Comparison of Immediate Neuromodulatory Effects between Focal Vibratory and Electrical Sensory Stimulations after Stroke
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Experimental Setup
2.3. Experimental Protocol
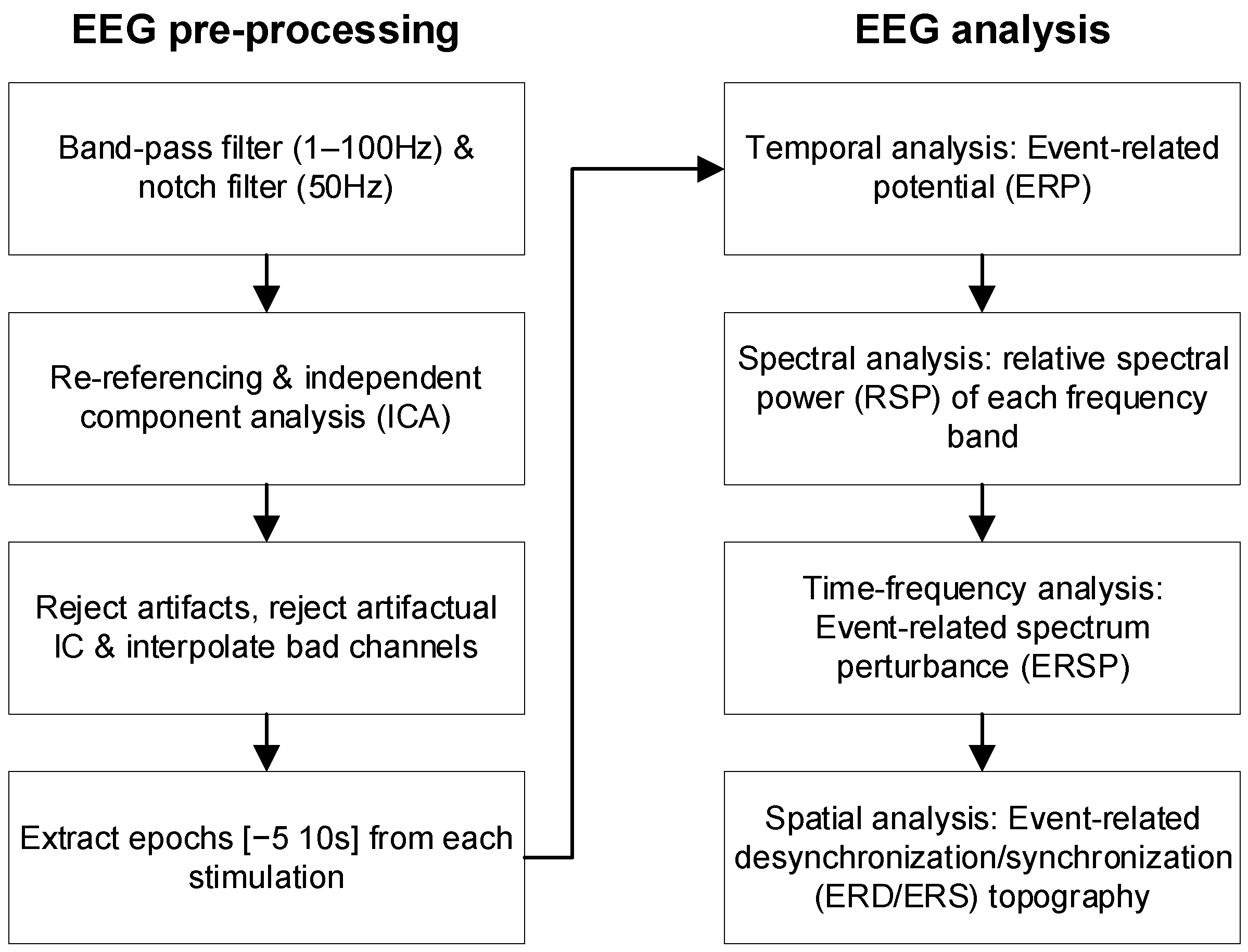
2.4. EEG Analysis
2.4.1. EEG Pre-Processing
2.4.2. EEG Temporal, Spectral, and Spatial Features
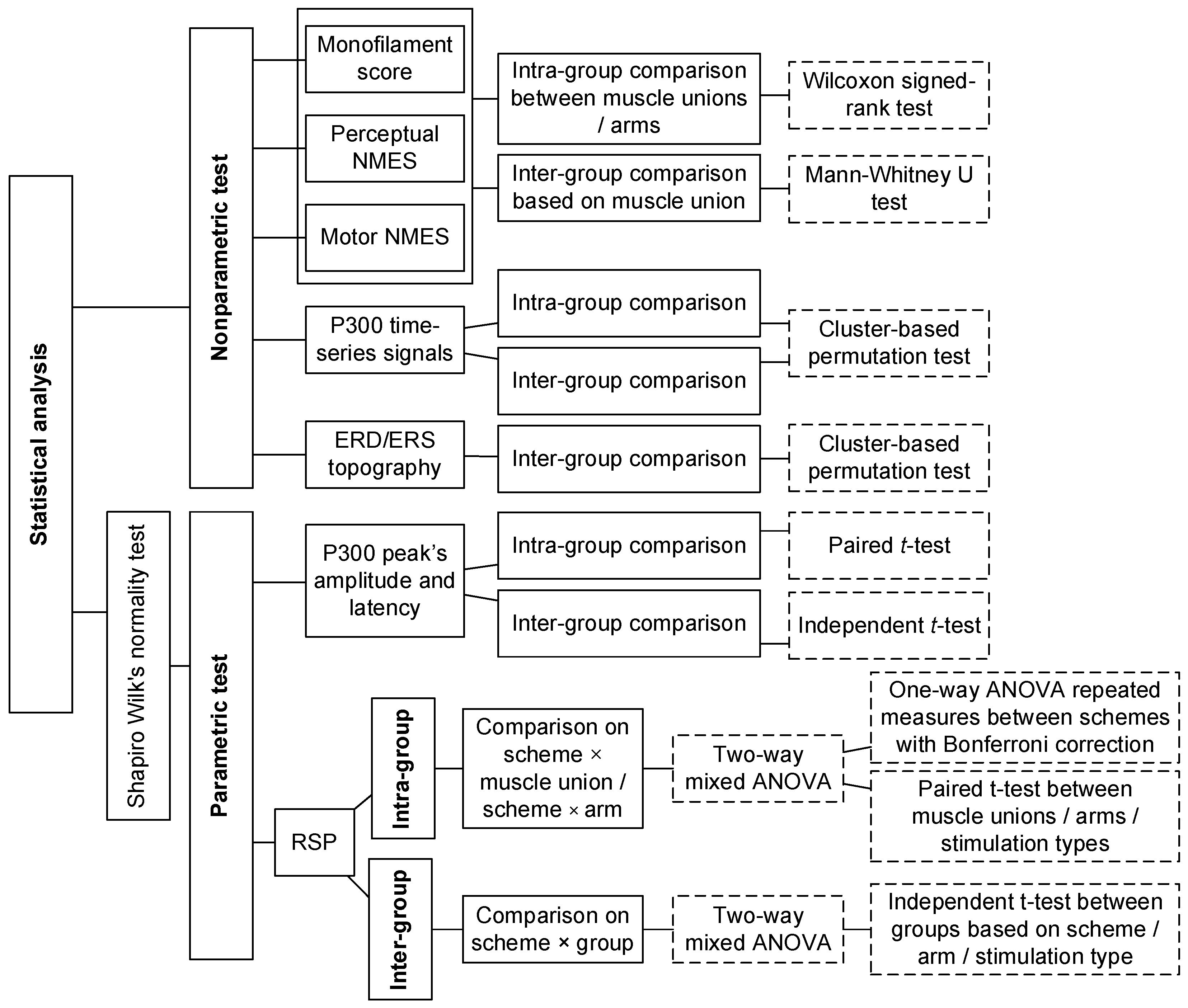
2.5. Statistical Analysis
3. Results
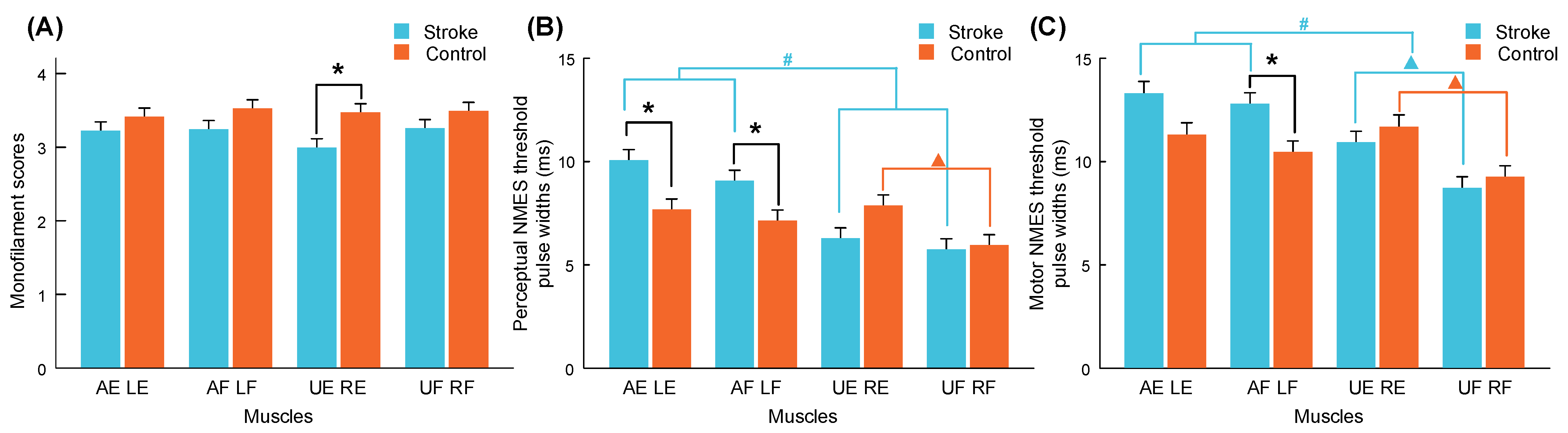
3.1. Monofilament Test and NMES Thresholds
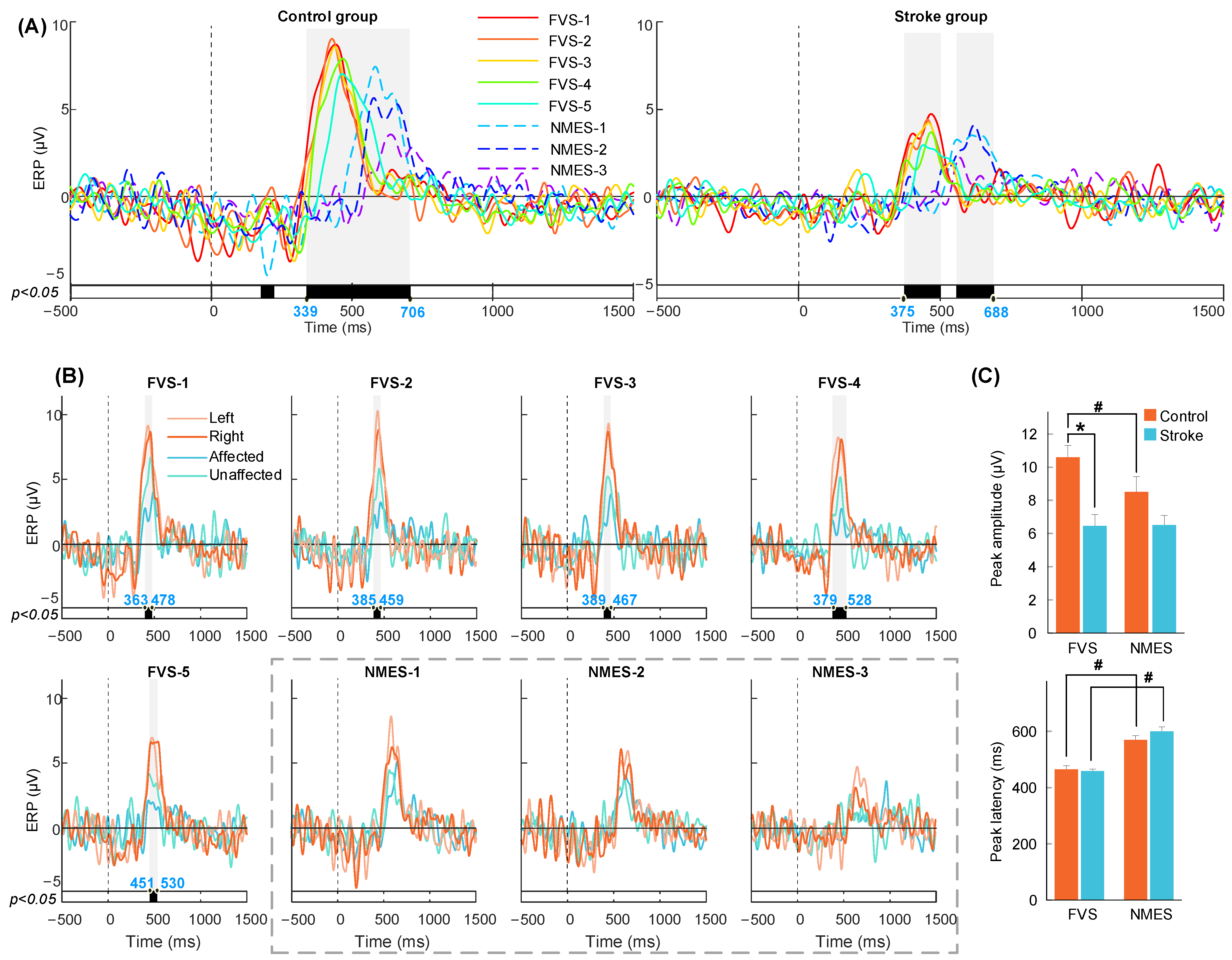
3.2. P300 in the ERP Response to FVS and NMES
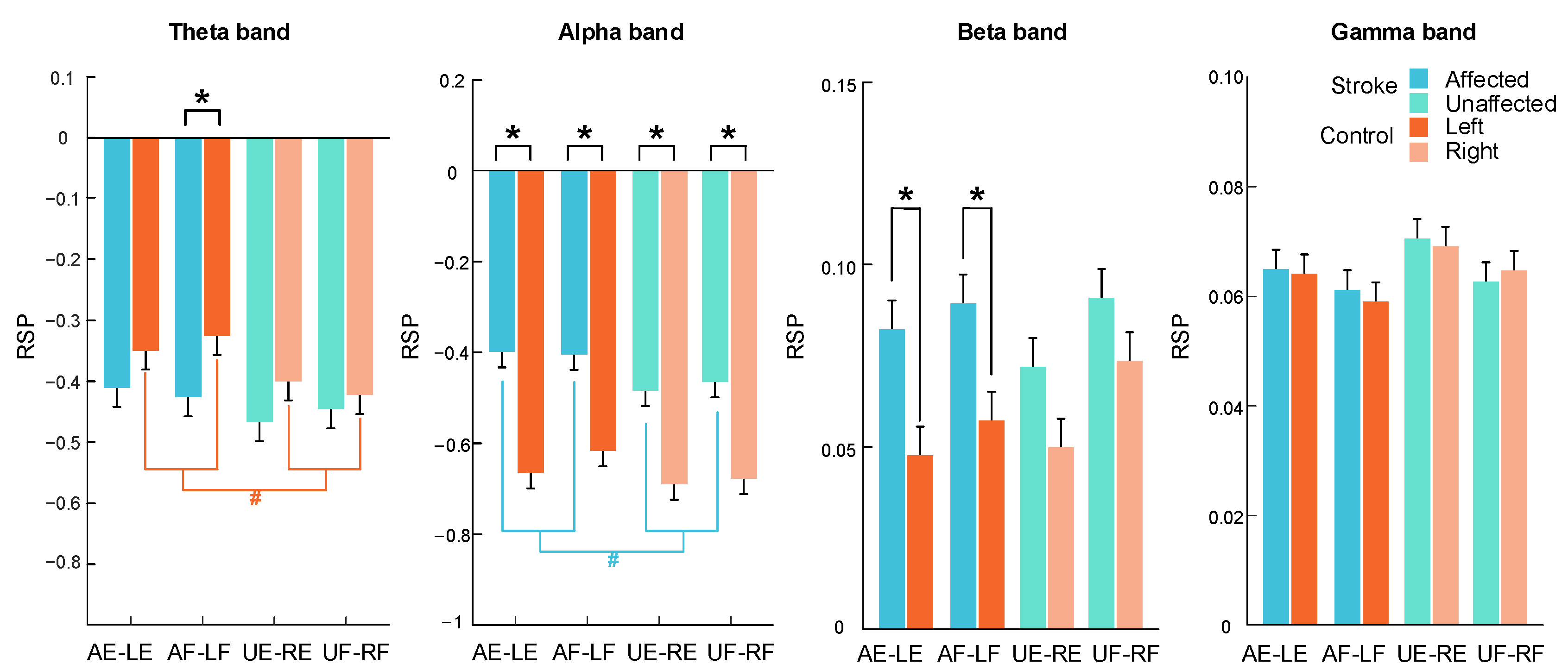
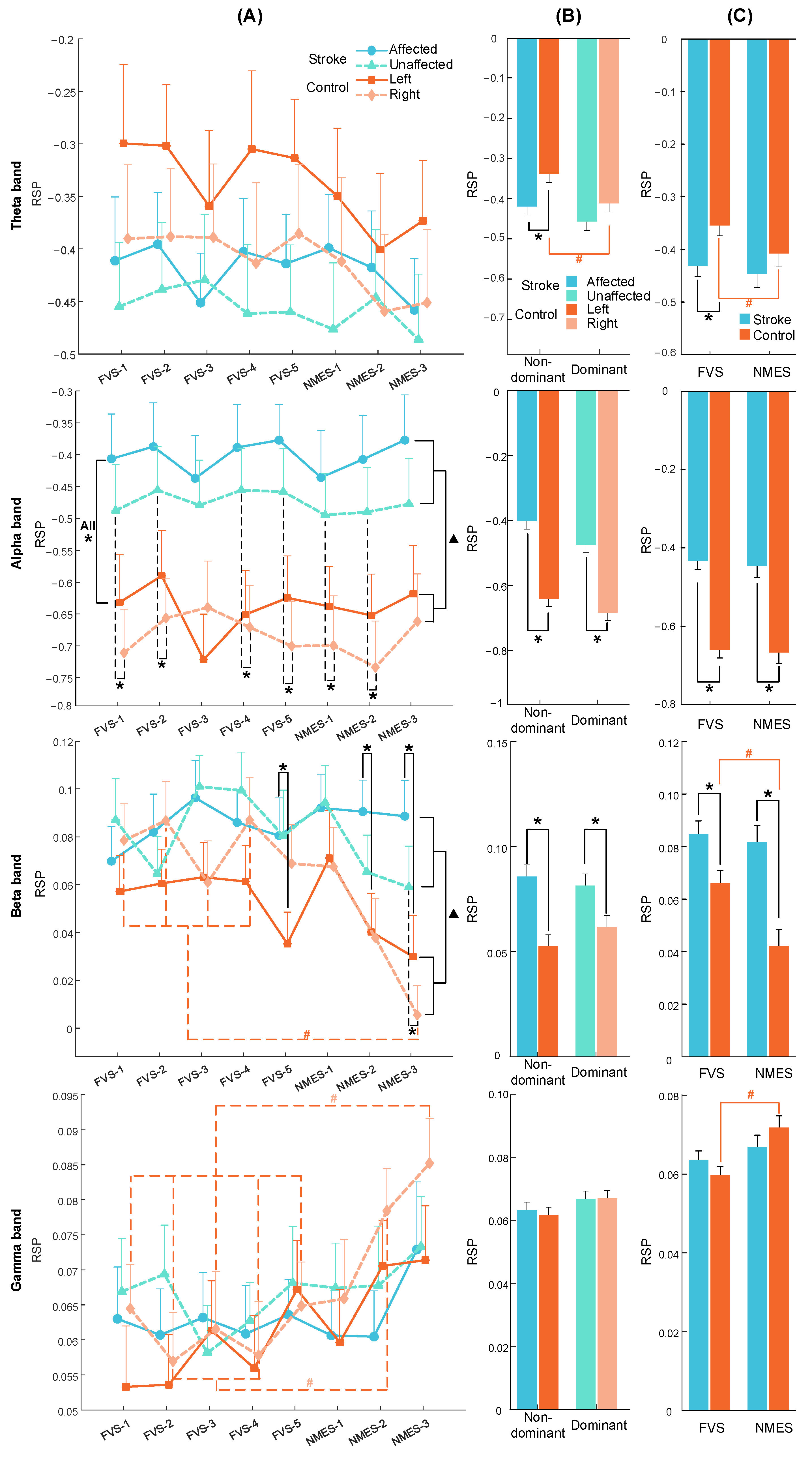
3.3. RSP Response on the Contralateral Sensorimotor Cortex
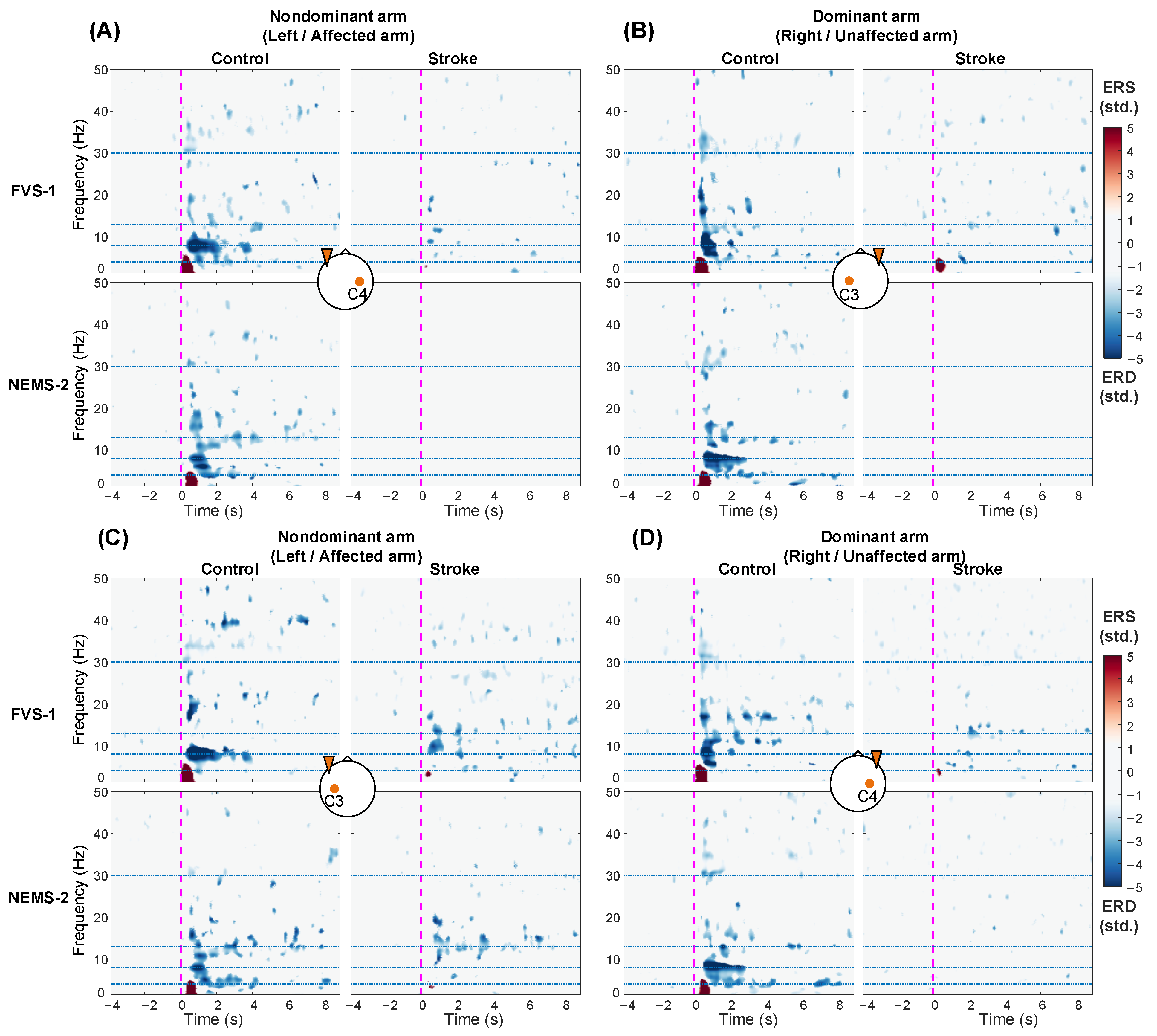
3.4. ERSP Response on the Bilateral Sensorimotor Cortex
3.5. Spatial Distribution of ERD/ERS Topography
4. Discussion
4.1. Altered Perceptual Sensitivity after Stroke
4.2. Earlier P300 Evoked by FVS Than NMES
4.3. Spectral Features of Cortical Responses to FVS and NMES
4.4. Topographical Patterns of Cortical Responses to FVS and NMES
4.5. Limitations and Future Work
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A

| Test Target | Stroke | Control | Mann–Whitney U Test | |
|---|---|---|---|---|
| Arm | Muscle Union | Mean ± SE | p (A) | |
| Nondominant (Affected/left) | ECU-ED | 3.23 ± 0.18 | 3.41 ± 0.10 | 0.412 (0.59) |
| FCR-FD | 3.24 ± 0.13 | 3.53 ± 0.10 | 0.137 (0.66) | |
| Wilcoxon signed-rank test—p (r) | 0.814 (0.06) | 0.129 (0.39) | - | |
| Dominant (Unaffected/right) | ECU-ED | 3.00 ± 0.12 | 3.47 ± 0.10 | 0.009 ** (0.22) |
| FCR-FD | 3.26 ± 0.10 | 3.49 ± 0.08 | 0.148 (0.66) | |
| Wilcoxon signed-rank test—p (r) | 0.068 (0.47) | 0.684 (0.10) | - | |
| Wilcoxon signed-rank test on arms—p (r) | 0.162 (0.26) | 0.720 (0.07) | - | |
| Stimulation Target | Stroke | Control | Mann–Whitney U Test | |
|---|---|---|---|---|
| Arm | Muscle Union | Mean ± SE | p (A) | |
| Nondominant (Affected/left) | ECU-ED | 10.1 ± 0.7 | 7.7 ± 0.5 | 0.009 ** (0.78) |
| FCR-FD | 9.1 ± 0.6 | 7.1 ± 0.6 | 0.037 * (0.72) | |
| Wilcoxon signed-rank test—p (r) | 0.099 (0.42) | 0.233 (0.31) | - | |
| Dominant (Unaffected/right) | ECU-ED | 6.3 ± 0.3 | 7.9 ± 0.6 | 0.07 (0.70) |
| FCR-FD | 5.7 ± 0.3 | 5.9 ± 0.3 | 0.775 (0.53) | |
| Wilcoxon signed-rank test—p (r) | 0.157 (3.87) | 0.010 ▲ (0.67) | - | |
| Wilcoxon signed-rank test on arms—p (r) | 0.000 ### (0.81) | 0.160 (0.26) | - | |
| Stimulation Target | Stroke | Control | Mann–Whitney U Test | |
|---|---|---|---|---|
| Arm | Muscle Union | Mean ± SE | p (A) | |
| Nondominant (Affected/left) | ECU-ED | 14.7 ± 1.1 | 12.1 ± 0.7 | 0.057 (0.72) |
| FCR-FD | 12.8 ± 0.7 | 10.5 ± 0.5 | 0.016 * (0.75) | |
| Wilcoxon signed-rank test—p (r) | 0.119 (0.40) | 0.087 (0.44) | ||
| Dominant (Unaffected/right) | ECU-ED | 10.9 ± 0.3 | 12.4 ± 0.7 | 0.413 (0.59) |
| FCR-FD | 8.7 ± 0.4 | 9.3 ± 0.4 | 0.486 (0.58) | |
| Wilcoxon signed-rank test—p (r) | 0.002 ▲▲ (0.81) | 0.004 ▲▲ (0.74) | ||
| Wilcoxon signed-rank test on arms—p (r) | 0.000 ### (0.76) | 0.246 (0.21) | - | |
| Stroke | Control | Independent t-Test | ||
|---|---|---|---|---|
| Peak | Stimulation | Mean ± SE | p (Cohen’s d) | |
| Amplitude | FVS | 6.45 ± 0.69 | 10.60 ± 0.72 | 0.000 *** (−1.52) |
| NMES | 6.51 ± 0.57 | 8.50 ± 0.92 | 0.077 (−0.67) | |
| Paired t-test—p (Cohen’s d) | 0.917 (−0.03) | 0.000 ### (1.08) | - | |
| Latency | FVS | 458.16 ± 7.28 | 464.61 ± 13.32 | 0.674 (−0.16) |
| NMES | 599.24 ± 16.24 | 569.09 ± 15.66 | 0.192 (0.49) | |
| Paired t-test—p (Cohen’s d) | 0.000 ### (−2.60) | 0.000 ### (−1.32) | - | |
| Frequency Bands | Group | Two-Way Mixed ANOVA | ||
|---|---|---|---|---|
| Scheme p (Partial η2) | Target Muscle Union p (Partial η2) | Scheme × Target Muscle Union p (Partial η2) | ||
| Theta | Stroke | 0.431 (0.02) | 0.957 (0.01) | 0.510 (0.05) |
| Control | 0.007 ## (0.05) | 0.871 (0.01) | 0.969 (0.02) | |
| Alpha | Stroke | 0.304 (0.02) | 0.889 (0.01) | 0.780 (0.04) |
| Control | 0.344 (0.02) | 0.947 (0.01) | 0.147 (0.07) | |
| Beta | Stroke | 0.237 (0.02) | 0.841 (0.02) | 0.775 (0.04) |
| Control | 0.000 ### (0.08) | 0.586 (0.03) | 0.766 (0.04) | |
| Gamma | Stroke | 0.314 (0.02) | 0.832 (0.02) | 0.314 (0.06) |
| Control | 0.000 ### (0.12) | 0.870 (0.01) | 0.954 (0.03) | |
| Frequency Bands | Two-Way Mixed ANOVA | ||
|---|---|---|---|
| Scheme p (Partial η2) | Target Arm p (Partial η2) | Scheme × Target Arm p (Partial η2) | |
| Theta | 0.003 ## (0.03) | 0.538 (0.02) | 0.669 (0.02) |
| Alpha | 0.075 (0.02) | 0.007 ## (0.10) | 0.581 (0.02) |
| Beta | 0.000 ### (0.04) | 0.082 (0.06) | 0.098 (0.04) |
| Gamma | 0.000 ### (0.05) | 0.881 (0.01) | 0.397 (0.03) |
| Frequency Bands | Two-Way Mixed ANOVA | ||
|---|---|---|---|
| Scheme p (Partial η2) | Group p (Partial η2) | Scheme × Group p (Partial η2) | |
| Theta | 0.003 ## (0.03) | 0.278 (0.01) | 0.245 (0.01) |
| Alpha | 0.076 (0.02) | 0.000 ### (0.09) | 0.877 (0.00) |
| Beta | 0.000 ### (0.04) | 0.012 # (0.05) | 0.054 (0.02) |
| Gamma | 0.000 ### (0.05) | 0.902 (0.00) | 0.065 (0.02) |
| Frequency Bands | Stimulation Schemes | Nondominant Arm (Affected/Left Arm) | Dominant Arm (Unaffected/Right Arm) | ||||
|---|---|---|---|---|---|---|---|
| Stroke-Affected Arm (A) | Control Left Arm (L) | Independent t-Test | Non-Stroke-Affected Arm (U) | Control Right Arm (R) | Independent t-Test | ||
| Mean ± SE | p (Cohen’s d) | Mean ± SE | p (Cohen’s d) | ||||
| Theta band | FVS-1 | −0.41 ± 0.06 | −0.3 ± 0.08 | 0.240 (−0.31) | −0.45 ± 0.06 | −0.39 ± 0.07 | 0.490 (−0.18) |
| FVS-2 | −0.4 ± 0.05 | −0.3 ± 0.06 | 0.215 (−0.32) | −0.44 ± 0.06 | −0.39 ± 0.06 | 0.577 (−0.15) | |
| FVS-3 | −0.45 ± 0.05 | −0.36 ± 0.07 | 0.285 (−0.28) | −0.43 ± 0.06 | −0.39 ± 0.07 | 0.661 (−0.11) | |
| FVS-4 | −0.4 ± 0.05 | −0.3 ± 0.07 | 0.282 (−0.28) | −0.46 ± 0.07 | −0.41 ± 0.07 | 0.629 (−0.13) | |
| FVS-5 | −0.41 ± 0.05 | −0.31 ± 0.06 | 0.175 (−0.36) | −0.46 ± 0.06 | −0.39 ± 0.07 | 0.411 (−0.21) | |
| NMES-1 | −0.4 ± 0.05 | −0.35 ± 0.06 | 0.511 (−0.16) | −0.48 ± 0.06 | −0.41 ± 0.07 | 0.512 (−0.17) | |
| NMES-2 | −0.42 ± 0.05 | −0.4 ± 0.07 | 0.847 (−0.05) | −0.45 ± 0.06 | −0.46 ± 0.07 | 0.892 (0.04) | |
| NMES-3 | −0.46 ± 0.05 | −0.37 ± 0.06 | 0.250 (−0.30) | −0.49 ± 0.06 | −0.45 ± 0.07 | 0.694 (−0.10) | |
| One-way ANOVA RM—p (Partial η2) | 0.346 (0.04) | 0.074 (0.07) | - | 0.667 (0.02) | 0.199 (0.05) | - | |
| Alpha band | FVS-1 | −0.41 ± 0.07 | −0.63 ± 0.07 | 0.032 * (0.57) | −0.49 ± 0.07 | −0.71 ± 0.07 | 0.028 * (0.58) |
| FVS-2 | −0.39 ± 0.07 | −0.59 ± 0.07 | 0.045 * (0.53) | −0.46 ± 0.07 | −0.66 ± 0.06 | 0.034 * (0.56) | |
| FVS-3 | −0.44 ± 0.07 | −0.72 ± 0.07 | 0.005 ** (0.75) | −0.48 ± 0.07 | −0.64 ± 0.07 | 0.120 (0.41) | |
| FVS-4 | −0.39 ± 0.07 | −0.65 ± 0.07 | 0.008 ** (0.70) | −0.46 ± 0.07 | −0.67 ± 0.07 | 0.024 * (0.60) | |
| FVS-5 | −0.38 ± 0.06 | −0.62 ± 0.07 | 0.006 ** (0.74) | −0.46 ± 0.07 | −0.7 ± 0.07 | 0.016 * (0.64) | |
| NMES-1 | −0.44 ± 0.07 | −0.64 ± 0.06 | 0.041 * (0.54) | −0.49 ± 0.07 | −0.7 ± 0.08 | 0.049 * (0.52) | |
| NMES-2 | −0.41 ± 0.07 | −0.65 ± 0.06 | 0.012 * (0.67) | −0.49 ± 0.07 | −0.73 ± 0.07 | 0.019 * (0.62) | |
| NMES-3 | −0.38 ± 0.07 | −0.62 ± 0.08 | 0.024 * (0.60) | −0.48 ± 0.07 | −0.66 ± 0.07 | 0.081 (0.46) | |
| One-way ANOVA RM—p (Partial η2) | 0.360 (0.04) | 0.213 (0.05) | - | 0.885 (0.02) | 0.241 (0.05) | - | |
| Beta band | FVS-1 | 0.07 ± 0.01 | 0.06 ± 0.02 | 0.546 (0.16) | 0.09 ± 0.02 | 0.08 ± 0.02 | 0.720 (0.09) |
| FVS-2 | 0.08 ± 0.02 | 0.06 ± 0.01 | 0.323 (0.26) | 0.06 ± 0.02 | 0.09 ± 0.02 | 0.333 (−0.25) | |
| FVS-3 | 0.1 ± 0.02 | 0.06 ± 0.01 | 0.126 (0.40) | 0.1 ± 0.01 | 0.06 ± 0.02 | 0.069 (0.48) | |
| FVS-4 | 0.09 ± 0.01 | 0.06 ± 0.02 | 0.235 (0.31) | 0.1 ± 0.02 | 0.09 ± 0.02 | 0.606 (0.13) | |
| FVS-5 | 0.08 ± 0.02 | 0.04 ± 0.01 | 0.031 * (0.57) | 0.08 ± 0.02 | 0.07 ± 0.02 | 0.643 (0.12) | |
| NMES-1 | 0.09 ± 0.01 | 0.07 ± 0.02 | 0.394 (0.22) | 0.09 ± 0.02 | 0.07 ± 0.02 | 0.241 (0.31) | |
| NMES-2 | 0.09 ± 0.01 | 0.04 ± 0.02 | 0.019 * (0.62) | 0.07 ± 0.02 | 0.04 ± 0.02 | 0.228 (0.31) | |
| NMES-3 | 0.09 ± 0.01 | 0.03 ± 0.02 | 0.013 * (0.67) | 0.06 ± 0.02 | 0.01 ± 0.01 | 0.015 * (0.65) | |
| One-way ANOVA RM—p (Partial η2) | 0.821 (0.02) | 0.230 (0.04) | - | 0.091 (0.06) | 0.000 ### (0.13) | - | |
| Gamma band | FVS-1 | 0.06 ± 0.01 | 0.05 ± 0.01 | 0.385 (0.23) | 0.07 ± 0.01 | 0.06 ± 0.01 | 0.807 (0.06) |
| FVS-2 | 0.06 ± 0.01 | 0.05 ± 0.01 | 0.460 (0.19) | 0.07 ± 0.01 | 0.06 ± 0.01 | 0.205 (0.33) | |
| FVS-3 | 0.06 ± 0.01 | 0.06 ± 0.01 | 0.846 (0.05) | 0.06 ± 0.01 | 0.06 ± 0.01 | 0.745 (−0.08) | |
| FVS-4 | 0.06 ± 0.01 | 0.06 ± 0.01 | 0.631 (0.13) | 0.06 ± 0.01 | 0.06 ± 0.01 | 0.596 (0.14) | |
| FVS-5 | 0.06 ± 0.01 | 0.07 ± 0.01 | 0.677 (−0.11) | 0.07 ± 0.01 | 0.06 ± 0.01 | 0.744 (0.09) | |
| NMES-1 | 0.06 ± 0.01 | 0.06 ± 0.01 | 0.923 (0.03) | 0.07 ± 0.01 | 0.07 ± 0.01 | 0.882 (0.04) | |
| NMES-2 | 0.06 ± 0.01 | 0.07 ± 0.01 | 0.270 (−0.29) | 0.07 ± 0.01 | 0.08 ± 0.01 | 0.289 (−0.28) | |
| NMES-3 | 0.07 ± 0.01 | 0.07 ± 0.01 | 0.900 (0.03) | 0.07 ± 0.01 | 0.09 ± 0.01 | 0.195 (−0.34) | |
| One-way ANOVA RM—p (Partial η2) | 0.615 (0.02) | 0.012 # (0.08) | - | 0.462 (0.03) | 0.000 ### (0.17) | - | |
| Bands | Stimulation Target Arm | Stroke | Control | Independent t-Test |
|---|---|---|---|---|
| Mean ± SE | p (Cohen’s d) | |||
| Theta band | Nondominant (Affected/left) | −0.42 ± 0.02 | −0.34 ± 0.02 | 0.006 ** (−0.25) |
| Dominant (Unaffected/right) | −0.46 ± 0.02 | −0.4 ± 0.02 | 0.162 (−0.13) | |
| Paired t-test p (Cohen’s d) | 0.330 (0.26) | 0.004 ## (0.89) | - | |
| Alpha band | Nondominant (Affected/left) | −0.4 ± 0.02 | −0.64 ± 0.02 | 0.000 *** (0.64) |
| Dominant (Unaffected/right) | −0.47 ± 0.02 | −0.68 ± 0.02 | 0.000 *** (0.55) | |
| Paired t-test p (Cohen’s d) | 0.103 (0.45) | 0.151 (0.39) | - | |
| Beta band | Nondominant (Affected/left) | 0.09 ± 0.01 | 0.05 ± 0.01 | 0.000 *** (0.40) |
| Dominant (Unaffected/right) | 0.08 ± 0.01 | 0.06 ± 0.01 | 0.017 * (0.22) | |
| Paired t-test p (Cohen’s d) | 0.706 (0.10) | 0.217 (−0.33) | - | |
| Gamma band | Nondominant (Affected/left) | 0.06 ± 0.002 | 0.06 ± 0.003 | 0.665 (0.04) |
| Dominant (Unaffected/right) | 0.07 ± 0.002 | 0.07 ± 0.002 | 0.959 (−0.01) | |
| Paired t-test p (Cohen’s d) | 0.445 (−0.20) | 0.087 (−0.48) | - | |
| Bands | Stimulation Type | Stroke | Control | Independent t-Test |
|---|---|---|---|---|
| Mean ± SE | p (Cohen’s d) | |||
| Theta band | FVS | −0.43 ± 0.02 | −0.35 ± 0.02 | 0.005 ** (−0.23) |
| NMES | −0.45 ± 0.02 | −0.41 ± 0.03 | 0.266 (−0.12) | |
| Paired t-test p (Cohen’s d) | 0.376 (0.24) | 0.000 ### (1.08) | - | |
| Alpha band | FVS | −0.43 ± 0.02 | −0.66 ± 0.02 | 0.000 *** (0.61) |
| NMES | −0.45 ± 0.03 | −0.67 ± 0.03 | 0.000 *** (0.57) | |
| Paired t-test p (Cohen’s d) | 0.340 (0.26) | 0.565 (0.15) | - | |
| Beta band | FVS | 0.085 ± 0.005 | 0.066 ± 0.005 | 0.008 ** (0.22) |
| NMES | 0.082 ± 0.006 | 0.042 ± 0.007 | 0.000 *** (0.45) | |
| Paired t-test p (Cohen’s d) | 0.340 (0.11) | 0.023 # (0.66) | - | |
| Gamma band | FVS | 0.064 ± 0.002 | 0.06 ± 0.002 | 0.199 (0.11) |
| NMES | 0.067 ± 0.003 | 0.072 ± 0.003 | 0.249 (−0.12) | |
| Paired t-test p (Cohen’s d) | 0.136 (−0.41) | 0.000 ### (−1.55) | - | |
References
- Kessner, S.S.; Bingel, U.; Thomalla, G. Somatosensory deficits after stroke: A scoping review. Top. Stroke Rehabil. 2016, 23, 136–146. [Google Scholar] [CrossRef] [PubMed]
- Edwards, L.L.; King, E.M.; Buetefisch, C.M.; Borich, M.R. Putting the “sensory” into sensorimotor control: The role of sensorimotor integration in goal-directed hand movements after stroke. Front. Integr. Neurosci. 2019, 13, 16. [Google Scholar] [CrossRef] [PubMed]
- Bolognini, N.; Russo, C.; Edwards, D.J. The sensory side of post-stroke motor rehabilitation. Restor. Neurol. Neurosci. 2016, 34, 571–586. [Google Scholar] [CrossRef] [PubMed]
- Conforto, A.B.; Dos Anjos, S.M.; Bernardo, W.M.; Da Silva, A.A.; Conti, J.; Machado, A.G.; Cohen, L.G. Repetitive Peripheral Sensory Stimulation and Upper Limb Performance in Stroke: A Systematic Review and Meta-analysis. Neurorehabil. Neural. Repair. 2018, 32, 863–871. [Google Scholar] [CrossRef] [PubMed]
- Sitaram, R.; Ros, T.; Stoeckel, L.; Haller, S.; Scharnowski, F.; Lewis-Peacock, J.; Weiskopf, N.; Blefari, M.L.; Rana, M.; Oblak, E.; et al. Closed-loop brain training: The science of neurofeedback. Nat. Rev. Neurosci. 2017, 18, 86–100. [Google Scholar] [CrossRef] [PubMed]
- Fleury, M.; Lioi, G.; Barillot, C.; Lécuyer, A. A Survey on the Use of Haptic Feedback for Brain-Computer Interfaces and Neurofeedback. Front. Neurosci. 2020, 14, 528. [Google Scholar] [CrossRef] [PubMed]
- Calabrò, R.S.; Naro, A.; Russo, M.; Milardi, D.; Leo, A.; Filoni, S.; Trinchera, A.; Bramanti, P. Is two better than one? Muscle vibration plus robotic rehabilitation to improve upper limb spasticity and function: A pilot randomized controlled trial. PLoS ONE 2017, 12, e0185936. [Google Scholar] [CrossRef] [PubMed]
- Corbet, T.; Iturrate, I.; Pereira, M.; Perdikis, S.; Del Millán, J.R. Sensory threshold neuromuscular electrical stimulation fosters motor imagery performance. Neuroimage 2018, 176, 268–276. [Google Scholar] [CrossRef]
- Paillard, T. Neuromuscular or Sensory Electrical Stimulation for Reconditioning Motor Output and Postural Balance in Older Subjects? Front. Physiol. 2021, 12, 779249. [Google Scholar] [CrossRef]
- Huang, Y.; Nam, C.; Li, W.; Rong, W.; Xie, Y.; Liu, Y.; Qian, Q.; Hu, X. A comparison of the rehabilitation effectiveness of neuromuscular electrical stimulation robotic hand training and pure robotic hand training after stroke: A randomized controlled trial. Biomed. Signal Process. Control 2020, 56, 101723. [Google Scholar] [CrossRef]
- Bao, S.-C.; Khan, A.; Song, R.; Kai-Yu Tong, R. Rewiring the Lesioned Brain: Electrical Stimulation for Post-Stroke Motor Restoration. J. Stroke 2020, 22, 47–63. [Google Scholar] [CrossRef] [PubMed]
- Hautasaari, P.; Kujala, U.M.; Tarkka, I.M. Detecting differences with magnetoencephalography of somatosensory processing after tactile and electrical stimuli. J. Neurosci. Methods 2019, 311, 331–337. [Google Scholar] [CrossRef] [PubMed]
- Shindo, K.; Fujiwara, T.; Hara, J.; Oba, H.; Hotta, F.; Tsuji, T.; Hase, K.; Liu, M. Effectiveness of hybrid assistive neuromuscular dynamic stimulation therapy in patients with subacute stroke: A randomized controlled pilot trial. Neurorehabil. Neural. Repair. 2011, 25, 830–837. [Google Scholar] [CrossRef] [PubMed]
- Insausti-Delgado, A.; López-Larraz, E.; Omedes, J.; Ramos-Murguialday, A. Intensity and Dose of Neuromuscular Electrical Stimulation Influence Sensorimotor Cortical Excitability. Front. Neurosci. 2020, 14, 593360. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Chandrashekhar, R.; Rippetoe, J.; Ghazi, M. Focal Muscle Vibration for Stroke Rehabilitation: A Review of Vibration Parameters and Protocols. Appl. Sci. 2020, 10, 8270. [Google Scholar] [CrossRef]
- Souron, R.; Besson, T.; Millet, G.Y.; Lapole, T. Acute and chronic neuromuscular adaptations to local vibration training. Eur. J. Appl. Physiol. 2017, 117, 1939–1964. [Google Scholar] [CrossRef] [PubMed]
- Amano, Y.; Noma, T.; Etoh, S.; Miyata, R.; Kawamura, K.; Shimodozono, M. Reaching exercise for chronic paretic upper extremity after stroke using a novel rehabilitation robot with arm-weight support and concomitant electrical stimulation and vibration: Before-and-after feasibility trial. Biomed. Eng. Online 2020, 19, 28. [Google Scholar] [CrossRef] [PubMed]
- Alsuradi, H.; Park, W.; Eid, M. EEG-Based Neurohaptics Research: A Literature Review. IEEE Access 2020, 8, 49313–49328. [Google Scholar] [CrossRef]
- Reuter, E.-M.; Voelcker-Rehage, C.; Vieluf, S.; Winneke, A.H.; Godde, B. Extensive occupational finger use delays age effects in tactile perception-an ERP study. Atten. Percept. Psychophys. 2014, 76, 1160–1175. [Google Scholar] [CrossRef]
- Bolton, D.A.E.; Staines, W.R. Age-related loss in attention-based modulation of tactile stimuli at early stages of somatosensory processing. Neuropsychologia 2012, 50, 1502–1513. [Google Scholar] [CrossRef]
- Schranz, C.; Vatinno, A.; Ramakrishnan, V.; Seo, N.J. Neuroplasticity after upper-extremity rehabilitation therapy with sensory stimulation in chronic stroke survivors. Brain Commun. 2022, 4, fcac191. [Google Scholar] [CrossRef] [PubMed]
- Yeh, I.-L.; Holst-Wolf, J.; Elangovan, N.; Cuppone, A.V.; Lakshminarayan, K.; Cappello, L.; Masia, L.; Konczak, J. Effects of a robot-aided somatosensory training on proprioception and motor function in stroke survivors. J. NeuroEng. Rehabil. 2021, 18, 77. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Jiao, J.; Hu, J.; Hsing, C.; Lai, Z.; Yang, Y.; Hu, X. Measurement of sensory deficiency in fine touch after stroke during textile fabric stimulation by electroencephalography (EEG). J. Neural Eng. 2020, 17, 45007. [Google Scholar] [CrossRef]
- Hu, X.; Tong, K.; Tsang, V.S.; Song, R. Joint-angle-dependent neuromuscular dysfunctions at the wrist in persons after stroke. Arch. Phys. Med. Rehabil. 2006, 87, 671–679. [Google Scholar] [CrossRef] [PubMed]
- Tombaugh, T.N.; McIntyre, N.J. The mini-mental state examination: A comprehensive review. J. Am. Geriatr. Soc. 1992, 40, 922–935. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, K.J.; Tilson, J.K.; Cen, S.Y.; Rose, D.K.; Hershberg, J.; Correa, A.; Gallichio, J.; McLeod, M.; Moore, C.; Wu, S.S.; et al. Fugl-Meyer assessment of sensorimotor function after stroke: Standardized training procedure for clinical practice and clinical trials. Stroke 2011, 42, 427–432. [Google Scholar] [CrossRef] [PubMed]
- Nam, C.; Zhang, B.; Chow, T.; Ye, F.; Huang, Y.; Guo, Z.; Li, W.; Rong, W.; Hu, X.; Poon, W. Home-based self-help telerehabilitation of the upper limb assisted by an electromyography-driven wrist/hand exoneuromusculoskeleton after stroke. J. NeuroEng. Rehabil. 2021, 18, 137. [Google Scholar] [CrossRef]
- Bohannon, R.W.; Smith, M.B. Interrater reliability of a modified Ashworth scale of muscle spasticity. Phys. Ther. 1987, 67, 206–207. [Google Scholar] [CrossRef]
- Suda, M.; Kawakami, M.; Okuyama, K.; Ishii, R.; Oshima, O.; Hijikata, N.; Nakamura, T.; Oka, A.; Kondo, K.; Liu, M. Validity and Reliability of the Semmes-Weinstein Monofilament Test and the Thumb Localizing Test in Patients with Stroke. Front. Neurol. 2020, 11, 625917. [Google Scholar] [CrossRef]
- Perle, S.M.; Schneider, M.J.; Seaman, D.R. Chiropractic Management of Peripheral Neuropathy: Pathophysiology, Assessment, and Treatment. Top. Clin. Chiropr. 1999, 6, 6–19. [Google Scholar]
- Seeck, M.; Koessler, L.; Bast, T.; Leijten, F.; Michel, C.; Baumgartner, C.; He, B.; Beniczky, S. The standardized EEG electrode array of the IFCN. Clin. Neurophysiol. 2017, 128, 2070–2077. [Google Scholar] [CrossRef] [PubMed]
- Lou, X.; Xiao, S.; Qi, Y.; Hu, X.; Wang, Y.; Zheng, X. Corticomuscular coherence analysis on hand movement distinction for active rehabilitation. Comput. Math. Method Med. 2013, 2013, 908591. [Google Scholar] [CrossRef] [PubMed]
- Nam, C.; Rong, W.; Li, W.; Cheung, C.; Ngai, W.; Cheung, T.; Pang, M.; Li, L.; Hu, J.; Wai, H.; et al. An Exoneuromusculoskeleton for Self-Help Upper Limb Rehabilitation After Stroke. Soft Robot. 2020, 9, 14–35. [Google Scholar] [CrossRef] [PubMed]
- Muraoka, Y. Development of an EMG recording device from stimulation electrodes for functional electrical stimulation. Front. Med. Biol. Eng. 2002, 11, 323–333. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.; Kuchenbecker, K.J. Vibrotactile Display: Perception, Technology, and Applications. Proc. IEEE 2013, 101, 2093–2104. [Google Scholar] [CrossRef]
- Nam, C.; Rong, W.; Li, W.; Xie, Y.; Hu, X.; Zheng, Y. The Effects of Upper-Limb Training Assisted with an Electromyography-Driven Neuromuscular Electrical Stimulation Robotic Hand on Chronic Stroke. Front. Neurol. 2017, 8, 679. [Google Scholar] [CrossRef] [PubMed]
- Murillo, N.; Valls-Sole, J.; Vidal, J.; Opisso, E.; Medina, J.; Kumru, H. Focal vibration in neurorehabilitation. Eur. J. Phys. Rehabil. Med. 2014, 50, 231–242. [Google Scholar]
- Wang, H.; Ghazi, M.; Chandrashekhar, R.; Rippetoe, J.; Duginski, G.A.; Lepak, L.V.; Milhan, L.R.; James, S.A. User Participatory Design of a Wearable Focal Vibration Device for Home-Based Stroke Rehabilitation. Sensors 2022, 22, 3308. [Google Scholar] [CrossRef]
- Seim, C.E.; Wolf, S.L.; Starner, T.E. Wearable vibrotactile stimulation for upper extremity rehabilitation in chronic stroke: Clinical feasibility trial using the VTS Glove. J. NeuroEng. Rehabil. 2021, 18, 14. [Google Scholar] [CrossRef]
- Genna, C.; Oddo, C.M.; Fanciullacci, C.; Chisari, C.; Jörntell, H.; Artoni, F.; Micera, S. Spatiotemporal Dynamics of the Cortical Responses Induced by a Prolonged Tactile Stimulation of the Human Fingertips. Brain Topogr. 2017, 30, 473–485. [Google Scholar] [CrossRef]
- EEGLAB Toolbox. Available online: https://sccn.ucsd.edu/eeglab/index.php (accessed on 24 October 2023).
- FieldTrip Toolbox. Available online: https://www.fieldtriptoolbox.org/ (accessed on 24 October 2023).
- Delorme, A.; Makeig, S. EEGLAB: An open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. J. Neurosci. Methods 2004, 134, 9–21. [Google Scholar] [CrossRef] [PubMed]
- Oostenveld, R.; Fries, P.; Maris, E.; Schoffelen, J.-M. FieldTrip: Open source software for advanced analysis of MEG, EEG, and invasive electrophysiological data. Comput. Intell. Neurosci. 2011, 2011, 156869. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Guo, Z.; Wong, K.; Zhu, H.; Huang, Y.; Hu, X.; Zheng, Y.-P. Pathway-specific cortico-muscular coherence in proximal-to-distal compensation during fine motor control of finger extension after stroke. J. Neural Eng. 2021, 18, 56034. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.; Qian, Q.; Wong, K.; Zhu, H.; Huang, Y.; Hu, X.; Zheng, Y. Altered Corticomuscular Coherence (CMCoh) Pattern in the Upper Limb During Finger Movements After Stroke. Front. Neurol. 2020, 11, 410. [Google Scholar] [CrossRef] [PubMed]
- Makeig, S.; Bell, A.; Jung, T.-P.; Sejnowski, T.J. Independent Component Analysis of Electroencephalographic Data. Adv. Neural Inf. Process. Syst. 1995, 8, 146–151. [Google Scholar]
- Pope, K.J.; Lewis, T.W.; Fitzgibbon, S.P.; Janani, A.S.; Grummett, T.S.; Williams, P.A.H.; Battersby, M.; Bastiampillai, T.; Whitham, E.M.; Willoughby, J.O. Managing electromyogram contamination in scalp recordings: An approach identifying reliable beta and gamma EEG features of psychoses or other disorders. Brain Behav. 2022, 12, e2721. [Google Scholar] [CrossRef] [PubMed]
- Bao, S.-C.; Leung, K.W.; Tong, K.-Y. Cortico-muscular interaction to monitor the effects of neuromuscular electrical stimulation pedaling training in chronic stroke. Comput. Biol. Med. 2021, 137, 104801. [Google Scholar] [CrossRef]
- Delorme, A.; Westerfield, M.; Makeig, S. Medial prefrontal theta bursts precede rapid motor responses during visual selective attention. J. Neurosci. 2007, 27, 11949–11959. [Google Scholar] [CrossRef]
- Grandchamp, R.; Delorme, A. Single-trial normalization for event-related spectral decomposition reduces sensitivity to noisy trials. Front. Psychol. 2011, 2, 236. [Google Scholar] [CrossRef]
- Remsik, A.B.; van Kan, P.L.E.; Gloe, S.; Gjini, K.; Williams, L.; Nair, V.; Caldera, K.; Williams, J.C.; Prabhakaran, V. BCI-FES With Multimodal Feedback for Motor Recovery Poststroke. Front. Hum. Neurosci. 2022, 16, 725715. [Google Scholar] [CrossRef]
- Mastrich, Z.; Hernandez, I. Results everyone can understand: A review of common language effect size indicators to bridge the research-practice gap. Health Psychol. 2021, 40, 727–736. [Google Scholar] [CrossRef] [PubMed]
- Otsuru, N.; Inui, K.; Yamashiro, K.; Urakawa, T.; Keceli, S.; Kakigi, R. Effects of prior sustained tactile stimulation on the somatosensory response to the sudden change of intensity in humans: An magnetoencephalography study. Neuroscience 2011, 182, 115–124. [Google Scholar] [CrossRef]
- Backes, W.H.; Mess, W.H.; van Kranen-Mastenbroek, V.; Reulen, J.P. Somatosensory cortex responses to median nerve stimulation: fMRI effects of current amplitude and selective attention. Clin. Neurophysiol. 2000, 111, 1738–1744. [Google Scholar] [CrossRef] [PubMed]
- Smith, G.V.; Alon, G.; Roys, S.R.; Gullapalli, R.P. Functional MRI determination of a dose-response relationship to lower extremity neuromuscular electrical stimulation in healthy subjects. Exp. Brain Res. 2003, 150, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Fraser, C.; Power, M.; Hamdy, S.; Rothwell, J.; Hobday, D.; Hollander, I.; Tyrell, P.; Hobson, A.; Williams, S.; Thompson, D. Driving plasticity in human adult motor cortex is associated with improved motor function after brain injury. Neuron 2002, 34, 831–840. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.-Y.; Kwon, H.; Yang, T.-H.; Kim, K. Vibration Alert to the Brain: Evoked and Induced MEG Responses to High-Frequency Vibrotactile Stimuli on the Index Finger of Dominant and Non-dominant Hand. Front. Hum. Neurosci. 2020, 14, 576082. [Google Scholar] [CrossRef] [PubMed]
- Jousmäki, V. Tracking functions of cortical networks on a millisecond timescale. Neural Netw. 2000, 13, 883–889. [Google Scholar] [CrossRef] [PubMed]
- Ortiz Alonso, T.; Santos, J.M.; Ortiz Terán, L.; Borrego Hernández, M.; Poch Broto, J.; de Erausquin, G.A. Differences in Early Stages of Tactile ERP Temporal Sequence (P100) in Cortical Organization during Passive Tactile Stimulation in Children with Blindness and Controls. PLoS ONE 2015, 10, e0124527. [Google Scholar] [CrossRef]
- Yakovlev, L.; Syrov, N.; Miroshnikov, A.; Lebedev, M.; Kaplan, A. Event-Related Desynchronization Induced by Tactile Imagery: An EEG Study. eNeuro 2023, 10, 1–12. [Google Scholar] [CrossRef]
- Carson, R.G.; Buick, A.R. Neuromuscular electrical stimulation-promoted plasticity of the human brain. J. Physiol. 2021, 599, 2375–2399. [Google Scholar] [CrossRef]
- Graczyk, E.L.; Delhaye, B.P.; Schiefer, M.A.; Bensmaia, S.J.; Tyler, D.J. Sensory adaptation to electrical stimulation of the somatosensory nerves. J. Neural Eng. 2018, 15, 46002. [Google Scholar] [CrossRef] [PubMed]
- Khajuria, A.; Joshi, D. EEG-explained cortical correlates of transfemoral amputees during balancing with vibrotactile feedback: A pilot study. Med. Eng. Phys. 2022, 101, 103772. [Google Scholar] [CrossRef] [PubMed]
- Wang, A.L.; Mouraux, A.; Liang, M.; Iannetti, G.D. Stimulus novelty, and not neural refractoriness, explains the repetition suppression of laser-evoked potentials. J. Neurophysiol. 2010, 104, 2116–2124. [Google Scholar] [CrossRef] [PubMed]
- Michail, G.; Dresel, C.; Witkovský, V.; Stankewitz, A.; Schulz, E. Neuronal Oscillations in Various Frequency Bands Differ between Pain and Touch. Front. Hum. Neurosci. 2016, 10, 182. [Google Scholar] [CrossRef] [PubMed]
- Williamson, J.N.; Sikora, W.A.; James, S.A.; Parmar, N.J.; Lepak, L.V.; Cheema, C.F.; Refai, H.H.; Wu, D.H.; Sidorov, E.V.; Dewald, J.P.A.; et al. Cortical Reorganization of Early Somatosensory Processing in Hemiparetic Stroke. J. Clin. Med. 2022, 11, 6449. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Huang, Y.; Jiao, J.; Hu, J.; Hsing, C.; Lai, Z.; Yang, Y.; Hu, X. Impairments of cortico-cortical connectivity in fine tactile sensation after stroke. J. NeuroEng. Rehabil. 2021, 18, 34. [Google Scholar] [CrossRef] [PubMed]
- Bauer, M.; Oostenveld, R.; Peeters, M.; Fries, P. Tactile spatial attention enhances gamma-band activity in somatosensory cortex and reduces low-frequency activity in parieto-occipital areas. J. Neurosci. 2006, 26, 490–501. [Google Scholar] [CrossRef]
- Aumann, T.D.; Prut, Y. Do sensorimotor β-oscillations maintain muscle synergy representations in primary motor cortex? Trends Neurosci. 2015, 38, 77–85. [Google Scholar] [CrossRef]
- Tyson, S.F.; Hanley, M.; Chillala, J.; Selley, A.B.; Tallis, R.C. Sensory loss in hospital-admitted people with stroke: Characteristics, associated factors, and relationship with function. Neurorehabil. Neural. Repair. 2008, 22, 166–172. [Google Scholar] [CrossRef]
- Celletti, C.; Suppa, A.; Bianchini, E.; Lakin, S.; Toscano, M.; La Torre, G.; Di Piero, V.; Camerota, F. Promoting post-stroke recovery through focal or whole body vibration: Criticisms and prospects from a narrative review. Neurol. Sci. 2020, 41, 11–24. [Google Scholar] [CrossRef]
- Perpetuini, D.; Russo, E.F.; Cardone, D.; Palmieri, R.; de Giacomo, A.; Pellegrino, R.; Merla, A.; Calabrò, R.S.; Filoni, S. Use and Effectiveness of Electrosuit in Neurological Disorders: A Systematic Review with Clinical Implications. Bioengineering 2023, 10, 680. [Google Scholar] [CrossRef]


| Group | No. of Participants | Stroke Type (Hemorrhage/ Ischemic) | Affected Arm (Left/Right) | Gender (Male/Female) | Age (Years, Mean ± SD) | Years after Stroke (Min/Max Years) |
|---|---|---|---|---|---|---|
| Stroke | 15 | 9/6 | 10/5 | 8/7 | 53 ± 11 | 2/18 |
| Control | 15 | −/− | −/− | 9/6 | 67 ± 3 | −/− |
| Clinical Assessment | FMA | MAS | Monofilament | ||||
|---|---|---|---|---|---|---|---|
| Upper Extremity | Wrist | Finger | Affected Extensor | Affected Flexor | Unaffected Extensor | Unaffected Flexor | |
| Score (mean ± SD) | 31.1 ± 11.5 | 1.1 ± 0.9 | 1.9 ± 0.8 | 3.23 ± 0.67 | 3.24 ± 0.49 | 3 ± 0.45 | 3.26 ± 0.38 |
| Group | Target Arm | Target Muscle Union | Stimulation Scheme/Trial |
|---|---|---|---|
| Stroke group | Nondominant/affected arm (A) | Affected ECU-ED (AE) | FVS-1,2,3,4,5; NMES-1,2,3 |
| Affected FCR-FD (AF) | FVS-1,2,3,4,5; NMES-1,2,3 | ||
| Dominant/unaffected arm (U) | Unaffected ECU-ED (UE) | FVS-1,2,3,4,5; NMES-1,2,3 | |
| Unaffected FCR-FD (UF) | FVS-1,2,3,4,5; NMES-1,2,3 | ||
| Control group | Nondominant/left arm (L) | Left ECU-ED (LE) | FVS-1,2,3,4,5; NMES-1,2,3 |
| Left FCR-FD (LF) | FVS-1,2,3,4,5; NMES-1,2,3 | ||
| Dominant/right arm (R) | Right ECU-ED (RE) | FVS-1,2,3,4,5; NMES-1,2,3 | |
| Right FCR-FD (RF) | FVS-1,2,3,4,5; NMES-1,2,3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, L.; Qing, W.; Huang, Y.; Ye, F.; Rong, W.; Li, W.; Jiao, J.; Hu, X. Comparison of Immediate Neuromodulatory Effects between Focal Vibratory and Electrical Sensory Stimulations after Stroke. Bioengineering 2024, 11, 286. https://doi.org/10.3390/bioengineering11030286
Lin L, Qing W, Huang Y, Ye F, Rong W, Li W, Jiao J, Hu X. Comparison of Immediate Neuromodulatory Effects between Focal Vibratory and Electrical Sensory Stimulations after Stroke. Bioengineering. 2024; 11(3):286. https://doi.org/10.3390/bioengineering11030286
Chicago/Turabian StyleLin, Legeng, Wanyi Qing, Yanhuan Huang, Fuqiang Ye, Wei Rong, Waiming Li, Jiao Jiao, and Xiaoling Hu. 2024. "Comparison of Immediate Neuromodulatory Effects between Focal Vibratory and Electrical Sensory Stimulations after Stroke" Bioengineering 11, no. 3: 286. https://doi.org/10.3390/bioengineering11030286






