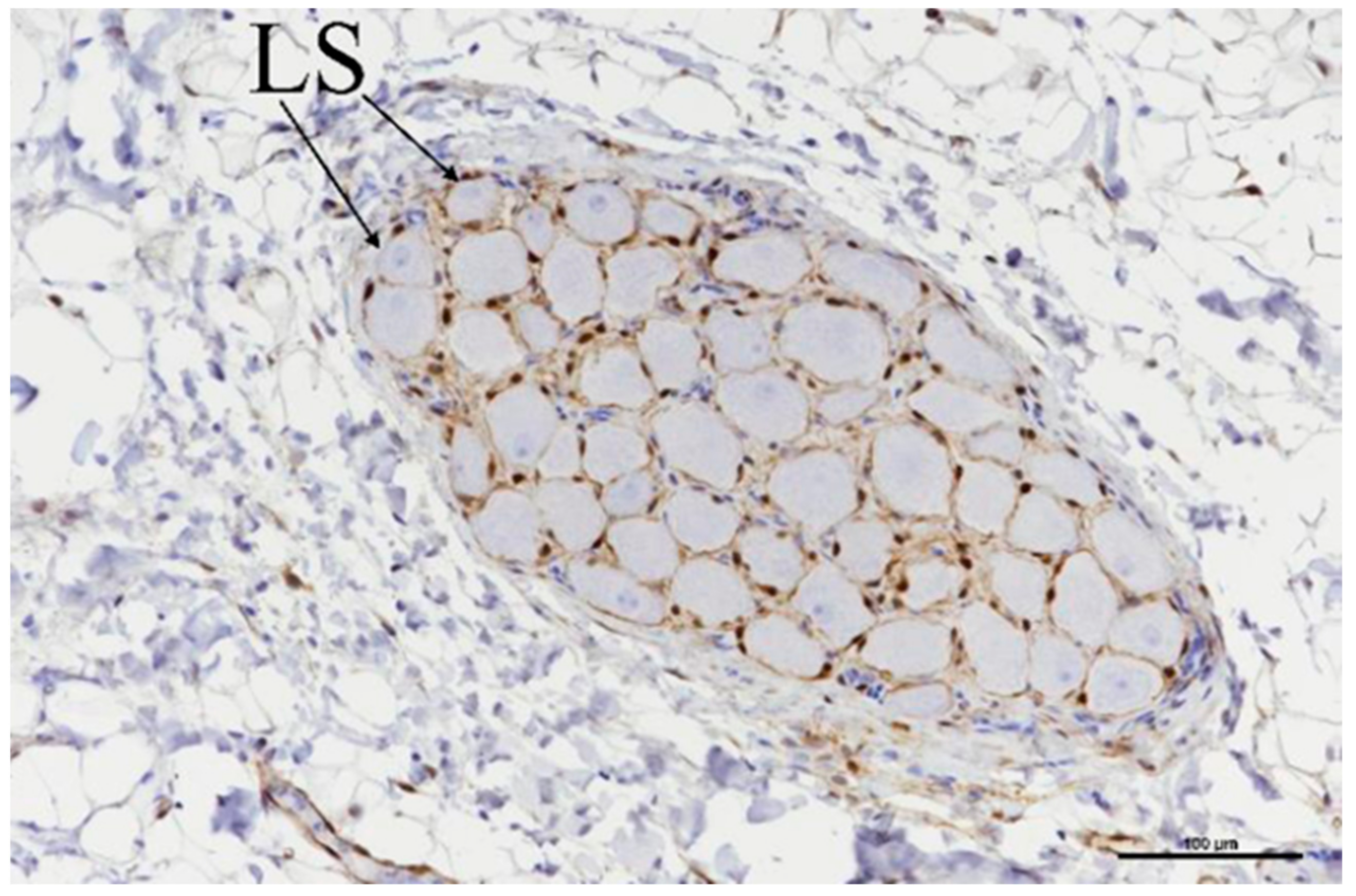
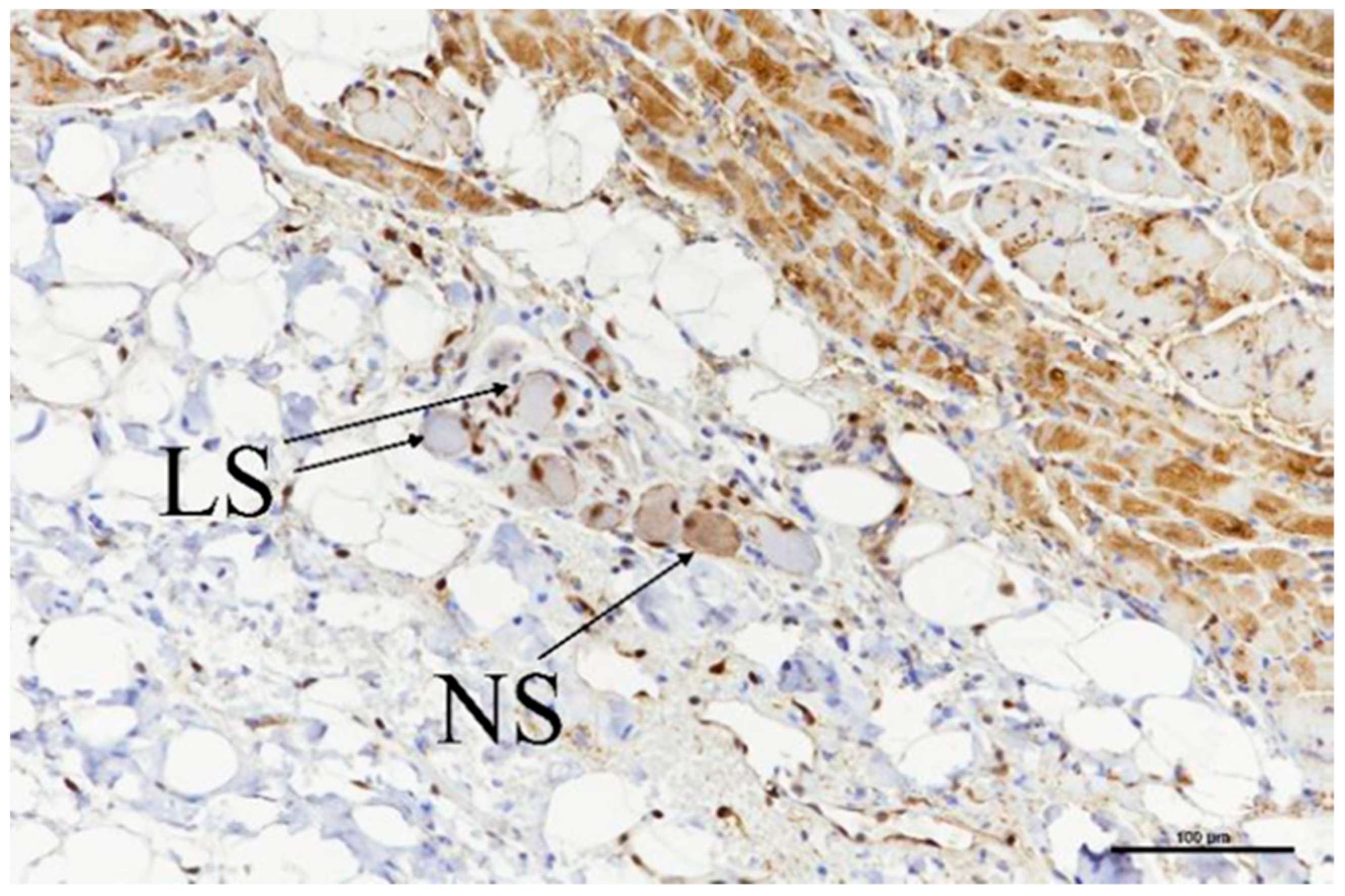
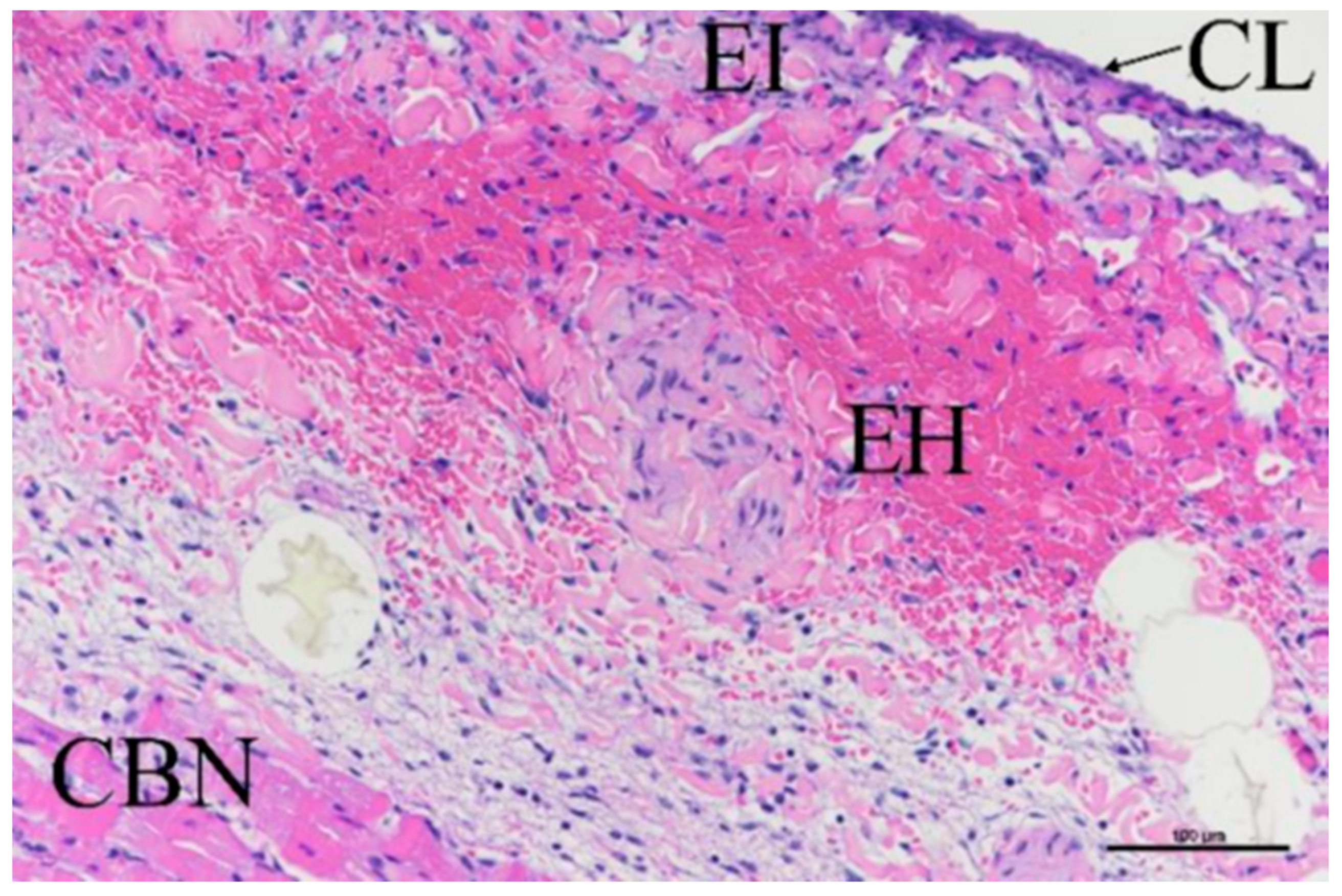
3.3. Histologic Evaluation
3.4. Porcine Study—Catheter Access and Navigation
4. Discussion
5. Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Hindricks, G.; Potpara, T.; Dagres, N.; Arbelo, E.; Bax, J.; Blomstrom-Lundqvist, C.; Boriani, G.; Castella, M.; Dan, G.-A.; Dilaveris, P.; et al. 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS): The Task Force for the diagnosis and management of atrial fibrillation of the European Society of Cardiology (ESC) Developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. Eur. Heart J. 2021, 42, 373–498. [Google Scholar] [PubMed]
- January, C.; Wann, S.; Calkins, H.; Chen, L.; Cigarro, J.; Cleveland, J., Jr.; Ellinor, P.; Ezekowitz, M.; Field, M.; Furie, K.; et al. 2019 AHA/ACC/HRS focused update of the 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation. A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. Heart Rhythm 2019, 16, e66–e93. [Google Scholar] [PubMed]
- Kuck, K.-H.; Brugada, J.; Furnkranz, A.; Metzner, A.; Ouyang, F.; Chun, J.; Elvan, A.; Arentz, T.; Bestehorn, K.; Pocock, S.; et al. Cryoballoon or Radiofrequency Ablation for Paroxysmal Atrial Fibrillation. N. Engl. J. Med. 2016, 374, 2235–2245. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, B.; Neuzil, P.; Luik, A.; Asensi, J.; Schrickel, J.; Deneke, T.; Bordignon, S.; Petru, J.; Merkel, M.; Sediva, L.; et al. Laser Balloon or Wide-Area Circumferential Irrigated Radiofrequency Ablation for Persistent Atrial Fibrillation: A Multicenter Prospective Randomized Study. Circ. Arrhythm. Electrophysiol. 2017, 10, e005767. [Google Scholar] [CrossRef]
- Chun, J.K.R.; Bordignon, S.; Last, J.; Mayer, L.; Tohoko, S.; Zanchi, S.; Bianchini, L.; Bologna, F.; Nagase, T.; Urbanek, L.; et al. Cryoballoon Versus Laserballoon: Insights from the First Randomized Balloon Trial in Catheter Ablation of Atrial Fibrillation. Circ. Arrhythm. Electrophysiol. 2021, 14, e009294. [Google Scholar] [CrossRef] [PubMed]
- Luik, A.; Kunzmann, K.; Hormann, P.; Schmidt, K.; Radzewitz, A.; Bramlage, P.; Schenk, T.; Schymik, G.; Merkel, M.; Kieser, M.; et al. Cryoballoon vs. open irrigated radiofrequency ablation for paroxysmal atrial fibrillation: Long-term FreezeAF outcomes. BMC Cardiovasc. Disord. 2017, 17, 135. [Google Scholar] [CrossRef]
- Barbhaiya, C.; Kumar, S.; Guo, Y.; Zhong, J.; John, R.; Tedrow, U.; Koplan, B.; Epstein, L.; Stevenson, W.; Michaud, G. Global Survey of Esophageal Injury in Atrial Fibrillation Ablation. J. Am. Coll. Cardiol. 2016, 2, 143–150. [Google Scholar] [CrossRef]
- Parikh, V.; Kowalski, M. Comparison of Phrenic Nerve Injury during Atrial Fibrillation Ablation between Different Modalities, Pathophysiology and Management. J. Atr. Fibrillation 2015, 8, 1314. [Google Scholar]
- Ekanem, E.; Reddy, V.; Schmidt, B.; Reichlin, T.; Neven, K.; Metzner, A.; Hansen, J.; Blaauw, Y.; Maury, P.; Arentz, T.; et al. Multinational survey on the methods, efficacy, and safety on the post-approval clinical use of pulsed field ablation (MANIFEST-PF). Europace 2022, 24, 1256–1266. [Google Scholar] [CrossRef]
- Reddy, V.; Gerstenfeld, E.; Natale, N.; Whang, W.; Cuoco, F.; Patel, C.; Mountantonakis, S.; Gibson, D.; Harding, J.; Ellis, C.; et al. Pulsed Field or Conventional Thermal Ablation for Paroxysmal Atrial Fibrillation. N. Engl. J. Med. 2023, 389, 1660–1671. [Google Scholar] [CrossRef]
- Aldaas, O.M.; Malladi, C.; Han, F.T.; Hoffmayer, K.S.; Krummen, D.; Ho, G.; Raissi, F.; Birgersdotter-Green, U.; Feld, G.K.; Hsu, J.C. Pulsed field ablation versus thermal energy ablation for atrial fibrillation: A systematic review and meta-analysis of procedural efficiency, safety, and efficacy. J. Interv. Card. Electrophysiol. 2023. [Google Scholar] [CrossRef] [PubMed]
- Reddy, V.; Neuzil, P.; Koruth, J.; Petru, J.; Funosako, M.; Cochet, H.; Sediva, L.; Chovanec, M.; Dukkipati, S.; Jais, P. Pulsed Field Ablation for Pulmonary Vein Isolation in Atrial Fibrillation. J. Am. Coll. Cardiol. 2019, 74, 315–326. [Google Scholar] [CrossRef] [PubMed]
- Kawamura, I.; Neuzil, P.; Shrivamurthy, P.; Petru, J.; Funasako, M.; Minami, K.; Kuroki, K.; Dukkipati, S.; Koruth, J.; Reddy, V. Does pulsed field ablation regress over time? A quantitative temporal analysis of pulmonary vein isolation. Heart Rhythm 2021, 18, 878–994. [Google Scholar] [CrossRef] [PubMed]
- Musikantow, D.; Neuzil, P.; Petru, P.; Koruth, J.; Kralovec, S.; Miller, M.; Funasako, M.; Chovanec, M.; Turugam, M.; Whang, W.; et al. Pulsed Field Ablation to Treat Atrial Fibrillation: Autonomic Nervous System Effects. JACC Clin. Electrophysiol. 2023, 9, 481–493. [Google Scholar] [CrossRef] [PubMed]
- Stojadinovic, P.; Wichterle, D.; Peichl, P.; Nakagawa, H.; Cihak, R.; Haskova, J.; Kautzner, J. Autonomic Changes Are More Durable after Radiofrequency than Pulsed Electric Field Pulmonary Vein Isolation. J. Am. Coll. Cardiol. 2022, 8, 895–904. [Google Scholar]
- Po, S.; Nakagawa, H.; Jackman, W. Localization of Left Atrial Ganglionated Plexi in Patients with Atrial Fibrillation. J. Cardiovasc. Electrophysiol. 2009, 20, 1186–1189. [Google Scholar] [CrossRef] [PubMed]
- Nakagawa, H.; Scherlag, B.; Patterson, E.; Ikeda, A.; Lockwood, D.; Jackman, W. Pathophysiologic basis of autonomic ganglionated plexus ablation in patients with atrial fibrillation. Heart Rhythm 2009, 6, s26–s34. [Google Scholar] [CrossRef]
- O’Brien, B.; Reilly, J.; Coffey, K.; Gonzalez-Suarez, A.; Quinlan, L.; van Zyl, M. Cardioneuroablation Using Epicardial Pulsed Field Ablation for the Treatment of Atrial Fibrillation. J. Cardiovasc. Dev. Dis. 2023, 10, 238. [Google Scholar] [CrossRef]
- Van Zyl, M.; Khabsa, M.; Tri, J.; Ladas, T.; Yasin, O.; Ladejobi, A.; Reilly, J.; O’Brien, B.; Coffey, K.; Asirvatham, S. Open-Chest Pulsed Electric Field Ablation of Cardiac Ganglionated Plexi in Acute Canine Models. J. Innov. Card. Rhythm Manag. 2022, 13, 5061–5069. [Google Scholar] [CrossRef]
- Musikantow, D.; Reddy, V.; Skalsky, I.; Shaburishvili, T.; van Zyl, M.; O’Brien, B.; Coffey, K.; Reilly, J.; Neuzil, P.; Asirvatham, S.; et al. Targeted ablation of epicardial ganglionated plexi during cardiac surgery with pulsed field electroporation (NEURAL AF). J. Interv. Card. Electrophysiol. 2023. [Google Scholar] [CrossRef]
- Yokokawa, M.; Sundaram, B.; Garg, A.; Stojanovska, J.; Oral, H.; Morady, F.; Chugh, A. Impact of mitral isthmus anatomy on the likelihood of achieving linear block in patients undergoing catheter ablation of persistent atrial fibrillation. Heart Rhythm 2011, 8, 1404–1410. [Google Scholar] [CrossRef] [PubMed]
- Sel, D.; Cukjati, D.; Batiuskaite, D.; Slivnik, T.; Mir, L.; Miklavcic, D. Sequential finite element model of tissue electropermeabilization. IEEE Trans. Biomed. Eng. 2005, 52, 816–827. [Google Scholar] [CrossRef] [PubMed]
- González-Suárez, A.; Pérez, J.J.; O’Brien, B.; Elahi, A. In silico modelling to assess the electrical and thermal disturbance provoked by a metal intracoronary stent during epicardial pulsed electric field ablation. J. Cardiovasc. Dev. Dis. 2022, 9, 458. [Google Scholar] [CrossRef] [PubMed]
- Avazzadeh, S.; Dehkordi, M.H.; Owens, P.; Jalali, A.; O’Brien, B.; Coffey, K.; O’Halloran, M.; Fernhead, H.O.; Keane, D.; Quinlan, L.R. Establishing electroporation thresholds for targeted cell specific cardiac ablation in a 2D culture model. J. Cardiavasc. Electrophysiol. 2022, 33, 2050–2061. [Google Scholar] [CrossRef] [PubMed]
- Kaminska, I.; Kotulska, M.; Stecka, A.; Saczko, J.; Drag-Zalesinska, M.; Wysocka, T.; Choromanska, A.; Skolucka, N.; Nowicki, R.; Marczak, J.; et al. Electroporation-induced changes in normal immature rat myoblasts (H9C2). Gen. Physiol. Biophys. 2012, 31, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Aksu, T.; Gopinathannair, R.; Gupta, D.; Pauza, D. Intrinsic cardiac autonomic nervous system: What do clinical electrophysiologists need to know about the “heart brain”. J. Cardiovasc. Electrophysiol. 2021, 32, 1737–1747. [Google Scholar] [CrossRef]
- Scherlag, B.; Nakagawa, H.; Jackman, W.; Yamanashi, W.; Patterson, E.; Po, S.; Lazzara, R. Electrical Stimulation to Identify Neural Elements on the Heart: Their Role in Atrial Fibrillation. J. Interv. Card. 2005, 13, 37–42. [Google Scholar] [CrossRef]
- Katritsis, D.G.; Pokushalov, E.; Romanov, A.; Giazitzoglou, E.; Siontis, G.; Po, S.; Camm, J.; Ioannidis, J. Autonomic Denervation Added to Pulmonary Vein Isolation for Paroxysmal Atrial Fibrillation. J. Am. Coll. Cardiol. 2013, 62, 2318–2325. [Google Scholar] [CrossRef]
- Driessen, A.; Berger, W.; Krul, S.; van den Berg, N.; Neefs, J.; Piersma, F.; Chan Pin Yin, D.R.P.P.; de Jong, J.; van Boven, W.J.; de Groot, J.R. Ganglion Plexus Ablation in Advanced Atrial Fibrillation. The AFACT Study. J. Am. Coll. Cardiol. 2016, 68, 1155–1165. [Google Scholar] [CrossRef]
- Padmanabhan, D.; Naksuk, N.; Killu, A.; Kapa, S.; Witt, C.; Sugrue, A.; DeSimone, C.; Madhavan, M.; de Groot, J.R.; O’Brien, B.; et al. Electroporation of epicardial autonomic ganglia: Safety and efficacy in medium-term models. J. Cardiovasc. Electrophysiol. 2019, 30, 607–615. [Google Scholar] [CrossRef]
- Pachon M, J.C.; Pachon, E.I.; Santillana, T.G.; Lobo, T.J.; Pachon, C.T.C.; Pachon M, J.C.; Albornoz, R.N.; Zerpa, J.C. Simplified Method for Vagal Effect Evaluation in Cardiac Ablation and Electrophysiological Procedure. J. Am. Coll. Cardiol. EP. 2015, 1, 451–460. [Google Scholar] [CrossRef] [PubMed]
- Pachon-M, J.C.; Pachon, E.I.; Pachon, C.T.C.; Santillana, T.G.; Lobo, T.J.; Pachon-M, J.C.; Zerpa, J.C.; Cunha, M.Z.; Higuti, C.; Ortencio, F.A.; et al. Long-term Evaluation of the Vagal Denervation by Cardioneuroablation Using Holter and Heart Rate Variability. Circ. Arrhythm. Electrophysiol. 2020, 13, e008703. [Google Scholar] [CrossRef] [PubMed]
- Julia, J.; Bokhari, F.; Uuetoa, H.; Derejko, P.; Traykov, V.; Gwizdala, A.; Sebag, F.; Hegbom, F.; Anfinsen, O.-G.; Al Quibbany, A.; et al. A New Era in Epicardial Access for the Ablation of Ventricular Arrhythmias–The Epi-CO2 Registry. J. Am. Coll. Cardiol. 2021, 7, 85–96. [Google Scholar]
- Ahmed, A.; Garg, J.; Kabra, R.; Charate, R.; Eliaz, R.; Demma, J.; Menshes, Z.; Hazan, O.; Lakkireddy, D. Feasibility and safety of a novel blunt tip concealed needle device for pericardial access in porcine models. J. Interv. Card. Electrophysiol. 2022. [Google Scholar] [CrossRef] [PubMed]
- Derejko, P.; Dzwonkowska, D.; Wrobel, K.; Kusnierz, J.; Bardyszewski, A.; Menshes, Z.; Hazan, O.; Leon, A.; Luria, D.; Lakkireddy, D. Safety and Efficacy of a Novel Blunt-Tip Concealed-Needle Epicardial Access Device: First-in-Human Feasibility Study. JACC Clin. Electrophysiol. 2022, 8, 908–912. [Google Scholar] [CrossRef] [PubMed]
- Reddy, V.; Dukkipati, S.; Neuzil, P.; Anic, A.; Petru, J.; Funasako, M.; Cochet, H.; Minami, K.; Breskovic, T.; Sikiric, I.; et al. Pulsed Field Ablation of Paroxysmal Atrial Fibrillation. 1-Year Outcomes of IMPULSE, PEFCAT, and PEFCAT II. JACC Clin. Electrophysiol. 2021, 7, 614–627. [Google Scholar] [CrossRef] [PubMed]
- Lemoine, M.; Fink, T.; Mencke, C.; Schleberger, R.; My, I.; Obergassel, J.; Bergau, L.; Sciacca, V.; Rottner, L.; Moser, J.; et al. Pulsed-field ablation-based pulmonary vein isolation: Acute safety, efficacy and short-term follow-up in a multi-center real world scenario. Clin. Res. Cardiol. 2023, 112, 795–806. [Google Scholar] [CrossRef]
- Bharati, S.; Levine, M.; Huang, S.; Handler, B.; Parr, G.; Bauernfeind, R.; Lev, M. The Conduction System of the Swine Heart. Chest 1991, 100, 207–212. [Google Scholar] [CrossRef]
- Crick, S.; Sheppard, M.; Ho, S.; Anderson, R. Localisation and quantification of autonomic innervation in the porcine heart I: Conduction system. J. Anat. 1999, 195, 341–357. [Google Scholar] [CrossRef]





| Animal | Target GPs | Bipolar Pairs/Sequence |
|---|---|---|
| 1 | ILGP, IRGP, RSGP, TSGP, LSGP, LMGP | 50 BP1 pulses + 40 BP2 pulses |
| 2 | ILGP, IRGP, RSGP, TSGP, LSGP, LMGP | 120 BP2 pulses |
| 3 | ILGP, IRGP | 60 BP2 pulses |
| RSGP, TSGP, LSGP, LMGP | 120 BP2 pulses |
| Isoline (V/cm) | Length (mm) | Width (mm) | Depth (mm) | Total Volume (mm3) | Fat Volume (mm3) | Myocardium Volume (mm3) | Total Current (A) | |
|---|---|---|---|---|---|---|---|---|
| Monopolar Finger | 400 | 33.84 | 19.80 | 6.38 | 2235.80 | 551.72 | 1020.90 | 3.47 |
| 1000 | 25.56 | 6.71 | 1.81 | 161.35 | 141.14 | 0.39 | ||
| Monopolar Glove | 400 | 30.44 | 24.16 | 6.93 | 2172.70 | 559.29 | 848.61 | 3.54 |
| 1000 | 17.95 | 11.77 | 1.32 | 219.47 | 197.17 | 6.41 | ||
| Bipolar BP1 | 400 | 29.08 | 11.77 | 4.09 | 868.88 | 297.48 | 381.84 | 2.03 |
| 1000 | 24.21 | 5.72 | 1.91 | 144.46 | 94.71 | 1.84 | ||
| Bipolar BP2 | 400 | 28.67 | 12.31 | 4.05 | 850.34 | 269.50 | 360.36 | 2.82 |
| 1000 | 24.45 | 5.92 | 1.91 | 202.08 | 111.96 | 2.58 |
| ILGP | IRGP | RSGP | TSGP | LSGP | LMGP | Overall Average | ||
|---|---|---|---|---|---|---|---|---|
| Animal 1 | BP1 Current (A) | 4.83 | 2.55 | 3.84 | 3.09 | 3.42 | 2.44 | 3.36 |
| BP1 Energy (J) | 0.483 | 0.255 | 0.384 | 0.309 | 0.342 | 0.244 | 0.336 | |
| BP2 Current (A) | 6.05 | 2.68 | 4.59 | 3.77 | 4.06 | 2.28 | 3.91 | |
| BP2 Energy (J) | 0.605 | 0.268 | 0.459 | 0.377 | 0.406 | 0.228 | 0.391 | |
| Animal 2 | BP2 Current (A) | 5.35 | 5.87 | 5.80 | 4.11 | 4.39 | 5.55 | 5.18 |
| BP2 Energy (J) | 0.535 | 0.587 | 0.580 | 0.411 | 0.439 | 0.555 | 0.518 | |
| Animal 3 | BP2 Current (A) | 3.93 | 3.72 | 2.21 | 4.31 | 3.86 | 3.76 | 3.63 |
| BP2 Energy (J) | 0.393 | 0.372 | 0.221 | 0.431 | 0.386 | 0.376 | 0.363 |
| Pulse Description | Overall Energy per GP (J) | Overall Energy per Animal (J) | |
|---|---|---|---|
| Animal 1 | 50 BP1 + 40 BP2 | 32.44 | 194.6 |
| Animal 2 | 120 BP2 | 62.16 | 373.0 |
| Animal 3 | 60 BP2 for ILGP and IRGP | 21.78 | 217.8 |
| 120 B2 for all other GPs | 43.56 |
| RA (ms) | CS (ms) | LAA (ms) | Average (ms) | AERP Extension (%) | ||
|---|---|---|---|---|---|---|
| Animal 1 | Baseline | 90 | 100 | 80 | 90 | 22 |
| Postablation | 120 | 140 | 70 | 110 | ||
| Animal 2 | Baseline | 110 | - | 110 | 110 | 68 |
| Postablation | 190 | - | 180 | 185 | ||
| Animal 3 | Baseline | 80 | 70 | 90 | 80 | 96 |
| Postablation | 140 | 140 | 190 | 157 |
| Target GP | No. of Slides with Evaluated GPs | Treatment |
|---|---|---|
| ILGP | 2 | 60 BP2, 50 BP1 + 40 BP2 |
| IRGP | 2 | Both 50 BP1 + 40 BP2 |
| TSGP | 2 | 120 BP2, 50 BP1 + 40 BP2 |
| LSGP | 1 | 50 BP1 + 40 BP2 |
| LMGP | 2 | 120 BP2, 50 BP1 + 40 BP2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
O’Brien, B.; Reilly, J.; Coffey, K.; González-Suárez, A.; Buchta, P.; Buszman, P.P.; Lukasik, K.; Tri, J.; van Zyl, M.; Asirvatham, S. Epicardial Pulsed Field Ablation of Ganglionated Plexi: Computational and Pre-Clinical Evaluation of a Bipolar Sub-Xiphoid Catheter for the Treatment of Atrial Fibrillation. Bioengineering 2024, 11, 18. https://doi.org/10.3390/bioengineering11010018
O’Brien B, Reilly J, Coffey K, González-Suárez A, Buchta P, Buszman PP, Lukasik K, Tri J, van Zyl M, Asirvatham S. Epicardial Pulsed Field Ablation of Ganglionated Plexi: Computational and Pre-Clinical Evaluation of a Bipolar Sub-Xiphoid Catheter for the Treatment of Atrial Fibrillation. Bioengineering. 2024; 11(1):18. https://doi.org/10.3390/bioengineering11010018
Chicago/Turabian StyleO’Brien, Barry, John Reilly, Ken Coffey, Ana González-Suárez, Piotr Buchta, Piotr P. Buszman, Karolina Lukasik, Jason Tri, Martin van Zyl, and Samuel Asirvatham. 2024. "Epicardial Pulsed Field Ablation of Ganglionated Plexi: Computational and Pre-Clinical Evaluation of a Bipolar Sub-Xiphoid Catheter for the Treatment of Atrial Fibrillation" Bioengineering 11, no. 1: 18. https://doi.org/10.3390/bioengineering11010018






